Dose Tapering of Advanced Therapies in Psoriatic Arthritis: Clinical Predictors and Outcomes in a Biosimilar-Dominant Real-Life Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Study Variables
2.3. Statistical Methodology
3. Results
3.1. Summary of Study Population
3.2. Differences Based on Sex
3.3. Differences Based on Drug Exposure
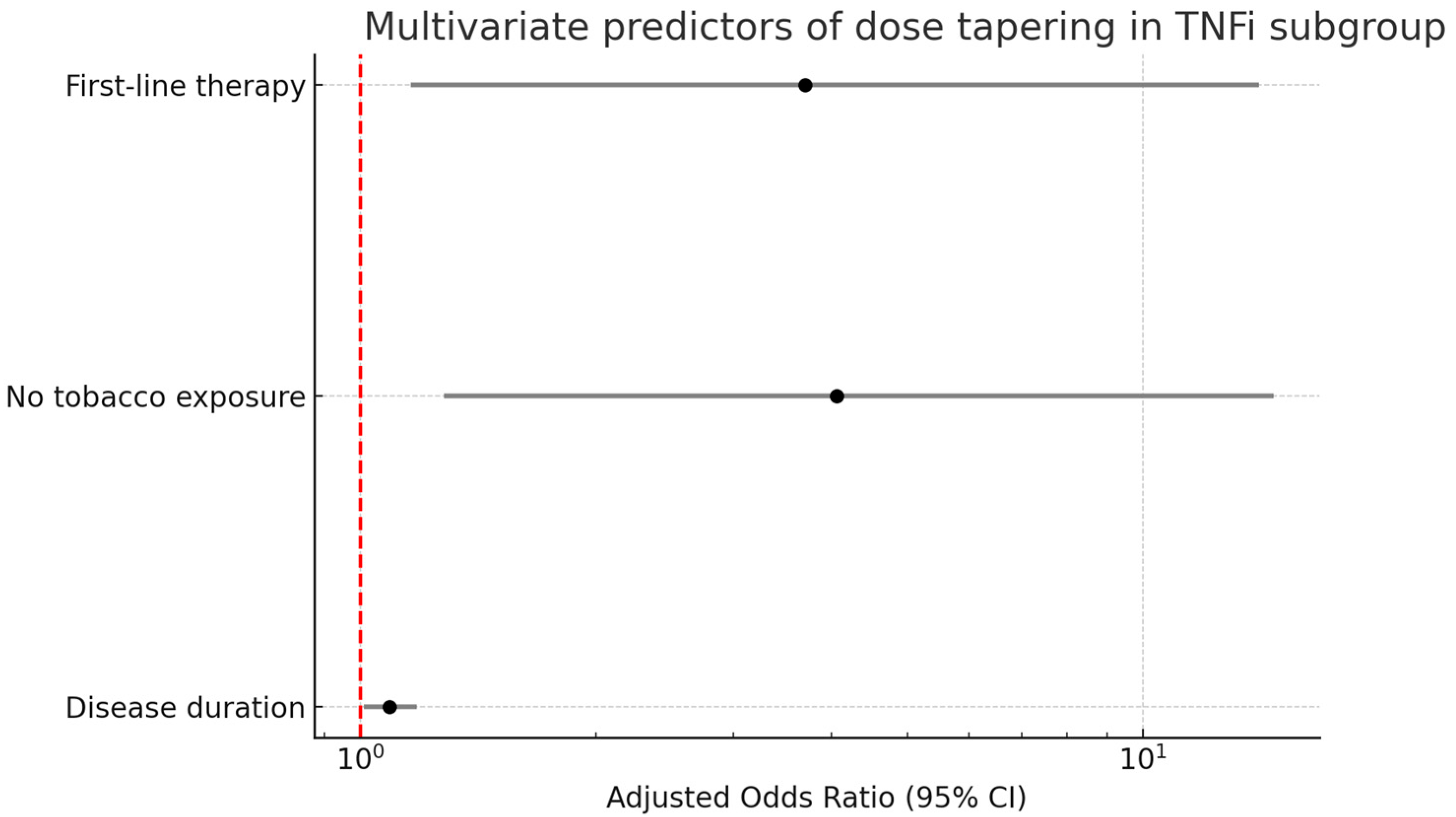
3.4. Dose Reduction-Associated Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerschbaumer, A.; Smolen, J.S.; Ferreira, R.J.O.; Bertheussen, H.; Baraliakos, X.; Aletaha, D.; McGonagle, D.G.; van der Heijde, D.; McInnes, I.B.; Esbensen, B.A.; et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: Systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann. Rheum. Dis. 2024, 83, 760–774. [Google Scholar] [CrossRef] [PubMed]
- Monte-Boquet, E.; Florez, Á.; Alcaín Martínez, G.J.; Sellas, A. Consensus statement on the use of biosimilar drugs in immune-mediated diseases in Spain. Reumatol. Clin. (Engl. Ed.) 2023, 19, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Tucker, L.J.; Coates, L.C. Tapering and Discontinuation of Biologics in Patients with Psoriatic Arthritis with Low Disease Activity. Drugs 2018, 78, 1705–1715. [Google Scholar] [CrossRef]
- Michielsens, C.A.; den Broeder, N.; van den Hoogen, F.H.; Mahler, E.A.; Teerenstra, S.; van der Heijde, D.; Verhoef, L.M.; den Broeder, A.A. Treat-to-target dose reduction and withdrawal strategy of TNF inhibitors in psoriatic arthritis and axial spondyloarthritis: A randomised controlled non-inferiority trial. Ann. Rheum. Dis. 2022, 81, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Ruwaard, J.; L’ Ami, M.J.; Kneepkens, E.L.; Krieckaert, C.; Nurmohamed, M.T.; Hooijberg, F.; van Kuijk, A.; van Denderen, J.C.; Burgemeister, L.; Rispens, T.; et al. Interval prolongation of etanercept in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: A randomized controlled trial. Scand. J. Rheumatol. 2023, 52, 129–136. [Google Scholar] [CrossRef]
- Gossec, L.; Kerschbaumer, A.; Ferreira, R.J.O.; Aletaha, D.; Baraliakos, X.; Bertheussen, H.; Boehncke, W.H.; Esbensen, B.A.; McInnes, I.B.; McGonagle, D.; et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 2024, 83, 706–719. [Google Scholar] [CrossRef]
- Uhrenholt, L.; Sørensen, M.E.R.; Lauridsen, K.B.; Duch, K.; Dreyer, L.; Christensen, R.; Hauge, E.M.; Loft, A.G.; Rasch, M.N.B.; Horn, H.C.; et al. Exploring TNFi drug-levels and anti-drug antibodies during tapering among patients with inflammatory arthritis: Secondary analyses from the randomised BIODOPT trial. Rheumatol. Int. 2024, 44, 1897–1908. [Google Scholar] [CrossRef]
