Treating IgA Nephropathy: Looking at the Future Without Forgetting the Past
Abstract
1. Introduction
1.1. Interest in Halting IgA Nephropathy Progression
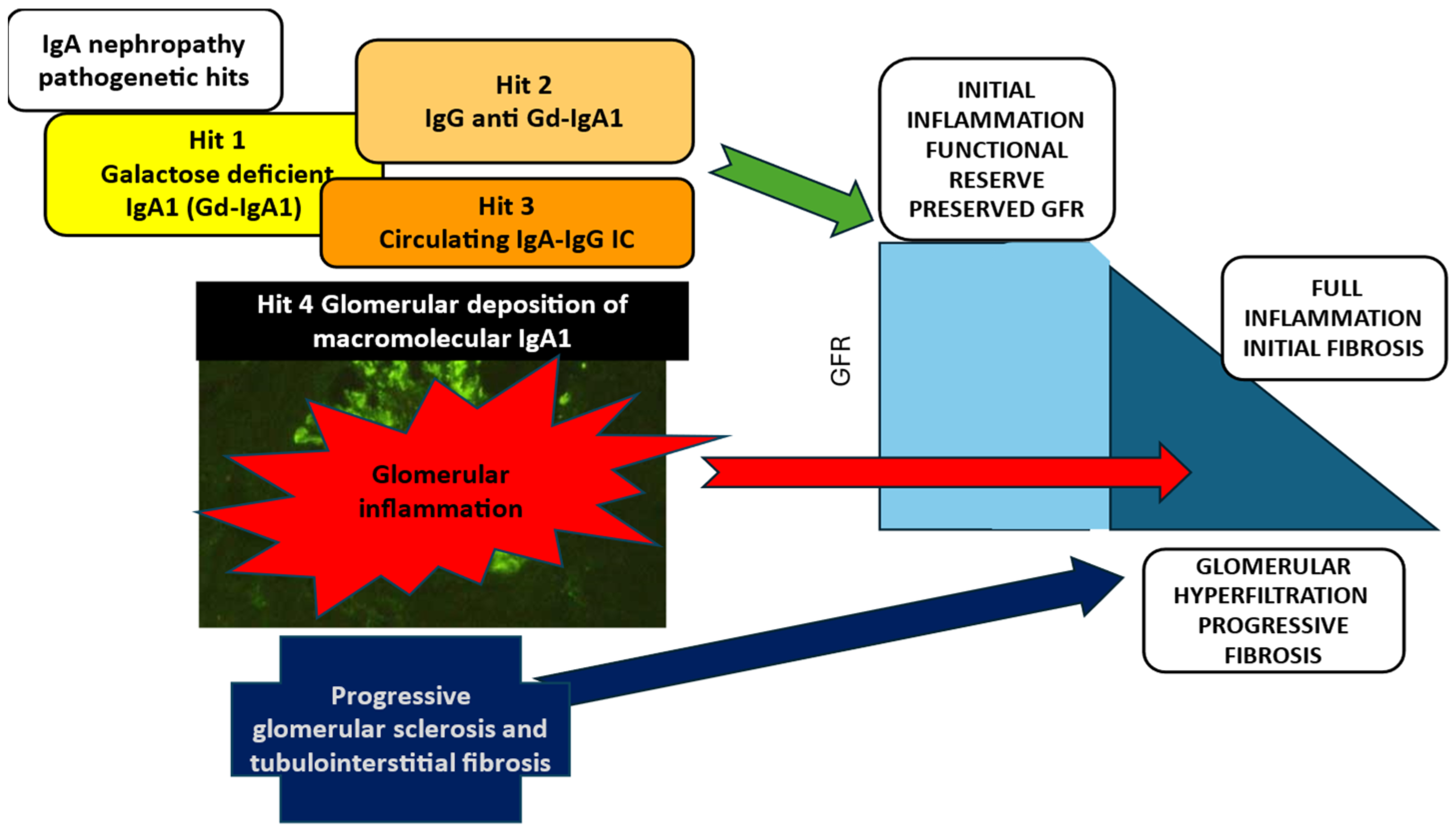
1.2. Pathogenesis of IgAN: The Initiating Events and the Progression
1.3. An Overview of the Evolving Recommendations for Treating IgAN
2. Treatment of IgAN with Corticosteroids (CSs)
2.1. Broad-Acting Systemic Glucocorticosteroids
2.2. A New Horizon for Corticosteroid Treatment in IgAN: Intestinal Immunity Targeted Formulation of Budesonide
3. New KDIGO to Be Published in 2025
4. Treatment Targeting the First Pathogenetic Hit of IgAN: The Synthesis of Gd-IgA1
5. Existing Therapies to Inhibit Lymphocyte Proliferation
6. New Treatments Targeting B Cells and Plasma Cells
7. New Treatments Targeting the Amplification of Glomerular Inflammation: The Complement Cascade
8. The Expanded Supportive Care (Non-Immunologic Treatment) for Patients with IgAN: SGLT2 Inhibitors and Anti-Endothelin A
9. Final Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, J.; Hinglais, N. Intercapillary deposits of IgA-IgG. J. Urol. Nephrol. 1968, 74, 694–695. [Google Scholar]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2021, 361, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.W.; Reinholdt, J.; Kilian, M. Anti-inflammatory activity of human IgA antibodies and their Fab alpha fragments: Inhibition of IgG-mediated complement activation. Eur. J. Immunol. 1989, 19, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- Peruzzi, L.; Coppo, R. IgAN Across the Age Spectrum: The Pediatric Perspective. Semin. Nephrol. 2024, 44, 151569. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.C.; Rauta, V.; Gronhagen-Riska, C.; Bartosik, L.P.; Jardine, A.G.; Ibels, L.S.; Pei, Y.; Cattran, D.C. A tricontinental view of IgA nephropathy. Nephrol. Dial. Transplant. 2003, 18, 1541–1548. [Google Scholar] [CrossRef]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Er, L.; Espino-Hernandez, G.; Kim, S.J.; et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern. Med. 2019, 179, 942–952. [Google Scholar] [CrossRef]
- Pitcher, D.; Braddon, F.; Hendry, B.; Mercer, A.; Osmaston, K.; Saleem, M.A.; Steenkamp, R.; Wong, K.; Turner, A.N.; Wang, K.; et al. Long-Term Outcomes in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2023, 18, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Coppo, R.; Er, L.; Russo, M.L.; Liu, Z.H.; Ding, J.; Katafuchi, R.; Yoshikawa, N.; Xu, H.; Kagami, S.; et al. International IgA Nephropathy Network. Updating the International IgA Nephropathy Prediction Tool for use in children. Kidney Int. 2021, 99, 1439–1450. [Google Scholar] [CrossRef]
