Exploring the Impact of Gender and Age of Onset on Psoriasis Treatment Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Definition of Cohort
2.3. Baseline Characteristics
2.4. Treatments
2.5. Statistical Analysis
2.6. Use of Generative AI Tools
3. Results
3.1. Patient Characteristics
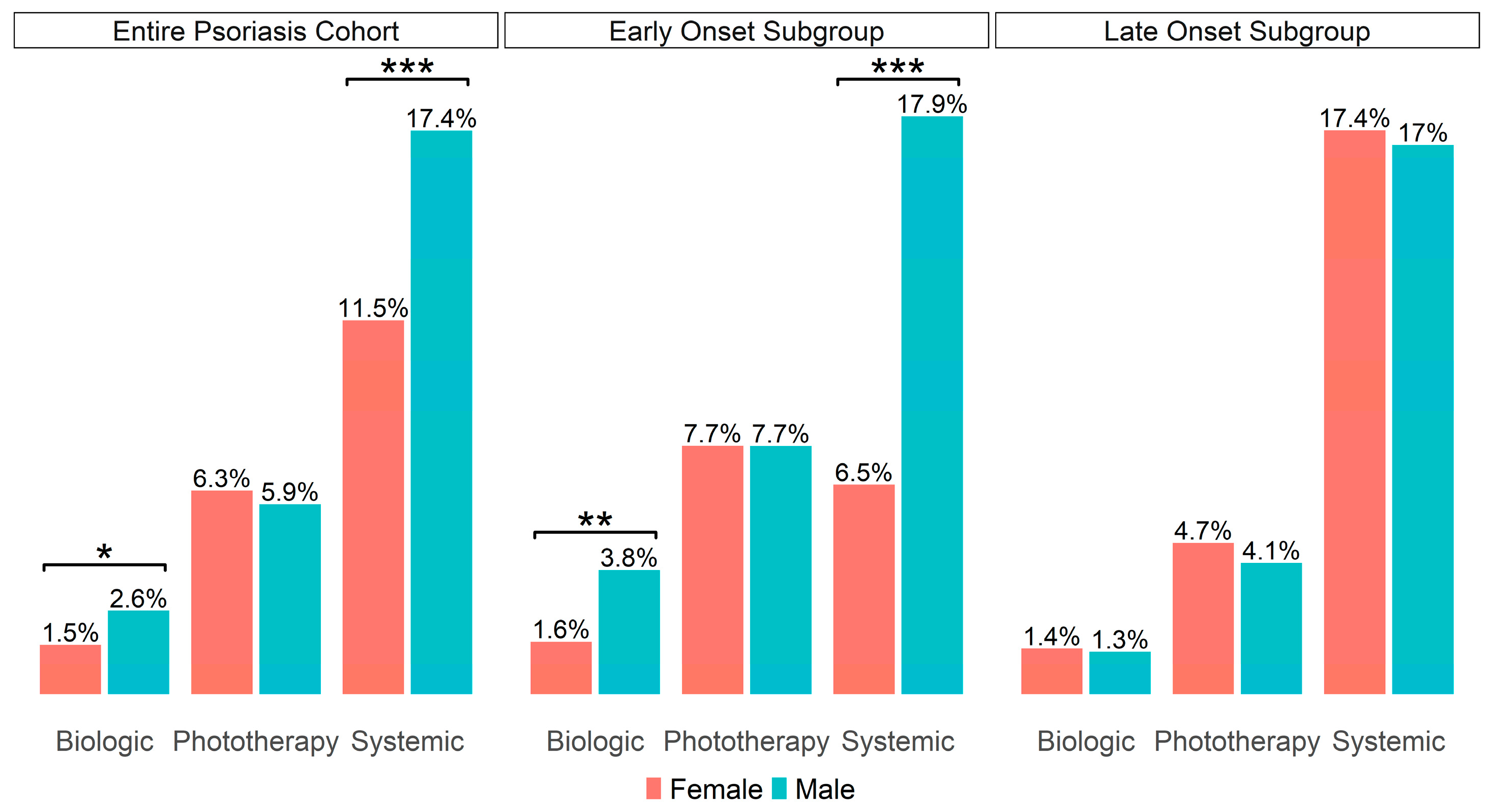
3.2. Comparison of Psoriasis Treatment Management and Time to Treatment Initiation Between Male and Female
3.3. Comparison of Time to Treatment Initiation Between Early-Onset and Late-Onset Patients
3.4. Cox Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHS | Clalit Health Services |
| PASI | Psoriasis Area Severity Index |
| EHR | Electronic Health Records |
| HMO | Health Maintenance Organization |
| IMIDs | Immune-Mediated Inflammatory Diseases |
References
- Eder, L.; Widdifield, J.; Rosen, C.F.; Cook, R.; Lee, K.; Alhusayen, R.; Paterson, M.J.; Cheng, S.Y.; Jabbari, S.; Campbell, W.; et al. Trends in the Prevalence and Incidence of Psoriasis and Psoriatic Arthritis in Ontario, Canada: A Population-Based Study. Arthritis Care Res. 2019, 71, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Schonmann, Y.; Ashcroft, D.M.; Iskandar, I.Y.K.; Parisi, R.; Sde-Or, S.; Comaneshter, D.; Batat, E.; Shani, M.; Vinker, S.; Griffiths, C.E.M.; et al. Incidence and Prevalence of Psoriasis in Israel between 2011 and 2017. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2075–2081. [Google Scholar] [CrossRef]
- Henseler, T.; Christophers, E. Psoriasis of Early and Late Onset: Characterization of Two Types of Psoriasis Vulgaris. J. Am. Acad. Dermatol. 1985, 13, 450–456. [Google Scholar] [CrossRef]
- Queiro, R.; Tejón, P.; Alonso, S.; Coto, P. Age at Disease Onset: A Key Factor for Understanding Psoriatic Disease. Rheumatology 2014, 53, 1178–1185. [Google Scholar] [CrossRef]
- Coto-Segura, P.; Vázquez-Coto, D.; Velázquez-Cuervo, L.; García-Lago, C.; Coto, E.; Queiro, R. The IFIH1/MDA5 Rs1990760 Gene Variant (946Thr) Differentiates Early- vs. Late-Onset Skin Disease and Increases the Risk of Arthritis in a Spanish Cohort of Psoriasis. Int. J. Mol. Sci. 2023, 24, 14803. [Google Scholar] [CrossRef]
- Mallon, E.; Bunker, C.B.; Newson, R. HLA-Cw6 and the Genetic Predisposition to Psoriasis: A Meta-Analysis of Published Serologic Studies. J. Investig. Dermatol. 1999, 113, 693–695. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Pujol, R.M.; García-Patos, V.; Bordas, X.; Smandía, J.A. Psoriasis of Early and Late Onset: A Clinical and Epidemiologic Study from Spain. J. Am. Acad. Dermatol. 2002, 46, 867–873. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis. JAMA 2020, 323, 1945. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, M. Challenges and Future Trends in the Treatment of Psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Ko, S.-H.; Chi, C.-C.; Yeh, M.-L.; Wang, S.-H.; Tsai, Y.-S.; Hsu, M.-Y. Lifestyle Changes for Treating Psoriasis. Cochrane Database Syst. Rev. 2019, 2019, CD011972. [Google Scholar] [CrossRef]
- Hägg, D.; Sundström, A.; Eriksson, M.; Schmitt-Egenolf, M. Severity of Psoriasis Differs Between Men and Women: A Study of the Clinical Outcome Measure Psoriasis Area and Severity Index (PASI) in 5438 Swedish Register Patients. Am. J. Clin. Dermatol. 2017, 18, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Hägg, D.; Eriksson, M.; Sundström, A.; Schmitt-Egenolf, M. The Higher Proportion of Men with Psoriasis Treated with Biologics May Be Explained by More Severe Disease in Men. PLoS ONE 2013, 8, e63619. [Google Scholar] [CrossRef]
- Stemmer, E.; Salmon-Divon, M.; Sinberger, L.A.; Lax, T.; Shrem, G.; Mor, I.; Zahavi, T. Sex-Based Transcriptomic Differences in Psoriatic Lesions: A Comprehensive Meta-Analysis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kim, N. Application of Sex/Gender-Specific Medicine in Healthcare. Korean J. Women Health Nurs. 2023, 29, 5–11. [Google Scholar] [CrossRef]
- Eder, L.; Widdifield, J.; Rosen, C.F.; Alhusayen, R.; Cheng, S.Y.; Young, J.; Campbell, W.; Bernatsky, S.; Gladman, D.D.; Paterson, M.; et al. Identifying and Characterizing Psoriasis and Psoriatic Arthritis Patients in Ontario Administrative Data: A Population-Based Study From 1991 to 2015. J. Rheumatol. 2020, 47, 1644–1651. [Google Scholar] [CrossRef]
- Na, S.J.; Jo, S.J.; Youn, J. Il Clinical Study on Psoriasis Patients for Past 30 Years (1982–2012) in Seoul National University Hospital Psoriasis Clinic. J. Dermatol. 2013, 40, 731–735. [Google Scholar] [CrossRef] [PubMed]
- El-Komy, M.H.M.; Mashaly, H.; Sayed, K.S.; Hafez, V.; El-Mesidy, M.S.; Said, E.R.; Amer, M.A.; AlOrbani, A.M.; Saadi, D.G.; El-Kalioby, M.; et al. Clinical and Epidemiologic Features of Psoriasis Patients in an Egyptian Medical Center. JAAD Int. 2020, 1, 81–90. [Google Scholar] [CrossRef]
- Remröd, C.; Sjöström, K.; Svensson, Å. Psychological Differences between Early- and Late-Onset Psoriasis: A Study of Personality Traits, Anxiety and Depression in Psoriasis. Br. J. Dermatol. 2013, 169, 344–350. [Google Scholar] [CrossRef]