- González-Álvaro, I.; Martínez-Fernández, C.; Dorantes-Calderón, B.; García-Vicuña, R.; Hernández-Cruz, B.; Herrero-Ambrosio, A.; Ibarra-Barrueta, O.; Martín-Mola, E.; Monte-Boquet, E.; Morell-Baladrón, A.; et al. Spanish Rheumatology Society and Hospital Pharmacy Society Consensus on recommendations for biologics optimization in patients with rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis. Rheumatology 2015, 54, 1200–1209. [Google Scholar] [CrossRef]
- Abad Hernández, M.Á.; Andreu, J.L.; Balsa Criado, A.; Díaz-González, F.; Moreno Muelas, J.V.; Queiro Silva, R.; Gómez-Reino, J.J. Update of the Position Paper of the Spanish Society of Rheumatology on Biosimilar Drugs. Reumatol. Clin. (Engl. Ed.) 2021, 17, 160–169. [Google Scholar] [CrossRef]
- García-Beloso, N.; Altabás-González, I.; Samartín-Ucha, M.; Gayoso-Rey, M.; De Castro-Parga, M.L.; Salgado-Barreira, Á.; Cibeira-Badia, A.; Piñeiro-Corrales, M.G.; González-Vilas, D.; Pego-Reigosa, J.M.; et al. Switching between reference adalimumab and biosimilars in chronic immune-mediated inflammatory diseases: A systematic literature review. Br. J. Clin. Pharmacol. 2022, 88, 1529–1550. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, H.R.; Lee, S.H.; Nam, B.; Shin, J.H.; Kim, T.H. Risk of disease flare in spondyloarthritis patients after tapering tumor necrosis factor inhibitors: A meta-analysis and literature review. Int. Immunopharmacol. 2024, 134, 112167. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Rosenø, N.A.L.; Aagaard, D.; Lørup, E.H.; Nielsen, M.L.; Nymand, L.; Kristensen, L.E.; Thyssen, J.P.; Thomsen, S.F.; Cordtz, R.L.; et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis—A nationwide cohort study from the DANBIO and DERMBIO registries. Semin. Arthritis. Rheum. 2022, 53, 151979. [Google Scholar] [CrossRef] [PubMed]
- Pezzolo, E.; Naldi, L. The relationship between smoking, psoriasis and psoriatic arthritis. Expert. Rev. Clin. Immunol. 2019, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, B.; Østergaard, M.; Dreyer, L.; Krogh, N.S.; Tarp, U.; Hansen, M.S.; Rifbjerg-Madsen, S.; Lorenzen, T.; Hetland, M.L. Treatment response, drug survival, and predictors thereof in 764 patients with psoriatic arthritis treated with anti-tumor necrosis factor alpha therapy: Results from the nationwide Danish DANBIO registry. Arthritis. Rheum. 2011, 63, 382–390. [Google Scholar] [CrossRef]
