Exploring the Impact of Beta-Blockers Post-Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loubeyre, C.; Lefèvre, T.; Louvard, Y.; Dumas, P.; Piéchaud, J.-F.; Lanore, J.-J.; Angellier, J.-F.; Le Tarnec, J.-Y.; Karrillon, G.; Margenet, A.; et al. Outcome after combined reperfusion therapy for acute myocardial infarction, combining pre-hospital thrombolysis with immediate percutaneous coronary intervention and stent. Eur. Heart J. 2001, 22, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Braunwald, E.; Moyé, L.A.; Basta, L.; Brown, E.J.; Cuddy, T.E.; Davis, B.R.; Geltman, E.M.; Goldman, S.; Flaker, G.C.; et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N. Engl. J. Med. 1992, 327, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Dargie, H.J. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001, 357, 1385–1390. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cannon, C.P.; Morrow, D.; Rifai, N.; Rose, L.M.; McCabe, C.H.; Pfeffer, M.A.; Braunwald, E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005, 352, 20–28. [Google Scholar] [CrossRef]
- McMurray, J.; Køber, L.; Robertson, M.; Dargie, H.; Colucci, W.; Lopez-Sendon, J.; Remme, W.; Sharpe, D.N.; Ford, I. Antiarrhythmic effect of carvedilol after acute myocardial infarction: Results of the Carvedilol Post-Infarct. Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J. Am. Coll. Cardiol. 2005, 45, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Doughty, R.N.; Whalley, G.A.; Walsh, H.A.; Gamble, G.D.; López-Sendón, J.; Sharpe, N. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: The CAPRICORN Echo Substudy. Circulation 2004, 109, 201–206. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Jr Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Kimura, K.; Kimura, T.; Ishihara, M.; Nakagawa, Y.; Nakao, K.; Miyauchi, K.; Sakamoto, T.; Tsujita, K.; Hagiwara, N.; Miyazaki, S.; et al. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ. J. 2019, 83, 1085–1196. [Google Scholar] [CrossRef]
- Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N. Engl. J. Med. 1981, 304, 801–807. [Google Scholar] [CrossRef]
- Hjalmarson, Å.; Herlitz, J.; Málek, I.; Rydén, L.; Vedin, A.; Waldenström, A.; Wedel, H.; Elmfeldt, D.; Holmberg, S.; Nyberg, G.; et al. Effect on mortality of metoprolol in acute myocardial infarction. A double-blind randomised trial. Lancet 1981, 2, 823–827. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982, 247, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Makani, H.; Radford, M.; Thakur, K.; Toklu, B.; Katz, S.D.; DiNicolantonio, J.J.; Devereaux, P.; Alexander, K.P.; Wetterslev, J.; et al. Clinical outcomes with β-blockers for myocardial infarction: A meta-analysis of randomized trials. Am. J. Med. 2014, 127, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Yndigegn, T.; Lindahl, B.; Mars, K.; Alfredsson, J.; Benatar, J.; Brandin, L.; Erlinge, D.; Hallen, O.; Held, C.; Hjalmarsson, P.; et al. Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction. N. Engl. J. Med. 2024, 390, 1372–1381. [Google Scholar] [CrossRef]
- Watanabe, H.; Ozasa, N.; Morimoto, T.; Shiomi, H.; Bingyuan, B.; Suwa, S.; Nakagawa, Y.; Izumi, C.; Kadota, K.; Ikeguchi, S.; et al. Long-term use of carvedilol in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. PLoS ONE 2018, 13, e0199347. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Baptista, R.M.; Monteiro, S.R.; Gonçalves, L.M. Usefulness of universal beta-blocker therapy in patients after ST-elevation myocardial infarction. Medicine 2021, 100, e23987. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://web.archive.org/web/20210716121605id_/http://www3.med.unipmn.it/dispense_ebm/2009-2010/Corso%20Perfezionamento%20EBM_Faggiano/NOS_oxford.pdf (accessed on 15 February 2025).
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials|Cochrane Bias. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 15 February 2025).