- Chularojanamontri, L.; Kulthanan, K.; Suthipinittharm, P.; Jiamton, S.; Wongpraparut, C.; Silpa-Archa, N.; Tuchinda, P.; Sirikuddta, W. Clinical Differences between Early- and Late-onset Psoriasis in Thai Patients. Int. J. Dermatol. 2015, 54, 290–294. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, T.-F. HLA-Cw6 and Psoriasis. Br. J. Dermatol. 2018, 178, 854–862. [Google Scholar] [CrossRef]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; De Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the Systemic Treatment of Psoriasis Vulgaris—Part 2: Specific Clinical and Comorbid Situations. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 281–317. [Google Scholar] [CrossRef] [PubMed]
- Puchner, A.; Gröchenig, H.P.; Sautner, J.; Helmy-Bader, Y.; Juch, H.; Reinisch, S.; Högenauer, C.; Koch, R.; Hermann, J.; Studnicka-Benke, A.; et al. Immunosuppressives and Biologics during Pregnancy and Lactation. Wien. Klin. Wochenschr. 2019, 131, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Gubbels Bupp, M.R. Sex, the Aging Immune System, and Chronic Disease. Cell Immunol. 2015, 294, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Regulatory Roles of Sex Hormones in Cutaneous Biology and Immunology. J. Dermatol. Sci. 2005, 38, 1–7. [Google Scholar] [CrossRef]
- Adachi, A.; Honda, T. Regulatory Roles of Estrogens in Psoriasis. J. Clin. Med. 2022, 11, 4890. [Google Scholar] [CrossRef]
- Murase, J.E.; Chan, K.K.; Garite, T.J.; Cooper, D.M.; Weinstein, G.D. Hormonal Effect on Psoriasis in Pregnancy and Post Partum. Arch. Dermatol. 2005, 141, 601–606. [Google Scholar] [CrossRef]
- Cemil, B.C.; Cengiz, F.P.; Atas, H.; Ozturk, G.; Canpolat, F. Sex Hormones in Male Psoriasis Patients and Their Correlation with the Psoriasis Area and Severity Index. J. Dermatol. 2015, 42, 500–503. [Google Scholar] [CrossRef]
- Stanczyk, F.Z.; Clarke, N.J. Measurement of Estradiol—Challenges Ahead. J. Clin. Endocrinol. Metab. 2014, 99, 56–58. [Google Scholar] [CrossRef]
- Mowad, C.M.; Margolis, D.J.; Halpern, A.C.; Suri, B.; Synnestvedt, M.; Guzzo, C.A. Hormonal Influences on Women with Psoriasis. Cutis 1998, 61, 257–260. [Google Scholar]
- Xiao, Y.; Yi, Y.; Jing, D.; Yang, S.; Guo, Y.; Xiao, H.; Kuang, Y.; Zhu, W.; Zhao, J.; Li, Y.; et al. Age at Natural Menopause, Reproductive Lifespan, and the Risk of Late-Onset Psoriasis and Psoriatic Arthritis in Women: A Prospective Cohort Study. J. Investig. Dermatol. 2024, 144, 1273–1281.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, M.; Jiang, J.; Duan, X.; Chai, B.; Zhang, J.; Tao, Q.; Chen, H. Triggers for the Onset and Recurrence of Psoriasis: A Review and Update. Cell Commun. Signal. 2024, 22, 108. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Jenkins, M. Gender Differences in Risk Assessment: Why Do Women Take Fewer Risks than Men? Judgm. Decis. Mak. 2006, 1, 48–63. [Google Scholar] [CrossRef]


