SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection?
Abstract
1. Introduction
2. Possible Additional Advantages of SGLT2 Inhibitors in Glomerulonephritis
3. Clinical Data from Randomised Clinical Trials and Real-World Experiences
3.1. Overall Effect on Glomerulonephritis
3.2. Evidence in Single Glomerulonephritis
3.2.1. IgA Nephropathy
3.2.2. Focal Segmental Glomerulosclerosis
3.2.3. Secondary Glomerulonephritis
4. Safety Issues Specific to Patients with Glomerulonephritis
5. The Missing Gap in the Knowledge
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rieg, T.; Masuda, T.; Gerasimova, M.; Mayoux, E.; Platt, K.; Powell, D.R.; Thomson, S.C.; Koepsell, H.; Vallon, V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am. J. Physiol. Physiol. 2014, 306, F188–F193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thai, K.; Kepecs, D.M.; Gilbert, R.E. Sodium-Glucose Linked Cotransporter-2 inhibition does not attenuate disease progression in the rat remnant kidney model of chronic kidney disease. PLoS ONE 2016, 11, e0144640. [Google Scholar] [CrossRef] [PubMed]
- Rajasekeran, H.; Reich, H.N.; Hladunewich, M.A.; Cattran, D.; Lovshin, J.A.; Lytvyn, Y.; Bjornstad, P.; Lai, V.; Tse, J.; Cham, L.; et al. Dapagliflozin in focal segmental glomerulosclerosis: A combined hu-man-rodent pilot study. Am. J. Physiol. Renal Physiol. 2018, 314, F412–F422. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: Cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Ćurčić, I.B.; Canecki-Varžić, S. Immunomodulatory effects of SGLT2 inhibitors-targeting inflammation and oxidative stress in aging. Int. J. Environ. Res. Public Health 2023, 20, 6671. [Google Scholar] [CrossRef]
- Aye, I.L.; Jansson, T.; Powell, T.L. Interleukin-1β inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol. Cell. Endocrinol. 2013, 381, 46–55. [Google Scholar] [CrossRef]
- Dror, E.; Dalmas, E.; Meier, D.T.; Wueest, S.; Thévenet, J.; Thienel, C.; Timper, K.; Nordmann, T.M.; Traub, S.; Schulze, F.; et al. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 2017, 18, 283–292. [Google Scholar] [CrossRef]
- La Grotta, R.; de Candia, P.; Olivieri, F.; Matacchione, G.; Giuliani, A.; Rippo, M.R.; Tagliabue, E.; Mancino, M.; Rispoli, F.; Ferroni, S.; et al. Anti-inflammatory effect of SGLT-2 inhibitors via uric acid and insulin. Cell. Mol. Life Sci. 2022, 79, 273. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. Anti-inflammatory potential of SGLT2 inhibitors: A systematic review and meta-analysis of preclinical studies in rodents. Eur. Hear. J. 2022, 43, ehac544.2683. [Google Scholar] [CrossRef]