- Al-Bawardy, R.; Alqarawi, W.; Al Suwaidi, J.; Almahmeed, W.; Zubaid, M.; Amin, H.; Sulaiman, K.; Al-Motarreb, A.; Alhabib, K. The Effect of Beta-Blocker Post-Myocardial Infarction with Ejection Fraction >40% Pooled Analysis from Seven Arabian Gulf Acute Coronary Syndrome Registries. Angiology 2024, 76, 33197241227025. [Google Scholar] [CrossRef]
- Yang, J.H.; Hahn, J.-Y.; Bin Song, Y.; Choi, S.-H.; Choi, J.-H.; Lee, S.H.; Kim, J.H.; Ahn, Y.-K.; Jeong, M.-H.; Choi, D.-J.; et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc. Interv. 2014, 7, 592–601. [Google Scholar] [CrossRef]
- Bao, B.; Ozasa, N.; Morimoto, T.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Iwabuchi, M.; Shizuta, S.; Shiomi, H.; Tada, T.; et al. β-Blocker therapy and cardiovascular outcomes in patients who have undergone percutaneous coronary intervention after ST-elevation myocardial infarction. Cardiovasc. Interv. Ther. 2013, 28, 139–147. [Google Scholar] [CrossRef]
- Konishi, H.; Miyauchi, K.; Kasai, T.; Tsuboi, S.; Ogita, M.; Naito, R.; Nishizaki, Y.; Okai, I.; Tamura, H.; Okazaki, S.; et al. Long-term effect of β-blocker in ST-segment elevation myocardial infarction in patients with preserved left ventricular systolic function: A propensity analysis. Heart Vessels 2016, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.-J.; Kim, S.-Y.; Choi, J.-H.; Park, H.K.; Beom, J.W.; Lee, J.-G.; Chae, S.C.; Kim, H.-S.; Kim, Y.J.; Cho, M.C.; et al. Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 475–482. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Park, J.-S.; Tahk, S.-J.; Hwang, G.-S.; Yoon, M.-H.; Choi, S.-Y.; Choi, B.-J.; Lim, H.-S.; Yang, H.-M.; Seo, K.-W.; et al. β-Blocker Therapy in the Era of Primary Percutaneous Intervention for ST Elevation Myocardial Infarction. Cardiology 2015, 132, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kernis, S.J.; Harjai, K.J.; Stone, G.W.; Grines, L.L.; Boura, J.A.; O’Neill, W.W.; Grines, C.L. Does beta-blocker therapy improve clinical outcomes of acute myocardial infarction after successful primary angioplasty? J. Am. Coll. Cardiol. 2004, 43, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Choo, E.H.; Chang, K.; Ahn, Y.; Jeon, D.S.; Lee, J.M.; Bin Kim, D.; Her, S.-H.; Park, C.S.; Kim, H.Y.; Yoo, K.-D.; et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart 2014, 100, 492–499. [Google Scholar] [CrossRef]
