Results of Cochlear Implantation in Patients with Congenital Rubell—Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Patients
2.3. Audiometric Evaluation
2.4. Surgical Procedure
3. Results
3.1. Clinical Characteristic of the Study Group
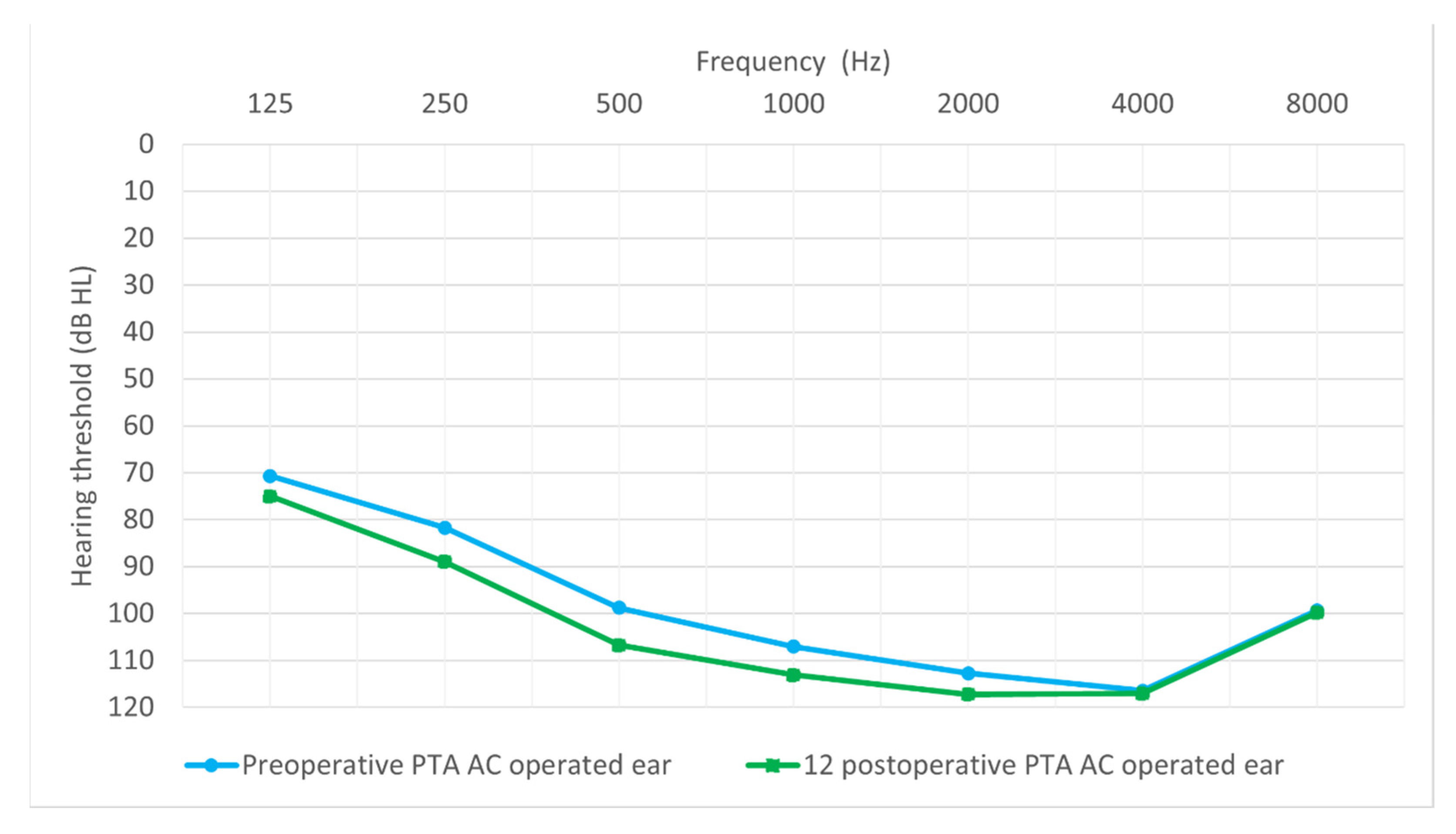
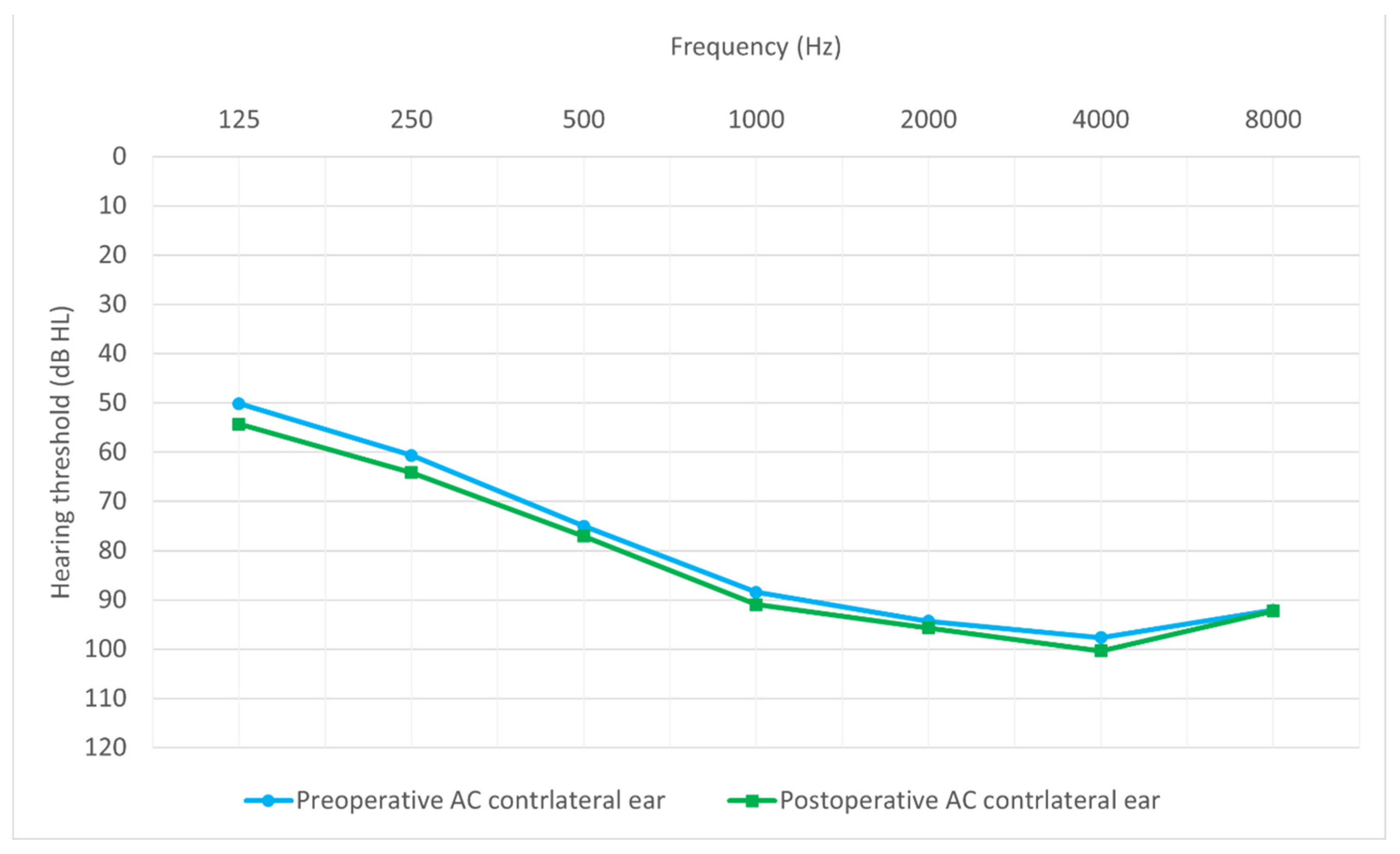
3.2. Comparison of Preoperative and Postoperative Hearing Thresholds
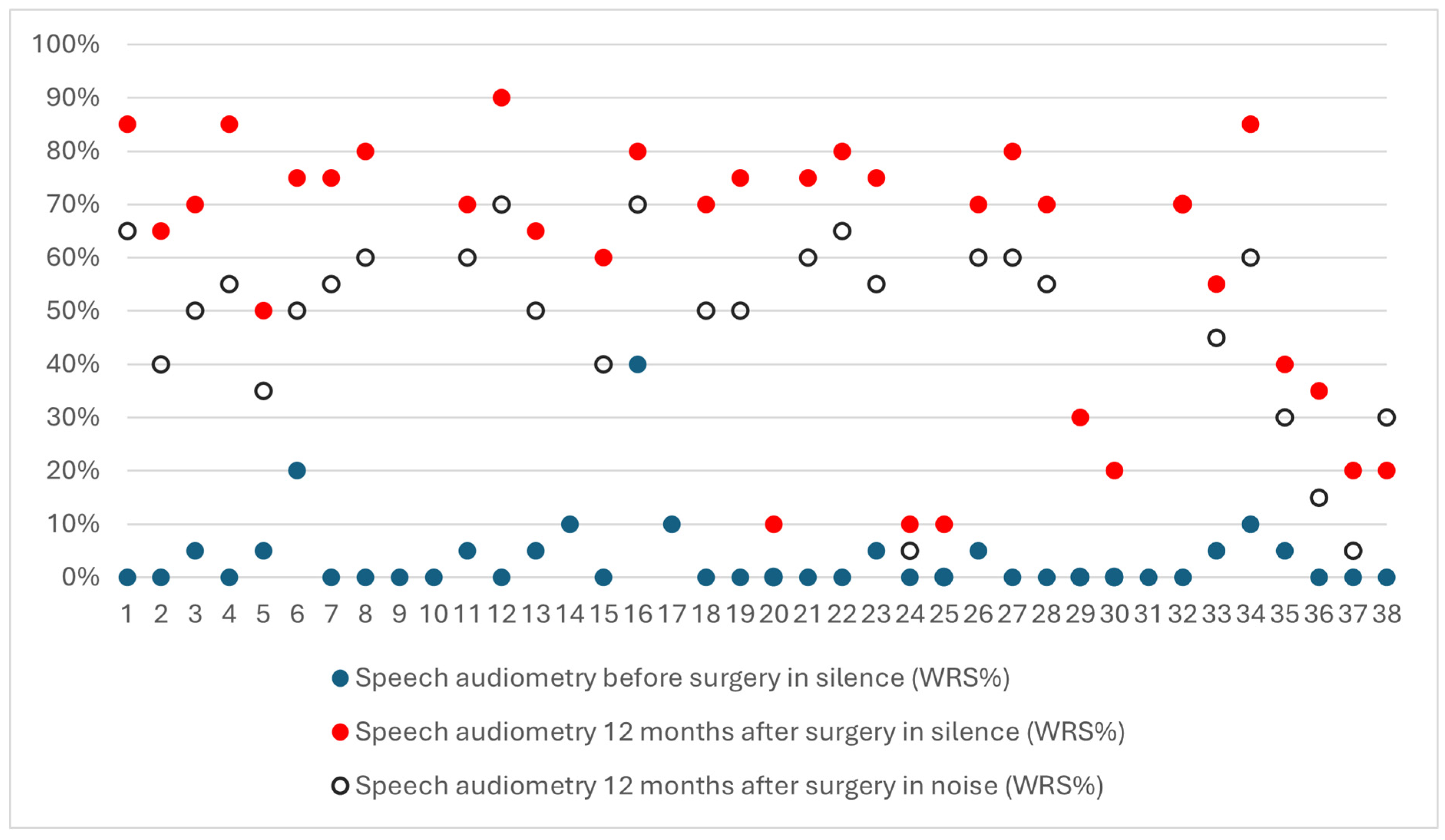
3.3. Speech Understanding
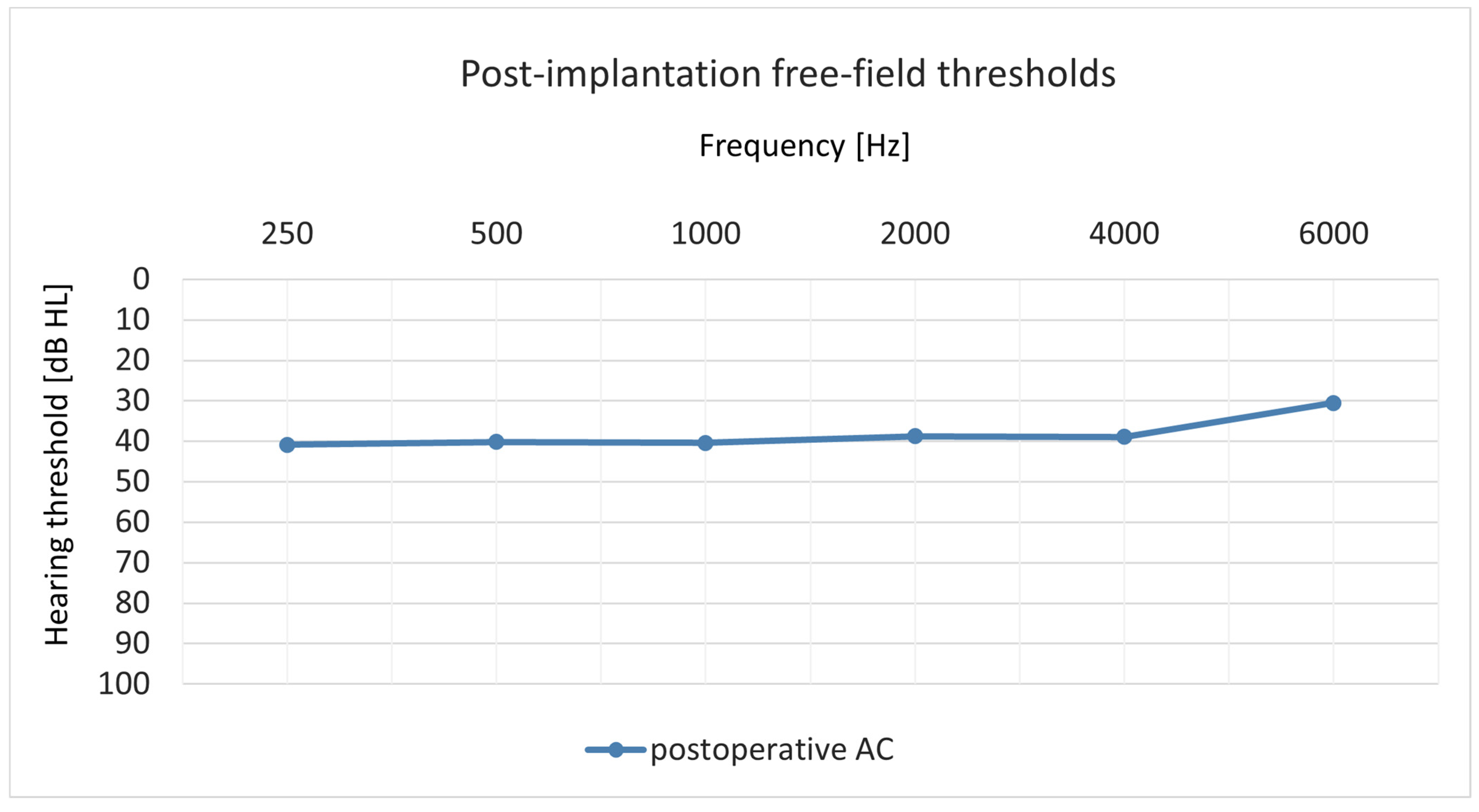
3.4. Hearing Preservation
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hon, K.L.; Leung, K.K.Y.; Leung, A.K.C.; Man, E.; Ip, P. Congenital Infections in Hong Kong: Beyond TORCH. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2020, 26, 318–322. [Google Scholar] [CrossRef]
- Winter, A.K.; Moss, W.J. Rubella. Lancet 2022, 399, 1336–1346. [Google Scholar] [CrossRef]
- Hess, A.F. German Measles (Rubella): An Experimental Study. Arch. Intern. Med. 1914, XIII, 913–916. [Google Scholar] [CrossRef]
- Gregg, N.M. Further Observations on Congenital Defects in Infants Following Maternal Rubella. Trans. Ophthalmol. Soc. Aust. 1944, 4, 119–131. [Google Scholar]
- Gregg, N.M. Congenital Cataract Following German Measles in the Mother. 1941. Aust. N. Z. J. Ophthalmol. 1991, 19, 267–276. [Google Scholar]