- Shihab, E.M.; Kadhim, H.M.; Shahooth, S.S. Dapagliflozin mitigates oxidative stress, inflammatory, and histopathological markers of aging in mice. J. Med. Life 2024, 17, 157–163. [Google Scholar] [CrossRef]
- Ke, Q.; Shi, C.; Lv, Y.; Wang, L.; Luo, J.; Jiang, L.; Yang, J.; Zhou, Y. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. FASEB J. 2022, 36, e22078. [Google Scholar] [CrossRef] [PubMed]
- Machado Júnior, P.A.B.; Lass, A.; Pilger, B.I.; Fornazari, R.; Moraes, T.P.; Pinho, R.A. SGLT2 inhibitors and NLRP3 inflam-masome: Potential target in diabetic kidney disease. J. Bras. Nefrol. 2024, 46, e20230187. [Google Scholar] [PubMed]
- Chou, Y.-L.; Chen, H.-L.; Hsu, B.-G.; Yang, C.-Y.; Chen, C.-H.; Lee, Y.-C.; Tsai, I.-L.; Sung, C.-C.; Wu, C.-C.; Yang, S.-R.; et al. Galectin-3 contributes to pathogenesis of IgA nephropathy. Kidney Int. 2024, 106, 658–670. [Google Scholar] [CrossRef]
- Lai, Y.; Zhuang, L.; Zhu, J.; Wang, S.; Guo, C.; Chen, B.; Li, J.; Shi, J.; Li, M.; Yang, N.; et al. Novel approach to alleviate lupus nephritis: Targeting the NLRP3 inflammasome in CD8+CD69+CD103+ TRM cells. J. Transl. Med. 2024, 22, 1139. [Google Scholar] [CrossRef]
- Jenkins, B.J.; Blagih, J.; Ponce-Garcia, F.M.; Canavan, M.; Gudgeon, N.; Eastham, S.; Hill, D.; Hanlon, M.M.; Ma, E.H.; Bishop, E.L.; et al. Canagliflozin impairs T cell effector function via metabolic suppression in autoimmunity. Cell Metab. 2023, 35, 1132–1146.e9. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Q.; Liu, A.; Leng, S.; Wang, S.; Li, C.; Ma, J.; Peng, J.; Xu, M. Empagliflozin modulates CD4+ T-cell differentiation via metabolic reprogramming in immune thrombocytopenia. Br. J. Haematol. 2022, 198, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Si, Z.; Yang, Y.; Li, S.; Xue, Y. Dapagliflozin reverses the imbalance of T helper 17 and T regulatory cells by inhibiting SGK1 in a mouse model of diabetic kidney disease. FEBS Open Bio 2021, 11, 1395–1405. [Google Scholar] [CrossRef]
- Cassis, P.; Locatelli, M.; Cerullo, D.; Corna, D.; Buelli, S.; Zanchi, C.; Villa, S.; Morigi, M.; Remuzzi, G.; Benigni, A.; et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. J. Clin. Investig. 2018, 3, e98720. [Google Scholar] [CrossRef]