- Infante, B.; Rossini, M.; Di Lorenzo, A.; Coviello, N.; Giuseppe, C.; Gesualdo, L.; Giuseppe, G.; Stallone, G. Recurrence of immunoglobulin A nephropathy after kidney transplantation: A narrative review of the incidence, risk factors, pathophysiology and management of immunosuppressive therapy. Clin. Kidney J. 2020, 13, 758–767. [Google Scholar] [CrossRef]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The pathophysiology of IgA nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Floege, J.; Coppo, R. Evaluating Progression Risk in Patients With Immunoglobulin A Nephropathy. Kidney Int. Rep. 2023, 8, 2515–2528. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Bolasco, P.G.; Fogazzi, G.B.; Andrulli, S.; Altieri, P.; Ponticelli, C.; Locatelli, F. Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 1999, 353, 883–887. [Google Scholar] [CrossRef]
- Manno, C.; Torres, D.D.; Rossini, M.; Pesce, F.; Schena, F.P. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol. Dial. Transplant. 2009, 24, 3694–3701. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, H.; Chen, Y.; Li, G.; Jiang, L.; Singh, A.K.; Wang, H. Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am. J. Kidney Dis. 2009, 53, 26–32. [Google Scholar] [CrossRef]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Peters, H.; Benck, U.; Mertens, P.R.; et al. STOP-IgAN Investigators. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N. Engl. J. Med. 2015, 373, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, H.; Wong, M.G.; Jardine, M.J.; Hladunewich, M.; Jha, V.; Monaghan, H.; Zhao, M.; Barbour, S.; Reich, H.; et al. TESTING Study Group. Effect of Oral Methylprednisolone on Clinical Outcomes in Patients with IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2017, 318, 432–442. [Google Scholar] [CrossRef]
- Floege, J.; Feehally, J. Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat. Rev. Nephrol. 2013, 9, 320–327. [Google Scholar] [CrossRef]
- Lv, J.; Wong, M.G.; Hladunewich, M.A.; Jha, V.; Hooi, L.S.; Monaghan, H.; Zhao, M.; Barbour, S.; Jardine, M.J.; Reich, H.N.; et al. TESTING Study Group. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA 2022, 327, 1888–1898. [Google Scholar] [CrossRef]
- Barratt, J.; Lafayette, R.; Kristensen, J.; Stone, A.; Cattran, D.; Floege, J.; Tesar, V.; Trimarchi, H.; Zhang, H.; Eren, N.; et al. NefIgArd Trial Investigators. Results from part A of the multi-center, double-blind, randomized, placebo-controlled NefIgArd trial, which evaluated targeted-release formulation of budesonide for the treatment of primary immunoglobulin A nephropathy. Kidney Int. 2023, 103, 391–402. [Google Scholar] [CrossRef]