- Forrest, J.M.; Menser, M.A.; Slinn, R.F.; Nowak, M.J.; Murphy, A.M.; Stout, M. Rubella vaccination trial. Med. J. Aust. 1971, 2, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Bouthry, E.; Picone, O.; Hamdi, G.; Grangeot-Keros, L.; Ayoubi, J.-M.; Vauloup-Fellous, C. Rubella and Pregnancy: Diagnosis, Management and Outcomes. Prenat. Diagn. 2014, 34, 1246–1253. [Google Scholar] [CrossRef]
- Gudeloglu, E.; Akillioglu, M.; Bedir Demirdag, T.; Unal, N.A.; Tapisiz, A.A. Congenital Rubella Syndrome: A Short Report and Literature Review. Trop. Dr. 2022, 53, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Muyassaroh, M.; Rahmi, N.A. Hearing Loss in Pediatric Patients with Congenital Rubella Syndrome. J. Med. Sci. Berk. Ilmu Kedokt. 2022, 54, 141–147. [Google Scholar] [CrossRef]
- WHO Rubella. Available online: https://www.who.int/news-room/fact-sheets/detail/rubella (accessed on 1 August 2024).
- Andrade, J.Q.; Bunduki, V.; Curti, S.P.; Figueiredo, C.A.; de Oliveira, M.I.; Zugaib, M. Rubella in Pregnancy: Intrauterine Transmission and Perinatal Outcome during a Brazilian Epidemic. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2006, 35, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ojala, P.; Vesikari, T.; Elo, O. Rubella During Pregnancy as a Cause of Congenital Hearing Loss1. Am. J. Epidemiol. 1973, 98, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Bukasa, A.; Campbell, H.; Brown, K.; Bedford, H.; Ramsay, M.; Amirthalingam, G.; Tookey, P. Rubella Infection in Pregnancy and Congenital Rubella in United Kingdom, 2003 to 2016. Eurosurveillance 2018, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.; Rowan, P.; Greisinger, A.; Mouzoon, M. Rubella Monitoring in Pregnancy as a Means for Evaluating a Possible Reemergence of Rubella. Am. J. Obstet. Gynecol. 