- Korbut, A.I.; Taskaeva, I.S.; Bgatova, N.P.; Muraleva, N.A.; Orlov, N.B.; Dashkin, M.V.; Khotskina, A.S.; Zavyalov, E.L.; Konenkov, V.I.; Klein, T.; et al. SGLT2 inhibitor empagliflozin and DPP4 inhibitor linagliptin reactivate glo-merular autophagy in db/db mice, a model of type 2 diabetes. Int. J. Mol. Sci. 2020, 21, 2987. [Google Scholar] [CrossRef]
- Yang, L.; Liang, B.; Li, J.; Zhang, X.; Chen, H.; Sun, J.; Zhang, Z. Dapagliflozin alleviates advanced glycation end product induced podocyte injury through AMPK/mTOR mediated autophagy pathway. Cell. Signal. 2022, 90, 110206. [Google Scholar] [CrossRef]
- Lv, X.; Wang, J.; Zhang, L.; Shao, X.; Lin, Y.; Liu, H.; Ma, G.; Li, J.; Zhou, S.; Yu, P. Canagliflozin reverses Th1/Th2 imbalance and promotes podocyte autophagy in rats with membranous nephropathy. Front. Immunol. 2022, 13, 993869. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Li, S.-S.; He, Y.-X.; Yan, L.-J.; Lv, F.; Liang, Q.-M.; Gan, Y.-H.; Han, L.-P.; Xu, H.-D.; Li, Y.-C.; et al. SGLT2 inhibitors alleviated podocyte damage in lupus nephritis by decreasing inflammation and enhancing autophagy. Ann. Rheum. Dis. 2023, 82, 1328–1340. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, L.; Xiao, J.-J.; Liu, Q.; Ni, L.; Hu, J.-W.; Yu, H.; Wu, X.; Zhang, B.-F. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med. 2023, 195, 89–102. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakano, T.; Torisu, K.; Aihara, S.; Wakisaka, M.; Kitazono, T. SGLT2 inhibition mitigates transition from acute kidney injury to chronic kidney disease by suppressing ferroptosis. Sci. Rep. 2024, 14, 20386. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Wang, S.; Chen, D.; Shu, J.; Chong, N.; Wang, Q.; Xu, Y. Dapagliflozin improves podocytes injury in diabetic nephropathy via regulating cholesterol balance through KLF5 targeting the ABCA1 signalling pathway. Diabetol. Metab. Syndr. 2024, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Molina, J.; Kim, J.-J.; Mallela, S.K.; Ahmad, A.; Santos, J.V.; Al-Ali, H.; Mitrofanova, A.; Sharma, K.; Fontanesi, F.; et al. Empagliflozin reduces podocyte lipotoxicity in experimental Alport syndrome. eLife 2023, 12, e83353. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.; McMurray, J.J.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Jongs, N.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; Stefansson, B.V.; Toto, R.D.; et al. Effect of dapagliflozin on the rate of decline in kidney function in patients with chronic kidney disease with and without type 2 diabetes: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 743–754. [Google Scholar] [CrossRef] [PubMed]