- Haddad, A.; Gazitt, T.; Feldhamer, I.; Feld, J.; Cohen, A.D.; Lavi, I.; Tatour, F.; Bergman, I.; Zisman, D. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res. Ther. 2021, 23, 44. [Google Scholar] [CrossRef]
- Prior-Español, A.; Sánchez-Piedra, C.; Campos, J.; Manero, F.J.; Pérez-García, C.; Bohórquez, C.; Busquets-Pérez, N.; Blanco-Madrigal, J.M.; Díaz-Torne, C.; Sánchez-Alonso, F.; et al. Clinical factors associated with discontinuation of ts/bDMARDs in rheumatic patients from the BIOBADASER III registry. Sci. Rep. 2021, 11, 11091. [Google Scholar] [CrossRef]
- Stober, C.; Ye, W.; Guruparan, T.; Htut, E.; Clunie, G.; Jadon, D. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology 2018, 57, 158–163. [Google Scholar] [CrossRef]
- Alonso, S.; Villa, I.; Fernández, S.; Martín, J.L.; Charca, L.; Pino, M.; Riancho, L.; Morante, I.; Santos, M.; Brandy, A.; et al. Multicenter Study of Secukinumab Survival and Safety in Spondyloarthritis and Psoriatic Arthritis: SEcukinumab in Cantabria and ASTURias Study. Front. Med. 2021, 8, 679009. [Google Scholar] [CrossRef]
- Uhrenholt, L.; Duch, K.; Christensen, R.; Dreyer, L.; Hauge, E.M.; Schlemmer, A.; Taylor, P.C.; Kristensen, S. Predicting successful biologics tapering in patients with inflammatory arthritis: Secondary analyses based on the BIOlogical Dose OPTimisation (BIODOPT) trial. Br. J. Clin. Pharmacol. 2023, 89, 3152–3164. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; He, D.; Chen, X.; Wang, H.; Zhang, Y.; Xue, Q.; Liu, W.; Xiang, G.; Li, Y.; et al. Disease activity guided stepwise tapering or discontinuation of rhTNFR:Fc, an etanercept biosimilar, in patients with ankylosing spondylitis: A prospective, randomized, open-label, multicentric study. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20929441. [Google Scholar] [CrossRef]
- Queiro, R.; Loredo, M.; Braña, I.; Pardo, E.; Alonso, S.; Alperi, M. Managing psoriatic arthritis in different clinical scenarios. Expert. Rev. Clin. Immunol. 2023, 19, 1469–1484. [Google Scholar] [CrossRef] [PubMed]

| Variable | N: 130 |
|---|---|
| Age, years, mean (SD) | 55.6 (11.2) |
| Male, n (%) | 64 (49.2) |
| Female, n (%) | 66 (51.8) |
| University degree, n (%) | 28 (21.5) |
| Disease duration, years, median (IQR) | 8.0 (3.0–13.0) |
| Smokers, n (%) | 37 (28.5) |
| Former smokers, n (%) | 29 (22.3) |
| Alcohol drinkers, n (%) | 22 (17) |
| Weight, mean (SD) | 77.7 (15.3) |
| CV comorbidity: | |
| Diabetes, n (%) | 15 (11.5) |
| Hypertension, n (%) | 39 (30) |