- López, A.P.; Alós-Almiñana, M.; Peris, J.E. Secondary adherence to beta-blockers after ST-elevation myocardial infarction without ventricular dysfunction. Med. Clin. 2020, 155, 242–248. [Google Scholar] [CrossRef]
- Ozasa, N.; Kimura, T.; Morimoto, T.; Hou, H.; Tamura, T.; Shizuta, S.; Nakagawa, Y.; Furukawa, Y.; Hayashi, Y.; Nakao, K.; et al. Lack of effect of oral beta-blocker therapy at discharge on long-term clinical outcomes of ST-segment elevation acute myocardial infarction after primary percutaneous coronary intervention. Am. J. Cardiol. 2010, 106, 1225–1233. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Abu-Assi, E.; Redondo-Diéguez, A.; González-Ferreiro, R.; López-López, A.; Bouzas-Cruz, N.; Castiñeira-Busto, M.; Gil, C.P.; García-Acuña, J.M.; González-Juanatey, J.R. Prognostic Benefit of Beta-blockers After Acute Coronary Syndrome with Preserved Systolic Function. Still Relevant Today? Rev. Esp. Cardiol. (Engl. Ed.) 2015, 68, 585–591. [Google Scholar] [CrossRef]
- Matsumoto, S.; Henderson, A.D.; Shen, L.; Kondo, T.; Yang, M.; Campbell, R.T.; Anand, I.S.; de Boer, R.A.; Desai, A.S.; Lam, C.S.; et al. Beta-blocker use and outcomes in patients with heart failure and mildly reduced and preserved ejection fraction. Eur. J. Heart Fail. 2024, 27, 124–139. [Google Scholar] [CrossRef]
- Wen, X.-S.; Luo, R.; Liu, J.; Duan, Q.; Qin, S.; Xiao, J.; Zhang, D.-Y. Short-term/long-term prognosis with or without beta-blockers in patients without heart failure and with preserved ejection fraction after acute myocardial infarction: A multicenter retrospective cohort study. BMC Cardiovasc. Disord. 2022, 22, 193. [Google Scholar] [CrossRef]
- Silvain, J.; Cayla, G.; Ferrari, E.; Range, G.; Puymirat, E.; Delarche, N.; Guedeney, P.; Cuisset, T.; Ivanes, F.; Lhermusier, T.; et al. Beta-Blocker Interruption or Continuation after Myocardial Infarction. N. Engl. J. Med. 2024, 391, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.T.; Huang, F.Y.; Zuo, Z.L.; Liao, Y.B.; Heng, Y.; Wang, P.J.; Gui, Y.Y.; Xia, T.L.; Xin, Z.M.; Liu, W.; et al. Meta-Analysis of Relation Between Oral β-Blocker Therapy and Outcomes in Patients with Acute Myocardial Infarction Who Underwent Percutaneous Coronary Intervention. Am. J. Cardiol. 