- EMPA-KIDNEY Collaborative Group. Impact of primary kidney disease on the effects of empagliflozin in patients with chronic kidney disease: Secondary analyses of the EMPA-KIDNEY trial. Lancet Diabetes Endocrinol. 2024, 12, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Stevens, K.; Padrón, M.; Huerta, A.; Montomoli, M.; Villa, J.; González, F.; Vega, C.; López Mendoza, M.; Fernández, L.; et al. Sodium-glucose cotransporter 2 inhibition in primary and secondary glo-merulonephritis. Nephrol. Dial. Transplant. 2024, 39, 328–340. [Google Scholar] [CrossRef] [PubMed]
- EMPA-KIDNEY Collaborative Group. Effects of empagliflozin on progression of chronic kidney disease: A prespecified sec-ondary analysis from the EMPA-KIDNEY trial. Lancet Diabetes Endocrinol. 2024, 12, 39–50. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Agrawal, N.; EMPA-KIDNEY Collaborative Group. Long-term effects of empagliflozin in pa-tients with chronic kidney disease. N. Engl. J. Med. 2025, 392, 777–787. [Google Scholar]
- Wheeler, D.C.; Toto, R.D.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.; Pecoits-Filho, R.; Correa-Rotter, R.; et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021, 100, 215–224. [Google Scholar] [CrossRef]
- Stamellou, E.; Nadal, J.; Hendry, B.; Mercer, A.; Seikrit, C.; Bechtel-Walz, W.; Schmid, M.; Moeller, M.J.; Schiffer, M.; Eckardt, K.-U.; et al. Long-term outcomes of patients with IgA nephropathy in the German CKD cohort. Clin. Kidney J. 2024, 17, sfae230. [Google Scholar] [CrossRef]
- Pozzi, C.; Baragetti, I.; Barruscotti, A.; Del Vecchio, L. The outcome of IgAN: Time of reflections in the perspective of new op-portunities. J. Nephrol. 2025; submitted. [Google Scholar]
- Wheeler, D.C.; Jongs, N.; Stefansson, B.V.; Chertow, G.M.; Greene, T.; Hou, F.F.; Langkilde, A.M.; McMurray, J.J.V.; Rossing, P.; Nowicki, M.; et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: A prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol. Dial. Transplant. 2022, 37, 1647–1656. [Google Scholar] [CrossRef]
- Rheault, M.N.; Alpers, C.E.; Barratt, J.; Bieler, S.; Canetta, P.; Chae, D.-W.; Coppock, G.; Diva, U.; Gesualdo, L.; Heerspink, H.J.; et al. Sparsentan versus irbesartan in focal segmental glomerulosclerosis. N. Engl. J. Med. 2023, 389, 2436–2445. [Google Scholar] [CrossRef]