| Dyslipidemia, n (%) | 49 (37.7) |
| Hyperuricemia, n (%) | 38 (29.2) |
| CV events, n (%) | 5 (3.8) |
| Family history: | |
| Psoriasis, n (%) | 55 (42.3) |
| PsA, n (%) | 12 (9.2) |
| CRP, median (IQR) | 0.20 [0.0–4.70] |
| CRP ≥ 0.5 mg/dL, n (%) | 31 (23.8) |
| PsA pattern: | |
| Peripheral, n (%) | 97 (74.6) |
| Mixed, n (%) | 26 (20) |
| Axial, n (%) | 6 (4.6) |
| PsA features: | |
| Dactylitis, n (%) | 53 (40.8) |
| Enthesitis, n (%) | 37 (28.5) |
| Uveitis, n (%) | 1 (0.8) |
| Nail disease, n (%) | 34 (26) |
| Outcomes: | |
| DAPSA, median (IQR) | 5.0 (0.4–10.0) |
| DAPSA remission, n (%) | 57 (43.8) |
| DAPSA low, n (%) | 55 (42.3) |
| Physician’s GDA, median (IQR) | 2.0 (0.0–4.0) |
| Treatment: | |
| Methotrexate, n (%) | 47 (36.2) |
| Leflunomide, n (%) | 12 (9.2) |
| TNF inhibitors, n (%) | 95 (73.1) |
| IL-17 inhibitors, n (%) | 14 (10.8) |
| Ustekinumab, n (%) | 2 (1.5) |
| Apremilast, n (%) | 13 (10) |
| Tofacitinib, n (%) | 6 (4.6) |
| Therapy line: | |
| 1, n (%) | 78 (60) |
| 2, n (%) | 24 (18.5) |
| ≥3, n (%) | 28 (21.5) |
| Median exposure to advanced therapies, years (IQR) | 1.7 (0.9–4.7) |
| * Dose tapering, n (%) | 32 (24.6) |
| Univariate Regression Model OR (95%CI), p-Value | Multivariate Regression Model OR (95%CI), p-Value |
|---|---|
| Male 2.8 (1.2–6.6), 0.018 | Male 3.26 (1.26–9.04), 0.018 |
| Age 1.02 (0.98–1.052), 0.430 | |
| Weight 0.99 (0.96–1.01), 0.347 | |
| Disease duration 1.08 (1.02–1.15), 0.008 | Disease duration 1.06 (0.99–1.13), 0.090 |
| No tobacco exposure 3.94 (1.4–11.5), 0.012 | No tobacco exposure 3.98 (1.30–14.20), 0.021 |
| Alcohol 1.23 (0.44–3.50), 0.696 | |
| University degree 1.70 (0.68–4.28), 0.259 | |
| Hypertension 0.75 (0.30–1.89), 0.539 | |
| Diabetes 0.45 (0.10–2.12), 0.313 | |
| Dyslipidemia 1.24 (0.55–2.83), 0.603 | |
| Hyperuricemia 1.45 (0.61–3.41), 0.400 | |
| Cardiovascular events 1.06 (0.11–10.50), 0.963 | |
| Nail disease 0.63 (0.23–1.71), 0.365 | |
| Enthesitis 0.65 (0.25–1.68), 0.374 | |
| Dactylitis 1.05 (0.46–2.38), 0.912 | |
| Axial disease 0.88 (0.32–2.42), 0.805 | |
| C-reactive protein 0.88 (0.77–1.00), 0.058 | |
| Physician’s global disease assessment 0.54 (0.40–0.73), <0.001 | |
| DAPSA 0.73 (0.63–0.85), <0.001 | |
| Methotrexate 1.62 (0.71–3.69), 0.249 | |
| Leflunomide 1.67 (0.47–5.96), 0.432 | |
| TNF inhibitors 15.2 (1.99–116.7), 0.009 | |
| First-line therapy 4.8 (1.7–13.5), 0.003 | First-line therapy 4.8 (1.7–16.7), 0.006 |
| Biosimilar 0.63 (0.13–3.02), 0.564 |