| Entire Psoriasis Cohort | Early-Onset Subgroup | Late-Onset Subgroup | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 3999) | Male (n = 2613) | Female (n = 1386) | p | Total (n = 2048) | Male (n = 1305) | Female (n = 743) | p | Total (n = 1951) | Male (n = 1308) | Female (n = 643) | p | |
| Onset age, Median (IQR) | 39.1 (26.6–54.4) | 40.1 (28.1–54.5) | 37.2 (24.0–54.1) | <0.001 b | 26.8 (19.8–32.5) | 28.1 (21.6–33.1) | 24.7 (17.5–31.3) | <0.001 b | 54.7 (47.8–62.2) | 54.5 (47.8–62.1) | 55.2 (48.1–62.3) | 0.9 b |
| Smoking % (n) | 35.2 (3911) | 40.9 (2558) | 24.5 (1353) | <0.001 a | 27.5 (1996) | 32.6 (1275) | 18.3 (721) | <0.001 a | 43.3 (1915) | 49 (1283) | 31.6 (632) | <0.001 a |
| Physical Activity % (n) | 17.3 (2160) | 18.4 (1321) | 15.5 (839) | 0.1 a | 13.4 (925) | 14.6 (522) | 11.9 (403) | 0.3 a | 20.2 (1235) | 20.9 (799) | 18.8 (436) | 0.4 a |
| BMI, Median (IQR, n) | 26 (22.6– 29.8, 3902) | 26.3 (23.3–29.8, 2550) | 25.3 (21.6–29.7, 1352) | <0.001 b | 23.9 (20.8–27.4, 1996) | 24.4 (21.6–27.8, 1272) | 22.7 (19.9–26.5, 724) | <0.001 b | 28 (25.1–31.4, 1906) | 28.1 (25.3–31.1, 1278) | 27.7 (24.4–32.5, 628) | 0.8 b |
| Obese (BMI > 30) % (n) | 24.1 (3902) | 24 (2550) | 24.5 (1352) | 0.7 a | 14.7 (1996) | 15.3 (1272) | 13.5 (724) | 0.3 a | 34.1 (1906) | 32.6 (1278) | 37.1 (628) | 0.055 a |
| Socioeconomic Distribution | 0.06 a | 0.051 a | 0.87 a | |||||||||
| Socioeconomic High % (n) | 23.7 (3933) | 24.4 (2574) | 22.6 (1359) | 22.9 (2016) | 23.9 (1289) | 21.0 (727) | 24.7 (1917) | 24.8 (1285) | 24.4 (632) | |||
| Socioeconomic Medium % (n) | 61.5 (3933) | 61.8 (2574) | 60.9 (1359) | 59.7 (2016) | 60.1 (1289) | 59.0 (727) | 63.4 (1917) | 63.5 (1285) | 63.1 (632) | |||
| Socioeconomic Low % (n) | 14.7 (3933) | 13.8 (2574) | 16.5 (1359) | 17.4 (2016) | 16 (1289) | 19.9 (727) | 11.9 (1917) | 11.7 (1285) | 12.5 (632) | |||
| One or more pregnancies after onset % | 28.8 (1386) | 52.8 (743) | 1.1 (643) | |||||||||
| Biological | Systemic | Phototherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Hazard Ratio (HR) | 95% CI | p-Value | n | Hazard Ratio (HR) | 95% CI | p-Value | n | Hazard Ratio (HR) | 95% CI | p-Value | |
| Late-Onset | 3940 | 0.4 | 0.3–0.7 | 0.0005 | 3897 | 1.23 | 1.04–1.45 | 0.02 | 3927 | 0.55 | 0.4–0.7 | <0.0001 |
| Gender-Male | 3940 | 1.9 | 1.2–3.2 | 0.01 | 3897 | 1.7 | 1.4–2 | <0.0001 | 3927 | 0.97 | 0.74–1.3 | 0.8 |