- Morales, E.; Galindo, M. SGLT2 inhibitors in lupus nephropathy, a new therapeutic strategy for nephroprotection. Ann. Rheum. Dis. 2022, 81, 1337–1338. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.; Sun, F.; Liu, Z.; Zhang, D.; Teng, X.; Morel, L.; Wang, X.; Ye, S. Safety and efficacy of the SGLT2 inhibitor dapagliflozin in patients with systemic lupus erythematosus: A phase I/II trial. RMD Open 2022, 8, e002686. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.-S.; Wang, S.-I.; Hsu, C.-C.; Hwu, C.-M.; Wei, J.C.-C. Sodium-glucose cotransporter-2 inhibitors and nephritis among patients with systemic lupus erythematosus. JAMA Netw. Open 2024, 7, e2416578. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Lo, J.E.; Kyttaris, V.C.; Tsokos, G.C.; Costenbader, K.H. Efficacy and safety of sodium-glucose cotransporter 2 inhib-itors for the primary prevention of cardiovascular, renal events, and safety outcomes in patients with systemic lupus erythe-matosus and comorbid type 2 diabetes: A population-based target trial emulation. Arthritis Rheumatol. 2024; Epub ahead of print. [Google Scholar]
- Kronbichler, A. Sodium-glucose cotransporter 2 (SGLT2) inhibition and autoimmunity. Semin. Arthritis Rheum. 2025, 72, 152663. [Google Scholar] [CrossRef]
- Elkeraie, A.; Zyada, R.; Elrggal, M.E.; Elrggal, M. Safety of SGLT2 inhibitors in patients with different glomerular diseases treated with immunosuppressive therapies. Eur. J. Clin. Pharmacol. 2023, 79, 961–966. [Google Scholar] [CrossRef]
- Cipriani, C.; Lauriero, G.; Tripepi, G.; Ferrari, S.; Bover, J.; Ravera, M.; Barbuto, S.; Cianciolo, G.; De Nicola, L.; Brandi, M.L.; et al. Effect of antidiabetic drugs on bone health in patients with normal renal function and in chronic kidney disease (CKD): Insight into clinical challenges in the treatment of type 2 diabetes. J. Clin. Med. 2023, 12, 7260. [Google Scholar] [CrossRef]
- Deng, G.; Wu, L.; Xiong, S.; Zhou, J.; Li, Z. The role of antidiabetic drugs in bone health: Assessing the risk of osteoporosis subtypes and fractures using mendelian randomization. Orthop. Res. Rev. 2025, 17, 129–145. [Google Scholar] [CrossRef]
- Locatelli, F.; Del Vecchio, L. Cardio-renoprotective effects of SGLT2 inhibitors—The role of anaemia correction. Nephrol. Dial. Transplant. 2024, 39, 904–906. [Google Scholar] [CrossRef]
- Koshino, A.; Schechter, M.; Chertow, G.M.; Vart, P.; Jongs, N.; Toto, R.D.; Rossing, P.; Correa-Rotter, R.; McMurray, J.J.; Górriz, J.L.; et al. Dapagliflozin and anemia in patients with chronic kidney disease. N. Engl. J. Med. Evid. 2023, 2, EVIDoa2300049. [Google Scholar] [CrossRef]