- Lafayette, R.; Kristensen, J.; Stone, A.; Floege, J.; Tesař, V.; Trimarchi, H.; Zhang, H.; Eren, N.; Paliege, A.; Reich, H.N.; et al. NefIgArd trial investigators. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet 2023, 402, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, C.; Andrulli, S.; Del Vecchio, L.; Melis, P.; Fogazzi, G.B.; Altieri, P.; Ponticelli, C.; Locatelli, F. Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J. Am. Soc. Nephrol. 2004, 15, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Feehally, J.; Cook, H.T.; Liu, Z.H.; Fervenza, F.C.; Mezzano, S.A.; Floege, J.; Nachman, P.H.; Gipson, D.S.; Praga, M.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int. Suppl. 2012, 2, 1–274. [Google Scholar]
- Rauen, T.; Wied, S.; Fitzner, C.; Eitner, F.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Budde, K.; Benck, U.; et al. STOP-IgAN Investigators. After ten years of follow-up, no difference between supportive care plus immunosuppression and supportive care alone in IgA nephropathy. Kidney Int. 2020, 98, 1044–1052. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. Executive summary of the KDIGO 2021 Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, 753–779. [Google Scholar] [CrossRef]
- Locatelli, F.; Del Vecchio, L.; Ponticelli, C. Systemic and targeted steroids for the treatment of IgA nephropathy. Clin. Kidney J. 2023, 16 (Suppl. S2), ii40–ii46. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef]
- Barratt, J.; Rovin, B.H.; Cattran, D.; Floege, J.; Lafayette, R.; Tesar, V.; Trimarchi, H.; Zhang, H.; NefIgArd Study Steering Committee. Why Target the Gut to Treat IgA Nephropathy? Kidney Int. Rep. 2020, 5, 1620–1624. [Google Scholar] [CrossRef]
- Brattsand, R.; Thalén, A.; Roempke, K.; Källström, L.; Gruvstad, E. Influence of 16 alpha, 17 alpha-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J. Steroid Biochem. 1982, 16, 779–786. [Google Scholar] [CrossRef]
- Peruzzi, L.; Coppo, R. Expected and verified benefits from old and new corticosteroid treatments in IgA nephropathy: From trials in adults to new IPNA-KDIGO guidelines. Pediatr. Nephrol. 2025, 40, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R.; Troyanov, S.; Bellur, S.; Cattran, D.; Cook, H.T.; Feehally, J.; Roberts, I.S.; Morando, L.; Camilla, R.; Tesar, V.; et al. VALIGA study of the ERA-EDTA Immunonephrology Working Group. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014, 86, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, L.; Di Leo, V.; Coppo, R. The mucosal immune system and IgA nephropathy. Semin. Immunopathol. 2021, 43, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Coppo, R. The intestine-renal connection in IgA nephropathy. Nephrol. Dial. Transplant. 2015, 30, 360–366. [Google Scholar] [CrossRef]
- Wimbury, D.; Muto, M.; Bhachu, J.S.; Scionti, K.; Brown, J.; Molyneux, K.; Seikrit, C.; Maixnerová, D.; Pérez-Alós, L.; Garred, P.; et al. Targeted-release budesonide modifies key pathogenic biomarkers in immunoglobulin A nephropathy: Insights from the NEFIGAN trial. Kidney Int. 2024, 105, 381–388. [Google Scholar] [CrossRef]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar] [CrossRef]