2014, 211, e1–e534. [Google Scholar] [CrossRef]
- Anvar, B.; Mencher, G.T.; Keet, S.J. Hearing Loss and Congenital Rubella in Atlantic Canada. Ear Hear. 1984, 5, 340–345. [Google Scholar] [CrossRef]
- Kobayashi, H.; Suzuki, A.; Nomura, Y. Unilateral Hearing Loss Following Rubella Infection in an Adult. Acta Oto-Laryngol. Suppl. 1994, 514, 49–51. [Google Scholar] [CrossRef]
- Parving, A.; Vejtorp, M.; Møller, K.; Møller, J.K. Congenital Hearing Loss and Rubella Infection. Acta Otolaryngol. 1980, 90, 262–266. [Google Scholar] [CrossRef]
- Brookhouser, P.E.; Bordley, J.E. Congenital Rubella Deafness: Pathology and Pathogenesis. Arch. Otolaryngol. 1973, 98, 252–257. [Google Scholar] [CrossRef]
- Ward, P.H.; Honrubia, V.; Moore, B.S. Inner Ear Pathology in Deafness Due to Maternal Rubella. Arch. Otolaryngol. Chic. Ill 1960 1968, 87, 22–28. [Google Scholar] [CrossRef]
- Obrycka, A.; Padilla, J.L.; Putkiewicz-Aleksandrowicz, J.; Lorens, A.; Skarzynski, H. Partial Deafness Treatment in Children: A Preliminary Report of the Parents’ Perspective. J. Hear. Sci. 2012, 2, 61–69. [Google Scholar] [CrossRef]