| Univariate Regression Model OR (95%CI), p-Value | Multivariate Regression Model OR (95%CI), p-Value |
|---|---|
| Male 2.41 (0.96–6.04), 0.061 | |
| Age 1.03 (0.99–1.07), 0.196 | |
| Weight 0.98 (0.95–1.01), 0.189 | |
| Disease duration 1.12 (1.04–1.20), 0.003 | Disease duration 1.09 (1.01–1.18), 0.030 |
| No tobacco exposure 4.05 (1.32–12.50), 0.015 | No tobacco exposure 4.06 (1.28–14.72), 0.023 |
| Alcohol 1.23 (0.41–3.71), 0.716 | |
| University degree 1.56 (0.59–4.16), 0.373 | |
| Hypertension 0.74 (0.27–2.0), 0.550 | |
| Diabetes 0.25 (0.03–2.06), 0.196 | |
| Dyslipidemia 1.22 (0.51–2.94), 0.653 | |
| Hyperuricemia 0.98 (0.39–2.44), 0.961 | |
| Cardiovascular events 2.21 (0.13–36.52), 0.580 | |
| Nail disease 0.56 (0.20–1.59), 0.277 | |
| Enthesitis 0.99 (0.36–2.77), 0.99 | |
| Dactylitis 1.01 (0.42–2.42), 0.981 | |
| Axial disease 1.10 (0.37–3.29), 0.859 | |
| C-reactive protein 0.90 (0.77–1.05), 0.170 | |
| Physician’s global disease assessment 0.57 (0.41–0.79), <0.001 | |
| DAPSA 0.73 (0.62–0.87), <0.001 | |
| Methotrexate 1.09 (0.46–2.59), 0.852 | |
| Leflunomide 9.85 (1.05–92.32), 0.045 | |
| First-line therapy 2.56 (0.86–7.60), 0.091 | First-line therapy 3.70 (1.16–14.10), 0.037 |
| Biosimilar 0.63 (0.13–3.02), 0.564 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loredo, M.; Pardo, E.; Braña, I.; Burger, S.; Chiminazzo, V.; Queiro, R. Dose Tapering of Advanced Therapies in Psoriatic Arthritis: Clinical Predictors and Outcomes in a Biosimilar-Dominant Real-Life Cohort. J. Clin. Med. 2025, 14, 4099. https://doi.org/10.3390/jcm14124099
Loredo M, Pardo E, Braña I, Burger S, Chiminazzo V, Queiro R. Dose Tapering of Advanced Therapies in Psoriatic Arthritis: Clinical Predictors and Outcomes in a Biosimilar-Dominant Real-Life Cohort. Journal of Clinical Medicine. 2025; 14(12):4099. https://doi.org/10.3390/jcm14124099
Chicago/Turabian StyleLoredo, Marta, Estefanía Pardo, Ignacio Braña, Stefanie Burger, Valentina Chiminazzo, and Rubén Queiro. 2025. "Dose Tapering of Advanced Therapies in Psoriatic Arthritis: Clinical Predictors and Outcomes in a Biosimilar-Dominant Real-Life Cohort" Journal of Clinical Medicine 14, no. 12: 4099. https://doi.org/10.3390/jcm14124099
APA StyleLoredo, M., Pardo, E., Braña, I., Burger, S., Chiminazzo, V., & Queiro, R. (2025). Dose Tapering of Advanced Therapies in Psoriatic Arthritis: Clinical Predictors and Outcomes in a Biosimilar-Dominant Real-Life Cohort. Journal of Clinical Medicine, 14(12), 4099. https://doi.org/10.3390/jcm14124099