- Cheung, C.K.; Barratt, J.; Liew, A.; Zhang, H.; Tesar, V.; Lafayette, R. The role of BAFF and APRIL in IgA nephropathy: Pathogenic mechanisms and targeted therapies. Front. Nephrol. 2024, 3, 1346769. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Zhu, L.; Shi, S.F.; Liu, L.J.; Lv, J.C.; Zhang, H. Increased APRIL Expression Induces IgA1 Aberrant Glycosylation in IgA Nephropathy. Medicine 2016, 95, e3099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, K.N.; Tang, S.C.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Primers 2016, 2, 16001. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Le, W.B.; Chen, N.; Wang, W.M.; Liu, Z.S.; Liu, D.; Chen, J.H.; Tian, J.; Fu, P.; Hu, Z.X.; et al. Mycophenolate Mofetil Combined With Prednisone Versus Full-Dose Prednisone in IgA Nephropathy With Active Proliferative Lesions: A Randomized Controlled Trial. Am. J. Kidney Dis. 2017, 69, 788–795. [Google Scholar] [CrossRef]
- Hou, F.F.; Xie, D.; Wang, J.; Xu, X.; Yang, X.; Ai, J.; Nie, S.; Liang, M.; Wang, G.; Jia, N.; et al. Effectiveness of Mycophenolate Mofetil Among Patients With Progressive IgA Nephropathy: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2254054. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Sun, J.; Xu, G.; Wang, C.; Zhou, S.; Nie, S.; Li, Y.; Su, L.; Chen, R.; et al. Immunosuppression versus Supportive Care on Kidney Outcomes in IgA Nephropathy in the Real-World Setting. Clin. J. Am. Soc. Nephrol. 2023, 18, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Duriseti, P.; Radhakrishnan, Y.; Vaughan, L.; Fervenza, F.C.; Zand, L. Mycophenolate Mofetil and Steroid for Treatment of Patients With IgA Nephropathy. Kidney Int. Rep. 2023, 9, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, R.A.; Canetta, P.A.; Rovin, B.H.; Appel, G.B.; Novak, J.; Nath, K.A.; Sethi, S.; Tumlin, J.A.; Mehta, K.; Hogan, M.; et al. A Randomized, Controlled Trial of Rituximab in IgA Nephropathy with Proteinuria and Renal Dysfunction. J. Am. Soc. Nephrol. 2017, 28, 1306–1313. [Google Scholar] [CrossRef]
- Uzzan, M.; Ko, H.M.; Rosenstein, A.K.; Pourmand, K.; Colombel, J.F.; Mehandru, S. Efficient long-term depletion of CD20+ B cells by rituximab does not affect gut-resident plasma cells. Ann. N. Y. Acad. Sci. 2018, 1415, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Maixnerova, D.; El Mehdi, D.; Rizk, D.V.; Zhang, H.; Tesar, V. New treatment strategies for IgA nephropathy: Targeting plasma cells as the main source of pathogenic antibodies. Clin. Med. 2022, 11, 2810. [Google Scholar] [CrossRef]
- Floege, J.; Lafayette, R.; Barratt, J.; Schwartz, B.; Manser, P.T.; Patel, U.D.; Pineda, L.; Faulhaber, N.; Boxhammer, R.; Haertle, S.; et al. Felzartamab (anti-CD38) in patients with IgA Nephropathy: Interim results from the IGNAZ study (abstract). Nephrol. Dial. Transplant. 2024, 39 (Suppl. S1), gfae069-0139. [Google Scholar] [CrossRef]