- Melvin, T.-A.N.; Della Santina, C.C.; Carey, J.P.; Migliaccio, A.A. The Effects of Cochlear Implantation on Vestibular Function. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2009, 30, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Joachims, H.Z.; Eliachar, I. Cochlear Hearing Loss Following Rubella in an Adult. Scand. Audiol. 1982, 11, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, R.T. Prognosis after Cochlear Implantation. BMJ 2004, 328, 419–420. [Google Scholar] [CrossRef]
- Prasad, K.; Borre, E.D.; Dillard, L.K.; Ayer, A.; Der, C.; Bainbridge, K.E.; McMahon, C.M.; Tucci, D.L.; Wilson, B.S.; Schmidler, G.D.S.; et al. Priorities for Hearing Loss Prevention and Estimates of Global Cause-Specific Burdens of Hearing Loss: A Systematic Rapid Review. Lancet Glob. Health 2024, 12, e217–e225. [Google Scholar] [CrossRef] [PubMed]
- Pruszewicz, A.; Demenko, G.; Richter, L.; Wika, T. Nowe listy artykulacyjne do badań audiometrycznych (cz. I). Otolaryngol. Pol. 1994, 48, 50–62. [Google Scholar] [PubMed]
- Skarzynski, H.; Lorens, A.; Piotrowska, A.; Skarzynski, P.H. Hearing Preservation in Partial Deafness Treatment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, CR555–CR562. [Google Scholar]
- Levy, D.A.; Lee, J.A.; Nguyen, S.A.; McRackan, T.R.; Meyer, T.A.; Lambert, P.R. Cochlear Implantation for Treatment of Tinnitus in Single-Sided Deafness: A Systematic Review and Meta-Analysis. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2020, 41, e1004–e1012. [Google Scholar] [CrossRef]
- Wilson, B.S. Partial Deafness Cochlear Implantation (PDCI) and Electric-Acoustic Stimulation (EAS). Cochlear Implants Int. 2010, 11 (Suppl. S1), 56–66. [Google Scholar] [CrossRef]