- Steinmetz, T.; Goldman, S.; Kagan, K.B.T.; Bielopolski, D.; Buchrits, S.; Schechter, A.; Kushnir, S.; Turjeman, A.; Agur, T.; Grossman, A.; et al. The beneficial effects of sodium-glucose cotransporter 2 inhibitors on anemia in type 2 diabetes-a real-world study. J. Clin. Endocrinol. Metab. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Abdallah, M.; Szuber, N.; Saliba, A.; Alkhateeb, H.; Al-Kali, A.; Begna, K.H.; Pardanani, A.; Tefferi, A. Sodium-glucose co-transporter-2 inhibitor use and JAK2 unmuted erythrocytosis in 100 cumulative cases. Am. J. Hematol. 2023, 98, E165–E167. [Google Scholar] [CrossRef] [PubMed]
- Peiti, S.; Del Vecchio, L.; Pucci Bella, G.; Uraghi, S. Terapia con SGLT2-I in pazienti con IgA Nephropathy, esperienza di centro. In Proceedings of the 65° Congress of the Italian Society of Nephrology, Riccione, Italy, 16–19 October 2024. (In Italian). [Google Scholar]
- Schwarz, Y.; Klein, P.; Lev-Shalem, L. Masked anemia and hematocrit elevation under sodium glucose transporter inhibitors: Findings from a large real-world study. Acta Diabetol. 2024, 61, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Del Vecchio, L.; Praga, M.; Floege, J.; Zoccali, C. Sodium glucose cotransporter 2 inhibitors in the treat-ment of glomerular diseases: A CKJ controversy. Clin. Kidney J. 2024, 17, sfae237. [Google Scholar] [CrossRef]
- Mudaliar, S.; Polidori, D.; Zambrowicz, B.; Henry, R.R. Sodium-Glucose Cotransporter Inhibitors: Effects on Renal and Intes-tinal Glucose Transport: From Bench to Bedside. Diabetes Care 2015, 38, 2344–2353. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of human sodium glucose transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Agarwal, R.; Bakris, G.L.; Cherney, D.Z.; Lam, C.S.; Neuen, B.L.; Sarafidis, P.A.; Tuttle, K.R.; Wanner, C.; Brinker, M.D.; et al. Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomised trial. Nephrol. Dial. Transplant. 2025, 40, 308–319. [Google Scholar] [CrossRef]
- Rovin, B.H.; Barratt, J.; Heerspink, H.J.L.; Alpers, C.E.; Bieler, S.; Chae, D.-W.; Diva, U.A.; Floege, J.; Gesualdo, L.; Inrig, J.K.; et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 2023, 402, 2077–2090. [Google Scholar] [CrossRef]

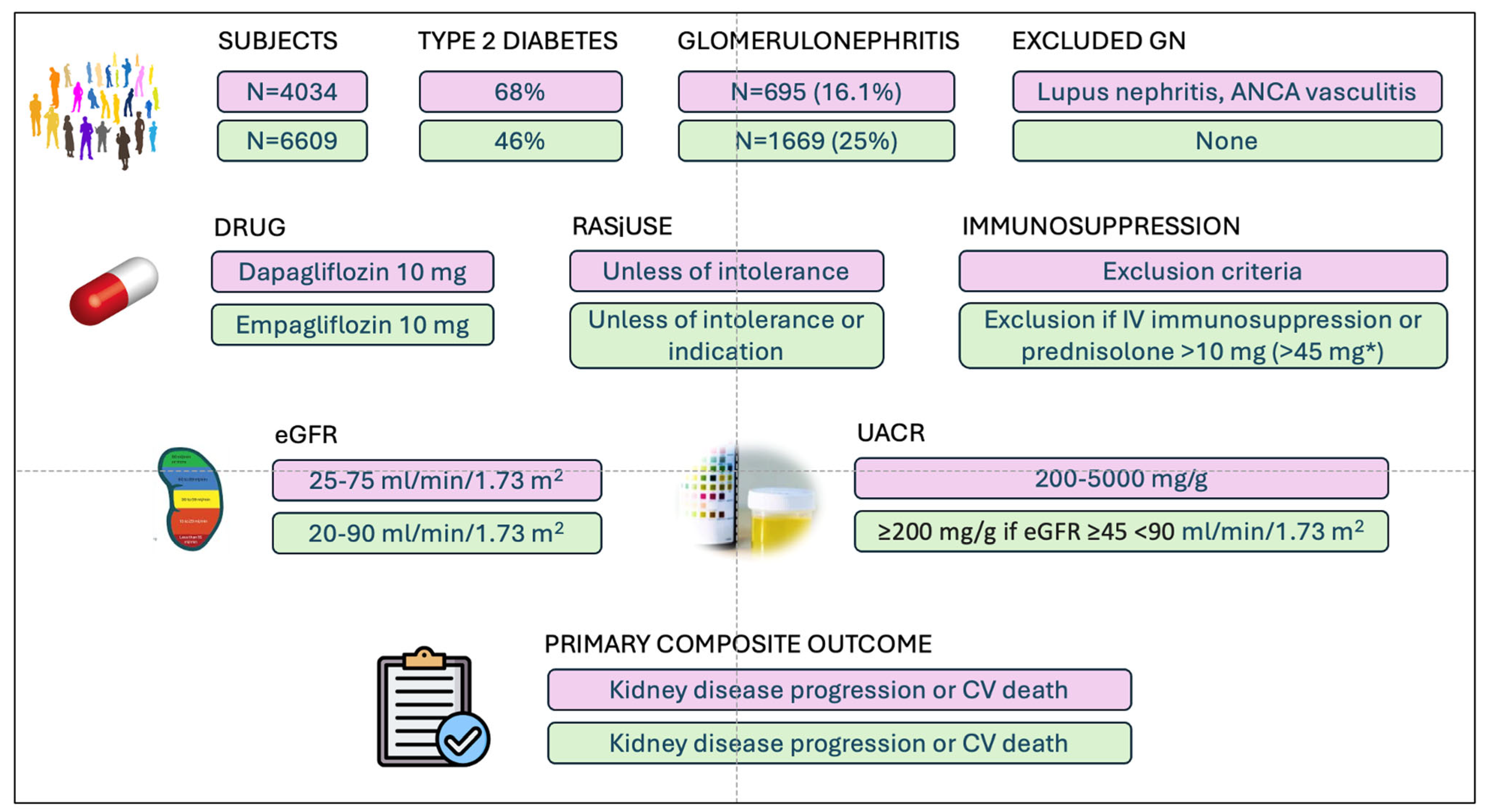
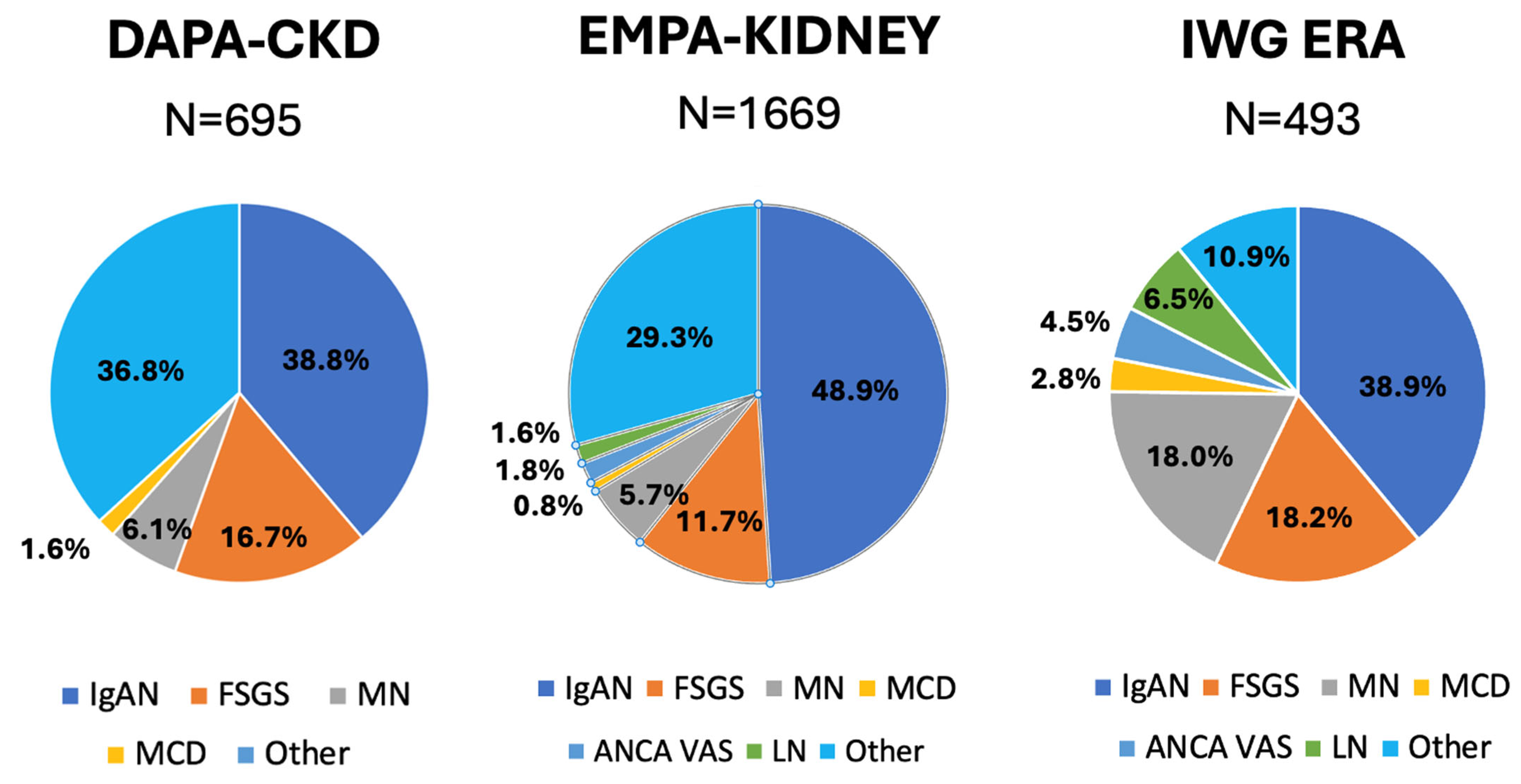
| DAPA-CKD N = 695 | EMPA-KIDNEY N = 1669 | IWG # N = 493 | |