- Mathur, M.; Barratt, J.; Chacko, B.; Chan, T.M.; Kooienga, L.; Oh, K.H.; Sahay, M.; Suzuki, Y.; Wong, M.G.; Yarbrough, J.; et al. ENVISION Trial Investigators Group. A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy. N. Engl. J. Med. 2024, 390, 20–31. [Google Scholar] [CrossRef]
- Barratt, J.; Hour, B.; Kooienga, L.; Roy, S.; Schwartz, B.; Siddiqui, A.; Tolentino, J.; Iyer, S.P.; Stromatt, C.; Endsley, A.; et al. POS-109 Interim results of phase 1 and 2 trials to investigate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of BION-1301 in patients with IgA nephropathy. Kidney Int. Rep. 2022, 7, S46. [Google Scholar] [CrossRef]
- Lafayette, R.; Barbour, S.; Israni, R.; Wei, X.; Eren, N.; Floege, J.; Jha, V.; Kim, S.G.; Maes, B.; Phoon, R.K.S.; et al. A phase 2b, randomized, double-blind, placebo-controlled, clinical trial of atacicept for treatment of IgA nephropathy. Kidney Int. 2024, 105, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Barbour, S.J.; Brenner, R.M.; Cooper, K.; Wei, X.; Eren, N.; Floege, J.; Jha, V.; Kim, S.G.; Maes, B.; et al. ORIGIN Phase 2b Investigators. Long-Term Results from an Open-Label Extension Study of Atacicept for the Treatment of IgA Nephropathy. J. Am. Soc. Nephrol. 2025, 36, 679–687. [Google Scholar] [CrossRef]
- Lv, J.; Liu, L.; Hao, C.; Li, G.; Fu, P.; Xing, G.; Zheng, H.; Chen, N.; Wang, C.; Luo, P.; et al. Randomized Phase 2 Trial of Telitacicept in Patients with IgA Nephropathy with Persistent Proteinuria. Kidney Int. Rep. 2023, 8, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Madan, A.; Yalavarthy, R.; Kim, D.K.; Moon Jy Park, I.; Mandayam, S.A.; Cortazar, F.B.; Kim, S.G.; Davies, R.H.; Enstrom, A.M.; Thomas, H. Results from longer follow-up with povetacicept, an enhanced dual BAFF/APRIL antagonist, in IgA nephropathy (RUBY-3 study). J. Am. Soc. Nephrol. Abstr. 2024, 35, FRPO854. [Google Scholar] [CrossRef]
- Caravaca-Fontán, F.; Gutiérrez, E.; Sevillano, Á.M.; Praga, M. Targeting complement in IgA nephropathy. Clin. Kidney J. 2023, 16 (Suppl. S2), ii28–ii39. [Google Scholar] [CrossRef]
- McCoy, R.C.; Abramowsky, C.R.; Tisher, C.C. IgA nephropathy. Am. J. Pathol. 1974, 76, 123–144. [Google Scholar]
- Zhang, H.; Rizk, D.V.; Perkovic, V.; Maes, B.; Kashihara, N.; Rovin, B.; Trimarchi, H.; Sprangers, B.; Meier, M.; Charney, A.; et al. Results of a randomized double-blind placebo-controlled Phase 2 study propose iptacopan as an alternative complement pathway inhibitor for IgA nephropathy. Kidney Int. 2024, 105, 189–199. [Google Scholar] [CrossRef]
- Perkovic, V.; Barratt, J.; Rovin, B.; Kashihara, N.; Maes, B.; Zhang, H.; Trimarchi, H.; Kollins, D.; Papachristofi, O.; Jacinto-Sanders, S.; et al. Alternative Complement Pathway Inhibition with Iptacopan in IgA Nephropathy. N. Engl. J. Med. 2025, 392, 531–543. [Google Scholar] [CrossRef]