- Octaviani, N.S.; Ekorini, H.M. Cochlear Implants Evaluation for Congenital Rubella Syndrome Patients in Dr. Soetomo Academic Medical Center. Int. J. Health Sci. 2022, 6, 320–327. [Google Scholar] [CrossRef]
- Al-Shawi, Y.; Mesallam, T.A.; Alfallaj, R.; Aldrees, T.; Albakheet, N.; Alshawi, M.; Alotaibi, T.; Algahtani, A. Inter-Rater Reliability and Validity of the Arabic Version of Categories of Auditory Performance-II (CAP-II) Among Children With Cochlear Implant. Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol. 2020, 41, e597–e602. [Google Scholar] [CrossRef]
- Toizumi, M.; Nguyen, G.T.H.; Motomura, H.; Nguyen, T.H.; Pham, E.; Kaneko, K.; Uematsu, M.; Nguyen, H.A.T.; Dang, D.A.; Hashizume, M.; et al. Sensory Defects and Developmental Delay among Children with Congenital Rubella Syndrome. Sci. Rep. 2017, 7, 46483. [Google Scholar] [CrossRef] [PubMed]
- Pramila, B.; Mohan, M.; Kavitha, K. Congenital Rubella Syndrome. TNOA J. Ophthalmic Sci. Res. 2018, 56, 254. [Google Scholar] [CrossRef]
- Calháu, C.M.D.F.; Lima Júnior, L.R.P.; Reis, A.M.d.C.d.S.; Capistrano, A.K.B.; Lima, D.d.V.S.P.; Calháu, A.C.D.F.; Júnior, F.d.A.R. Etiology Profile of the Patients Implanted in the Cochlear Implant Program. Braz. J. Otorhinolaryngol. 2015, 77, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.A.; Sousa, H.; Barros, E. Health-Related Quality of Life after Pediatric Cochlear Implantation. Int. J. Pediatr. Otorhinolaryngol. 2022, 155, 111087. [Google Scholar] [CrossRef]