2015, 115, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Aarvik, M.D.; Sandven, I.; Dondo, T.B.; Gale, C.P.; Ruddox, V.; Munkhaugen, J.; Atar, D.; Otterstad, J.E. Effect of oral β-blocker treatment on mortality in contemporary post-myocardial infarction patients: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 12–20. [Google Scholar] [CrossRef]
- Rashid, M.; Stevens, C.; Wijeysundera, H.C.; Curzen, N.; Khoo, C.W.; Mohamed, M.O.; Aktaa, S.; Wu, J.; Ludman, P.F.; Mamas, M.A. Rates of Elective Percutaneous Coronary Intervention in England and Wales: Impact of COURAGE and ORBITA Trials. J. Am. Heart Assoc. 2022, 11, e025426. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ono, M.; Dai, Z.; Saito, A.; Kanie, T.; Takaoka, Y.; Mizuno, A.; Yoneoka, D.; Komiyama, N. Temporal trends in clinical outcomes after percutaneous coronary intervention: A systematic review of 66,327 patients from 25 all-comers trials. EuroIntervention 2022, 17, 1318–1329. [Google Scholar] [CrossRef]
- Dondo, T.B.; Hall, M.; West, R.M.; Jernberg, T.; Lindahl, B.; Bueno, H.; Danchin, N.; Deanfield, J.E.; Hemingway, H.; Fox, K.A.; et al. β-Blockers and Mortality After Acute Myocardial Infarction in Patients Without Heart Failure or Ventricular Dysfunction. J. Am. Coll. Cardiol. 2017, 69, 2710–2720. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, W.; Han, Q.; Xiao, R.P. Emerging concepts and therapeutic implications of beta-adrenergic receptor subtype signaling. Pharmacol. Ther. 2005, 108, 257–268. [Google Scholar] [CrossRef]
- Lubbe, W.F.; Podzuweit, T.; Opie, L.H. Potential arrhythmogenic role of cyclic adenosine monophosphate (AMP) and cytosolic calcium overload: Implications for prophylactic effects of beta-blockers in myocardial infarction and proarrhythmic effects of phosphodiesterase inhibitors. J. Am. Coll. Cardiol. 1992, 19, 1622–1633. [Google Scholar] [CrossRef]