- Barratt, J.; Liew, A.; Yeo, S.C.; Fernström, A.; Barbour, S.J.; Sperati, C.J.; Villanueva, R.; Wu, M.J.; Wang, D.; Borodovsky, A.; et al. Cemdisiran Phase 2 Study Investigators and Collaborators. Phase 2 Trial of Cemdisiran in Adult Patients with IgA Nephropathy: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2024, 19, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, R.; Tumlin, J.; Fenoglio, R.; Kaufeld, J.; Pérez Valdivia, M.Á.; Wu, M.S.; Susan Huang, S.H.; Alamartine, E.; Kim, S.G.; Yee, M.; et al. SANCTUARY Study Investigators. Efficacy and Safety of Ravulizumab in IgA Nephropathy: A Phase 2 Randomized Double-Blind Placebo-Controlled Trial. J. Am. Soc. Nephrol. 2025, 36, 645–656. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Toto, R.D.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Pecoits-Filho, R.; Correa-Rotter, R.; et al. DAPA-CKD Trial Committees and Investigators. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021, 100, 215–224. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Radhakrishnan, J.; Alpers, C.E.; Barratt, J.; Bieler, S.; Diva, U.; Inrig, J.; Komers, R.; Mercer, A.; Noronha, I.L.; et al. PROTECT Investigators. Sparsentan in patients with IgA nephropathy: A prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet 2023, 401, 1584–1594. [Google Scholar] [CrossRef]
- Rovin, B.H.; Barratt, J.; Heerspink, H.J.L.; Alpers, C.E.; Bieler, S.; Chae, D.W.; Diva, U.A.; Floege, J.; Gesualdo, L.; Inrig, J.K.; et al. DUPRO steering committee and PROTECT Investigators. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 2023, 402, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Jardine, M.; Kohan, D.E.; Lafayette, R.A.; Levin, A.; Liew, A.; Zhang, H.; Lodha, A.; Gray, T.; Wang, Y.; et al. ALIGN Study Investigators. Atrasentan in Patients with IgA Nephropathy. N. Engl. J. Med. 2025, 392, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Du, X.; Xu, Y.; Zhang, Y.; Liu, B.; Bi, G.; Xu, C.; Luo, Q.; Wu, H.; Wan, J.; et al. The Selective Endothelin Receptor Antagonist SC0062 in IgA Nephropathy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Am. Soc. Nephrol. 2025, 392, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Si, F.L.; Chen, P.; Hou, W.Y.; Yang, H.Y.; Lv, J.C.; Shi, S.F.; Zhou, X.J.; Liu, L.J.; Zhang, H. Effectiveness and safety of finerenone in non-diabetic patients with IgA nephropathy. J. Nephrol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Agarwal, R.; Bakris, G.L.; Cherney, D.Z.I.; Lam, C.S.P.; Neuen, B.L.; Sarafidis, P.A.; Tuttle, K.R.; Wanner, C.; Brinker, M.D.; et al. Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomized trial. Nephrol. Dial. Transplant. 2025, 40, 308–319. [Google Scholar] [CrossRef]