|---|---|---|---|
| Age a, years | ~51.8 (13.8) | 53·5 (13.6) | 55 (42–65) |
| Sex female, N (%) | 254 (36.5) | 596 (35.7) | 157 (32) |
| Race, N (%) | |||
| 341 (49) 9 (1.2) 326 (47) 19 (2.7) | 765 (45.8) 2.2 (1.3) 863 (51.7) 19 (1.1) | Mainly NA NA NA |
| BMI a, kg/m2 | NA | 27.2 (5.8) | 29 (26–33) |
| Kidney biopsy, N (%) | 1312 (78.6) | 493 (100) | |
| RAS inhibitors, N (%) | 691 (99) | 1535 (92) | 493 (100) |
| Diabetes, N (%) | 97 (13.9) | 172 (10.3) | 147 (30) |
| Hypertension, N (%) | NA | NA | 357 (72) |
| Previous history of CVD, N (%) | 24 (3.4) * | 144 (8.6) | 81 (16) |
| Maintenance immunosuppression, N (%) | 0 (0) c | 139 (8.3) | 79 (16) |
| eGFR a, ml/min/1.73 m2 | ~42.9 (11.9) | 42.4 (17.8) | 56 (39–82) |
| UACR b, mg/g | ~978 (540–1750) | 700 (306–1428) | 1287 (729–2294) |
| Outcome | Events | Absolute Risk Reduction (%; 95% CI) | NNT (n; 95% CI) | |
|---|---|---|---|---|
| SGLT2i | Placebo | |||
| EMPA-KIDNEY | ||||
| Primary composite outcome | 117/853 (13.72%) | 142/816 (17.40%) | 3.69; 0.21–7.16 | 28; 14–481.8 |
| Any kidney disease progression | 115/853 (13.48%) | 139/816 (17.30%) | 3.55; 0.10–7.00 | 29; 14.3–980.8 |
| ESKF | 48/853 (5.6%) | 58/816 (7.10%) | 1.48; −0.86–3.83 | NS |
| Sustained ≥40% eGFR decline | 107/853 (12.54%) | 136/816 (16.67%) | 4.12; 0.73–7.51 | 25; 13.3–136.1 |
| DAPA-CKD | ||||
| Primary composite outcome | 22/343 (6.41%) | 49/352 (13.92%) | 7.51; 3.06–11.96 | 14; 8.4–32.7 |
| Kidney specific composite outcome | 21/343 (6.12%) | 46/352 (13.07%) | 6.95; 2.61–11.29 | 15; 8.9–38.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Vecchio, L.; Peiti, S.; Pucci Bella, G.; Locatelli, F. SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection? J. Clin. Med. 2025, 14, 3533. https://doi.org/10.3390/jcm14103533
Del Vecchio L, Peiti S, Pucci Bella G, Locatelli F. SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection? Journal of Clinical Medicine. 2025; 14(10):3533. https://doi.org/10.3390/jcm14103533
Chicago/Turabian StyleDel Vecchio, Lucia, Silvia Peiti, Giulio Pucci Bella, and Francesco Locatelli. 2025. "SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection?" Journal of Clinical Medicine 14, no. 10: 3533. https://doi.org/10.3390/jcm14103533
APA StyleDel Vecchio, L., Peiti, S., Pucci Bella, G., & Locatelli, F. (2025). SGLT2 Inhibitors in Glomerulonephritis: Beyond Nephroprotection? Journal of Clinical Medicine, 14(10), 3533. https://doi.org/10.3390/jcm14103533