- Kopecky, S.L. Effect of beta blockers, particularly carvedilol, on reducing the risk of events after acute myocardial infarction. Am. J. Cardiol. 2006, 98, 1115–1119. [Google Scholar] [CrossRef]
- Bonow, R.O.; Udelson, J.E. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management. Ann. Intern. Med. 1992, 117, 502–510. [Google Scholar] [CrossRef]
- Basu, S.; Senior, R.; Raval, U.; van der Does, R.; Bruckner, T.; Lahiri, A. Beneficial effects of intravenous and oral carvedilol treatment in acute myocardial infarction. A placebo-controlled, randomized trial. Circulation 1997, 96, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Borrello, F.; Beahan, M.; Klein, L.; Gheorghiade, M. Reappraisal of beta-blocker therapy in the acute and chronic post-myocardial infarction period. Rev. Cardiovasc. Med. 2003, 4 (Suppl. S3), S13–S24. [Google Scholar]
- Frishman, W.H.; Chang, C.M. Beta-adrenergic blockade in the prevention of myocardial infarction: A new theory. J. Hypertens. 1991, 9, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Grandi, E.; Ripplinger, C.M. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol. Res. 2019, 146, 104274. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Freemantle, N.; Cleland, J.; Young, P.; Mason, J.; Harrison, J. beta Blockade after myocardial infarction: Systematic review and meta regression analysis. BMJ 1999, 318, 1730–1737. [Google Scholar] [CrossRef]
- Misumida, N.; Harjai, K.; Kernis, S.; Kanei, Y. Does Oral Beta-Blocker Therapy Improve Long-Term Survival in ST-Segment Elevation Myocardial Infarction with Preserved Systolic Function? A Meta-Analysis. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 280–285. [Google Scholar] [CrossRef]
- Canton, L.; Suma, N.; Amicone, S.; Impellizzeri, A.; Bodega, F.; Marinelli, V.; Ciarlantini, M.; Casuso, M.; Bavuso, L.; Belà, R.; et al. Clinical impact of multimodality assessment of myocardial viability. Echocardiography 2024, 41, e15854. [Google Scholar] [CrossRef]
- Joseph, P.; Swedberg, K.; Leong, D.P.; Yusuf, S. The Evolution of β-Blockers in Coronary Artery Disease and Heart Failure (Part 1/5). J. Am. Coll. Cardiol. 2019, 74, 672–682. [Google Scholar] [CrossRef]
- Spadafora, L.; Betti, M.; D’Ascenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Post-Discharge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2025, 85, 322–328. [Google Scholar] [CrossRef]