| Smoking | 3855 | 1.1 | 0.7–1.7 | 0.7 | 3813 | 1.5 | 1.3–1.8 | <0.0001 | 3845 | 1.1 | 0.84–1.45 | 0.5 |
| Physical Activity | 2121 | 0.7 | 0.3–1.7 | 0.4 | 2096 | 0.75 | 0.5–1 | 0.07 | 2115 | 0.46 | 0.24–0.88 | 0.02 |
| BMI | 3849 | 1.01 | 0.98–1.05 | 0.5 | 3808 | 1.02 | 1.01–1.04 | 0.0004 | 3838 | 0.96 | 0.94–0.98 | 0.001 |
| Obese (BMI > 30) | 3849 | 1.3 | 0.8–2.08 | 0.3 | 3808 | 1.3 | 1.07–1.5 | 0.008 | 3838 | 0.9 | 0.66–1.2 | 0.5 |
| Socioeconomic Low | 3875 | 1.7 | 0.86–3.4 | 0.1 | 3832 | 1.5 | 1.2–2 | 0.002 | 3862 | 0.7 | 0.45–1.06 | 0.09 |
| Socioeconomic Medium | 3875 | 1.2 | 0.7–2.1 | 0.45 | 2832 | 1.2 | 0.99–1.5 | 0.06 | 3862 | 0.65 | 0.5–0.9 | 0.003 |
| Hazard Ratio (HR) | 95% CI | p-Value | |
|---|---|---|---|
| Biological (n = 3940) | |||
| Late-Onset | 0.4 | 0.3–0.7 | 0.00035 |
| Gender-Male | 2 | 1.2–3.3 | 0.006 |
| Systemic (n = 3722) | |||
| Late-Onset | 1.05 | 0.9–1.25 | 0.6 |
| Gender-Male | 1.6 | 1.3–1.9 | <0.0001 |
| Smoking | 1.4 | 1.15–1.6 | 0.0004 |
| BMI | 1 | 0.99–1.03 | 0.3 |
| Obese (BMI > 30) | 1.1 | 0.8–1.5 | 0.5 |
| Socioeconomic Low | 1.5 | 1.2–2 | 0.002 |
| Socioeconomic Medium | 1.2 | 0.96–1.5 | 0.1 |
| Phototherapy (n = 2075) | |||
| Late-Onset | 0.7 | 0.5–1.04 | 0.07 |
| BMI | 0.96 | 0.93–0.99 | 0.046 |
| Physical Activity | 0.48 | 0.25–0.9 | 0.03 |
| Socioeconomic Low | 0.64 | 0.3–1.2 | 0.2 |
| Socioeconomic Medium | 0.83 | 0.5–1.3 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lax, T.; Stemmer, E.; Fallach, N.; Shrem, G.; Schreiber-Divon, M.; Ayalon, S.; Giat, E.; Mor, I.; Salmon-Divon, M. Exploring the Impact of Gender and Age of Onset on Psoriasis Treatment Management. J. Clin. Med. 2025, 14, 4090. https://doi.org/10.3390/jcm14124090
Lax T, Stemmer E, Fallach N, Shrem G, Schreiber-Divon M, Ayalon S, Giat E, Mor I, Salmon-Divon M. Exploring the Impact of Gender and Age of Onset on Psoriasis Treatment Management. Journal of Clinical Medicine. 2025; 14(12):4090. https://doi.org/10.3390/jcm14124090
Chicago/Turabian StyleLax, Tair, Edia Stemmer, Noga Fallach, Guy Shrem, Michal Schreiber-Divon, Snait Ayalon, Eitan Giat, Inbal Mor, and Mali Salmon-Divon. 2025. "Exploring the Impact of Gender and Age of Onset on Psoriasis Treatment Management" Journal of Clinical Medicine 14, no. 12: 4090. https://doi.org/10.3390/jcm14124090
APA StyleLax, T., Stemmer, E., Fallach, N., Shrem, G., Schreiber-Divon, M., Ayalon, S., Giat, E., Mor, I., & Salmon-Divon, M. (2025). Exploring the Impact of Gender and Age of Onset on Psoriasis Treatment Management. Journal of Clinical Medicine, 14(12), 4090. https://doi.org/10.3390/jcm14124090