- El Karoui, K.; Fervenza, F.C.; De Vriese, A.S. Treatment of IgA Nephropathy: A Rapidly Evolving Field. J. Am. Soc. Nephrol. 2024, 35, 103–116. [Google Scholar] [CrossRef]
- Floege, J.; Bernier-Jean, A.; Barratt, J.; Rovin, B. Treatment of patients with IgA nephropathy: A call for a new paradigm. Kidney Int. 2025, 107, 640–651. [Google Scholar] [CrossRef]
- Baragetti, I.; Del Vecchio, L.; Ferrario, F.; Alberici, F.; Amendola, A.; Russo, E.; Ponti, S.; Di Palma, A.M.; Pani, A.; Rollino, C.; et al. Italian Group of Steroids in IgAN. The safety of corticosteroid therapy in IGA nephropathy: Analysis of a real-life Italian cohort. J. Nephrol. 2025, 38, 225–234. [Google Scholar] [CrossRef]
- Watanabe-Kusunoki, K.; Nakazawa, D.; Yamamoto, J.; Matsuoka, N.; Kaneshima, N.; Nakagaki, T.; Yamamoto, R.; Maoka, T.; Iwasaki, S.; Tsuji, T.; et al. Comparison of administration of single- and triple-course steroid pulse therapy combined with tonsillectomy for immunoglobulin A nephropathy. Medicine 2021, 100, e27778. [Google Scholar] [CrossRef]
| Treatment Target | Mechanism of Action | Drug | Study | Phase | Identifier |
|---|---|---|---|---|---|
| B cells and plasma cells | |||||
| CD38 | Feltarzamab | IGNAZ | IIa | NCT05065970 | |
| APRIL | Sibeprenlimab | ENVISION | II | NCT04287985 | |
| APRIL | Sibeprenlimab | VISIONARY | III | NCT05248646 | |
| APRIL | Zigakibart | BEYOND | II | NCT05852938 | |
| BAFF and APRIL | Ataticept | ORIGIN-3 | IIb | NCT04716231 | |
| BAFF and APRIL | Telitaticept | III | NCT04905212 | ||
| BAFF and APRIL | Povetacicept | I/II | NCT05732402 | ||
| Complement | |||||
| MASP-2 | Narsoplimab | ARTEMIS-IgAN | III | NCT03608033 | |
| C Factor B | Iptacopan | APPLAUSE-IgAN | III | NCT04578834 | |
| C5 | Cemdisiran | ALN-CC5 | II | NCT03841448 | |
| C5 | Ravulizumab | SANCTUARY | II | NCT04564339 | |
| Supportive care | |||||
| Dual Endothelin and Angiotensin (DEARA) | Sparsentan | PROTECT | III | NCT03762850 | |
| Endothelin A | Atrasentan | AFFINITY | II | NCT04573920 | |
| Atrasentan | ALIGN | III | NCT04573478 | ||
| Endothelin A | SC 0062 | II | NCT05687890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppo, R. Treating IgA Nephropathy: Looking at the Future Without Forgetting the Past. J. Clin. Med. 2025, 14, 4045. https://doi.org/10.3390/jcm14124045
Coppo R. Treating IgA Nephropathy: Looking at the Future Without Forgetting the Past. Journal of Clinical Medicine. 2025; 14(12):4045. https://doi.org/10.3390/jcm14124045
Chicago/Turabian StyleCoppo, Rosanna. 2025. "Treating IgA Nephropathy: Looking at the Future Without Forgetting the Past" Journal of Clinical Medicine 14, no. 12: 4045. https://doi.org/10.3390/jcm14124045
APA StyleCoppo, R. (2025). Treating IgA Nephropathy: Looking at the Future Without Forgetting the Past. Journal of Clinical Medicine, 14(12), 4045. https://doi.org/10.3390/jcm14124045