- Rossello, X.; Raposeiras-Roubin, S.; Latini, R.; Dominguez-Rodriguez, A.; Barrabés, J.A.; Sánchez, P.L.; Anguita, M.; Fernández-Vázquez, F.; Pascual-Figal, D.; Hernandez, J.M.D.l.T.; et al. Rationale and design of the pragmatic clinical trial tREatment with Beta-blockers after myOcardial infarction withOut reduced ejection fracTion (REBOOT). Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.M.D.; Munkhaugen, J.; Halvorsen, S.; Olsen, M.H.; Bakken, A.; Sehested, T.S.G.; Ruddox, V.; Lange, T.; Fagerland, M.W.; Torp-Pedersen, C.; et al. The Danish-Norwegian randomized trial on beta-blocker therapy after myocardial infarction: Design, rationale, and baseline characteristics. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Kim, J.; Kang, D.; Doh, J.-H.; Kim, J.; Park, Y.H.; Ahn, S.G.; Kim, W.; Park, J.P.; Kim, S.M.; et al. Discontinuation of β-blocker therapy in stabilised patients after acute myocardial infarction (SMART-DECISION): Rationale and design of the randomised controlled trial. BMJ Open 2024, 14, e086971. [Google Scholar] [CrossRef] [PubMed]
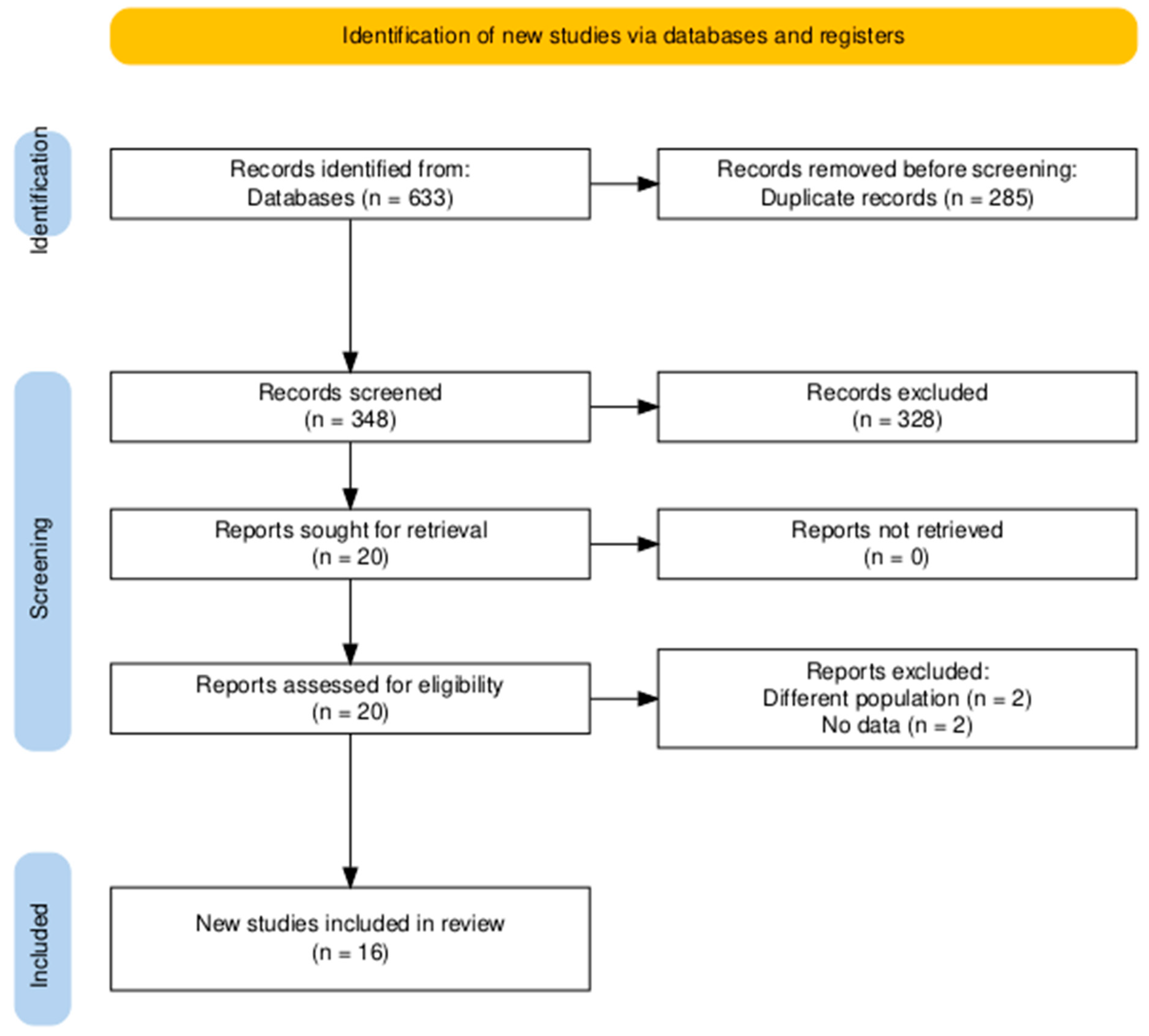
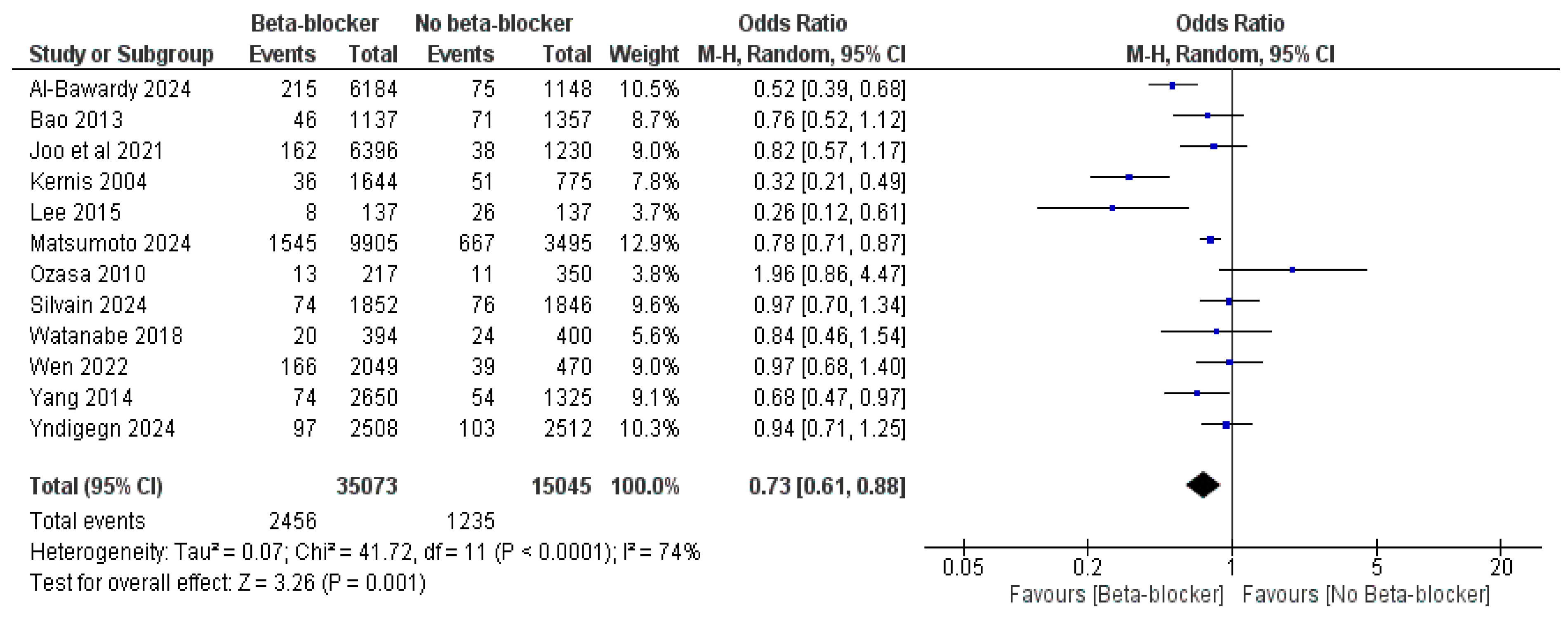

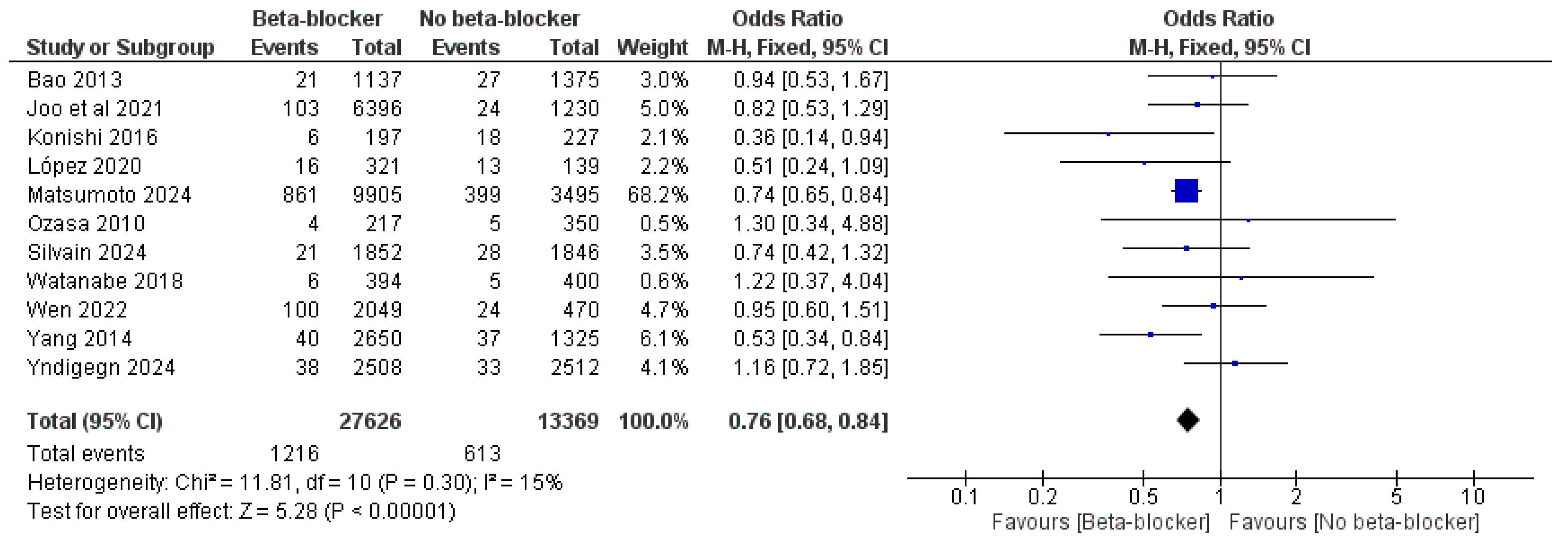
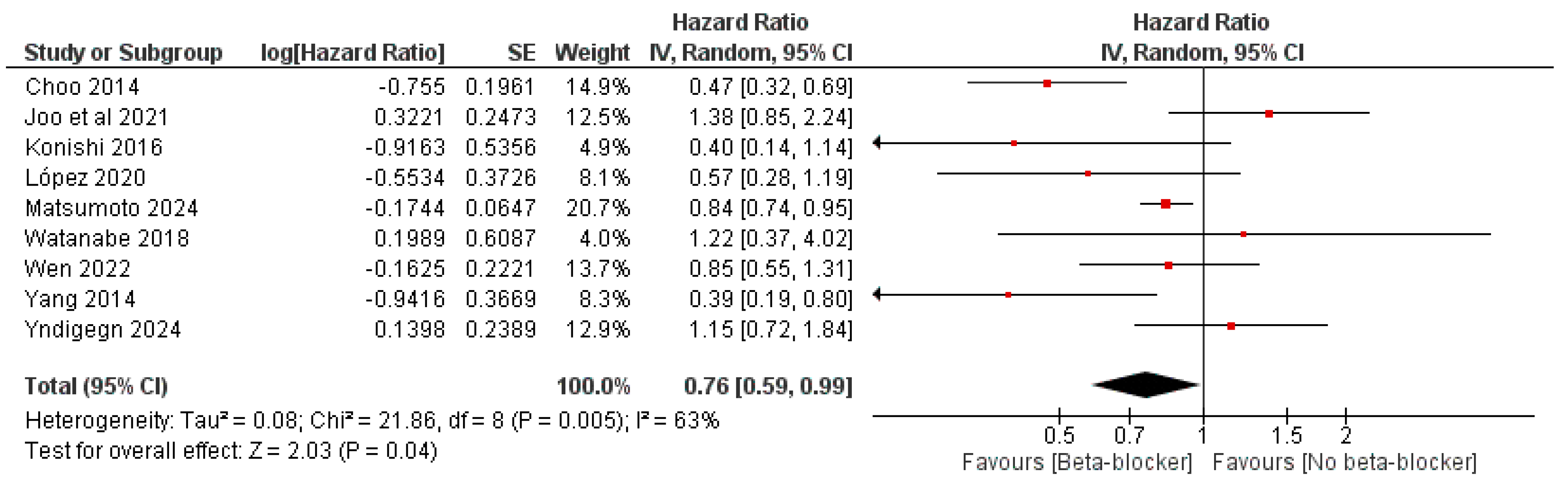
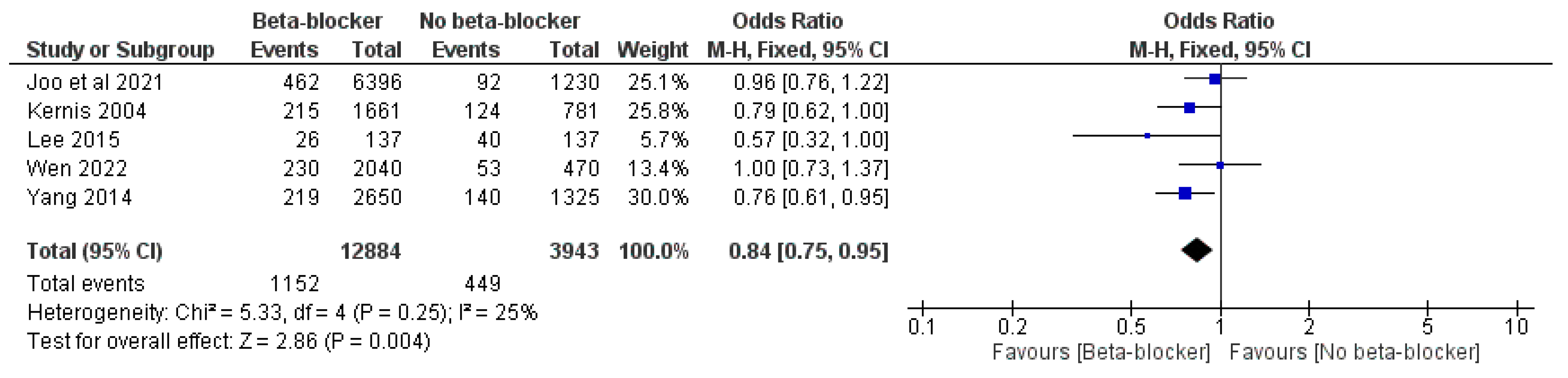
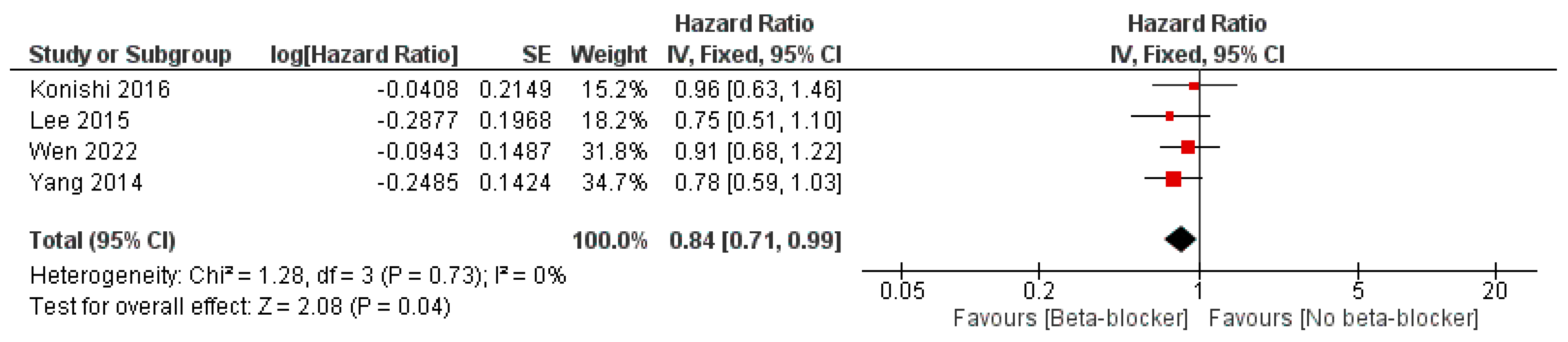
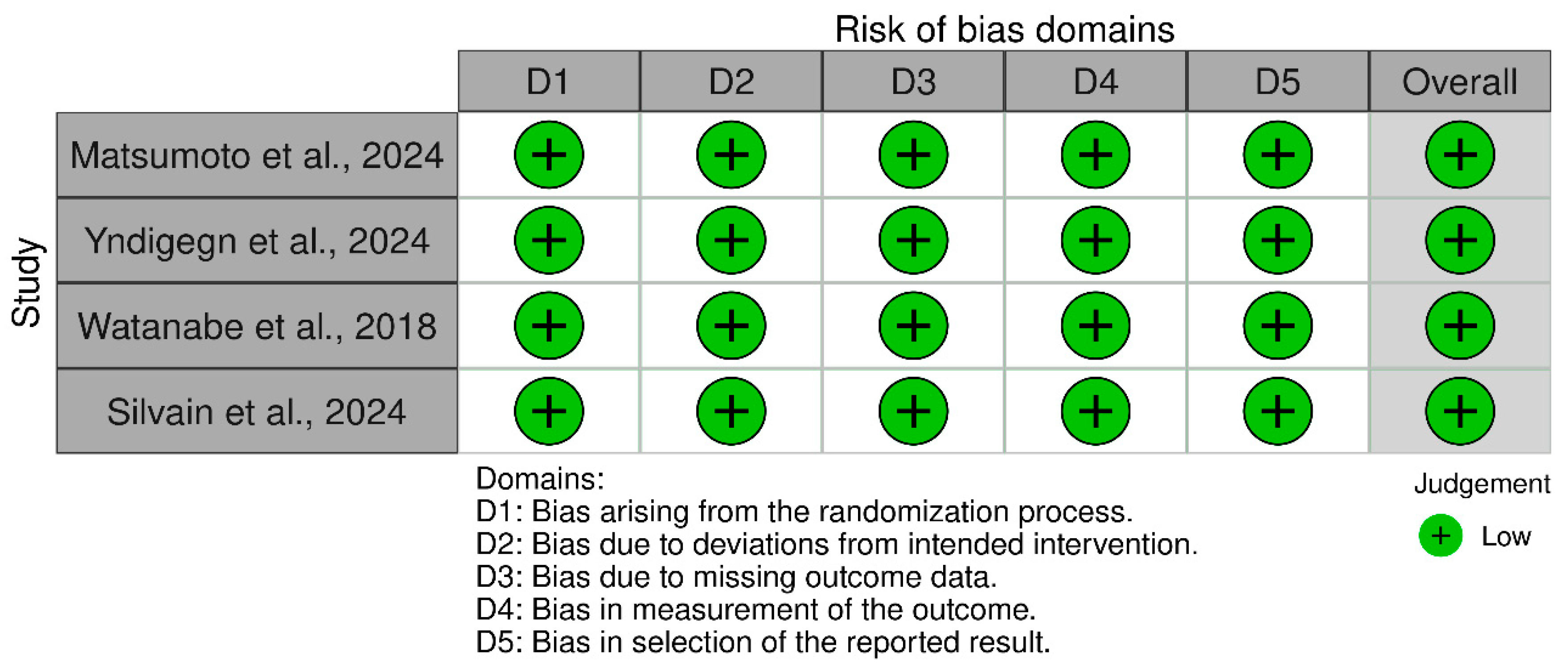
| Study ID | Design | Sample Size | Age | Male, n (%) | LVEF, Mean (SD) | Previous PCI | Follow-Up, Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-Blocker | No Beta-Blocker | Beta-Blocker | No Beta-Blocker | Beta-Blocker | No Beta-Blocker | Beta-Blocker | No Beta-Blocker | Beta-Blocker | No Beta-Blocker | |||
| Ozasa et al., 2010 [29] | Cohort | 349 | 561 | 66.4 (11.5) | 68 (11.6) | 264 (75) | 430 (76) | 51 (11.9) | 53.2 (12.2) | 41 (11) | 79 (14) | 12 |
| López et al., 2020 [28] | Cohort | 417 | 43 | 63.8 (13.8) | 317 (76) | 31 (70.1) | NR | NR | 396 (95) | 36 (90.7) | 47.7 | |
| Al-Bawardy et al., 2024 [20] | Cohort | 15,541 | 2798 | 55.3 (12.1) | 27.4 (13.5) | 12,510 (80.5) | 2029 (72.5) | NR | NR | 1756 (22.2) | 154 (12.4) | 1–12 |