- Contrera, K.J.; Betz, J.; Li, L.; Blake, C.R.; Sung, Y.K.; Choi, J.S.; Lin, F.R. Quality of Life after Intervention with a Cochlear Implant or Hearing Aid. Laryngoscope 2016, 126, 2110–2115. [Google Scholar] [CrossRef]
- Skarzynski, P.H.; Lorens, A.; Gos, E.; Kolodziejak, A.; Obrycka, A.; Skarżyńska, M.B.; Czajka, N.; Porowski, M.; Skarzynski, H. Outcomes of Cochlear Implantation Using FLEX26 Electrode: Audiological Results and Quality of Life after 12 Months. Audiol. Neurotol. 2023, 28, 458–465. [Google Scholar] [CrossRef]
| Operated ear | Right | 26 (68%) |
| Left | 12 (32%) | |
| Tinnitus in operated ear | Yes | 18 (47%) |
| No | 20 (53%) | |
| Tinnitus in the opposite ear | Yes | 9 (76%) |
| No | 29 (24%) | |
| Vertigo | Yes | 5 (13%) |
| No | 33 (87%) | |
| Hearing aids (HAs) | Yes | 35 (92%) |
| No | 3 (8%) | |
| HAs from what age? | Range (years) | 0.42–8 |
| M (SD) | 2.62 (1.87) | |
| Computed tomography | Yes | 22 (58%) |
| No | 16 (42%) | |
| Surgical approach | Posterior tympanotomy, round window | 31 (82%) |
| Posterior tympanotomy, cochleostomy | 7 (18%) | |
| Implant | Med-El | 27 (71%) |
| Advanced Bionics | 2 (5%) | |
| Oticon | 1 (3%) | |
| Cochlear | 8 (21%) | |
| Type of implant | Concerto | 6 (16%) |
| Sonata | 14 (37%) | |
| Synchrony | 4 (11%) | |
| Pulsar | 3 (8%) | |
| HiRes 90 k | 2 (5%) | |
| Neuro Zti | 1 (3%) | |
| CI422 | 5 (13%) | |