| Choo et al., 2014 [27] | Cohort | 2424 | 595 | 60.9 (12.1) | 63.1 (12.8) | 1799 (74.2) | 412 (69.2) | NR | NR | NR | NR | 1–12 |
| Matsumoto et al., 2024 [31] | Cohort | 9905 | 3495 | 72.1 (8.4) | 43.6 (8.5) | 4510 (45.5) | 1512 (43.3) | 59.4 (7.1) | 61 (7.8) | 1681 (21.6) | 280 (14) | 34.1 |
| Kernis et al., 2004 [26] | Cohort | 1661 | 781 | 60 (12) | 62 (12) | 1240 (75) | 560 (72) | 49.2 (12) | 48.1 (13) | 146 (8.8) | 98 (13) | 6 |
| Lee et al., 2015 [25] | Cohort | 589 | 303 | 56 (12) | 61 (13) | 491 (82.1) | 225 (74.3) | 53 (10) | 49 (12) | 40 (6.7) | 25 (8.3) | 54 |
| Yndigegn et al., 2024 [14] | RCT | 2508 | 2512 | 65 (2.3) | 65 (2.3) | 1945 (77.6) | 1944 (77.4) | NR | NR | 147 (5.9) | 175 (7) | 13 |
| Watanabe et al., 2018 [15] | RCT | 394 | 400 | 63.9 (11.2) | 64.5 (11.3) | 327 (83) | 312 (78) | 58.1 (8.6) | 58 (8.9) | 20 (5.1) | 21 (5.3) | 12 |
| Joo et al., 2021 [24] | Cohort | 10,251 | 1949 | 63.2 (12.5) | 65.6 (12.9) | 7655 (74.7) | 1414 (72.4) | 52.2 (10.8) | 52.5 (12.1) | NR | NR | 24 |
| Konishi et al., 2016 [23] | Cohort | 197 | 227 | 64 (12.1) | 64.2 (11.1) | 152 (77.2) | 184 (81.1) | 55.6 (9.5) | 57.4 (9.9) | NR | NR | 6 |
| Wen et al., 2022 [32] | Cohort | 2049 | 470 | 62 (2.6) | 64 (3) | 1624 (79.5) | 101 (21.5) | 58 (1.02) | 60 (1.2) | 1673 (81.6) | 331 (70.4) | 1 |
| Bao et al., 2013 [22] | Cohort | 1614 | 2078 | 65.8 (12.2) | 68 (12.1) | 1255 (77.8) | 1500 (72.2) | 52.4 (12.6) | 54.3 (12.2) | NR | NR | 3 |
| Raposeiras-Roubin et al., 2015 [30] | Cohort | 2277 | 959 | 63.8 (12) | 67.8 (11.5) | 1692 (74.3) | 407 (68.4) | NR | NR | 1667 (73.2) | 340 (57.1) | 62 |
| Yang et al., 2014 [21] | Cohort | 6873 | 1637 | 62 (2.7) | 65 (2.8) | 5182 (75.4) | 1217 (74.3) | 51 (2) | 50 (2.5) | 285 (4.1) | 83 (5.1) | 12–24 |
| Silvain et al., 2024 [33] | RCT | 1852 | 1846 | 63.5 (10.9) | 63.5 (11.2) | 1531 Silvain et al., 2024 (82.7) | 1530 (82.9) | 57.2 (5.9) | 57.2 (5.9) | 1693 (91.4) | 1709 (92.5) | 6 |
| Study Name | The Level of Representation of the Affected Cohort (★) | Identification of the Unexposed Cohort (★) | Determination of Exposure (★) | Evidence That the Outcome of Interest Was Absent at the Commencement of the Research (★) | Comparison of Cohorts Based on Design or Assessment (Max★★) | Was the Follow-Up Duration Sufficient for the Consequences to Manifest? (★) | Evaluation of Results (★) | Assessment of Cohort Follow-Up Sufficiency (★) | Quality Level |
|---|---|---|---|---|---|---|---|---|---|
| Ozasa et al., 2010 [29] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| López et al., 2020 [28] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Al-Bawardy et al., 2024 [20] | ★ | - | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Choo et al., 2014 [27] | ★ | ★ | ★ | ★ | ★★ | - | ★ | ★ | High |
| Kernis et al., 2004 [26] | ★ | - | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Lee et al., 2015 [25] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Joo et al., 2021 [24] | ★ | - | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Konishi et al., 2016 [23] | ★ | - | ★ | ★ | ★★ | - | ★ | ★ | High |
| Wen et al., 2022 [32] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Bao et al., 2013 [22] | ★ | - | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Raposeiras-Roubin et al., 2015 [30] | ★ | - | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Yang et al., 2014 [21] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
| Matsumoto et al., 2024 [31] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Alnemer, K. Exploring the Impact of Beta-Blockers Post-Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: A Meta-Analysis. J. Clin. Med. 2025, 14, 3969. https://doi.org/10.3390/jcm14113969
A. Alnemer K. Exploring the Impact of Beta-Blockers Post-Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: A Meta-Analysis. Journal of Clinical Medicine. 2025; 14(11):3969. https://doi.org/10.3390/jcm14113969
Chicago/Turabian StyleA. Alnemer, Khalid. 2025. "Exploring the Impact of Beta-Blockers Post-Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: A Meta-Analysis" Journal of Clinical Medicine 14, no. 11: 3969. https://doi.org/10.3390/jcm14113969
APA StyleA. Alnemer, K. (2025). Exploring the Impact of Beta-Blockers Post-Acute Myocardial Infarction in Patients with Preserved Ejection Fraction: A Meta-Analysis. Journal of Clinical Medicine, 14(11), 3969. https://doi.org/10.3390/jcm14113969