| CI522 | 1 (3%) | |
| CI512 | 2 (5%) | |
| Electrode | Standard | 7 (18%) |
| Medium | 1 (3%) | |
| FlexSoft | 4 (11%) | |
| Flex28 | 10 (26%) | |
| Flex26 | 5 (13%) | |
| SlimJ | 2 (5%) | |
| Evo | 1 (3%) | |
| CI 512 | 2 (5%) | |
| CI 422/CI 522 | 6 (16%) |
| Minimal | Partial | Complete | |
|---|---|---|---|
| Hearing preservation | 8 (23%) | 15 (43%) | 12 (34%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolodziejak, A.; Czajka, N.; Zdanowicz, R.; Skarżyński, H.; Skarżyński, P.H. Results of Cochlear Implantation in Patients with Congenital Rubell—Retrospective Study. J. Clin. Med. 2025, 14, 3999. https://doi.org/10.3390/jcm14113999
Kolodziejak A, Czajka N, Zdanowicz R, Skarżyński H, Skarżyński PH. Results of Cochlear Implantation in Patients with Congenital Rubell—Retrospective Study. Journal of Clinical Medicine. 2025; 14(11):3999. https://doi.org/10.3390/jcm14113999
Chicago/Turabian StyleKolodziejak, Aleksandra, Natalia Czajka, Rita Zdanowicz, Henryk Skarżyński, and Piotr Henryk Skarżyński. 2025. "Results of Cochlear Implantation in Patients with Congenital Rubell—Retrospective Study" Journal of Clinical Medicine 14, no. 11: 3999. https://doi.org/10.3390/jcm14113999
APA StyleKolodziejak, A., Czajka, N., Zdanowicz, R., Skarżyński, H., & Skarżyński, P. H. (2025). Results of Cochlear Implantation in Patients with Congenital Rubell—Retrospective Study. Journal of Clinical Medicine, 14(11), 3999. https://doi.org/10.3390/jcm14113999









