Effect of Complete Revascularization in STEMI: Ischemia-Driven Rehospitalization and Cardiovascular Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
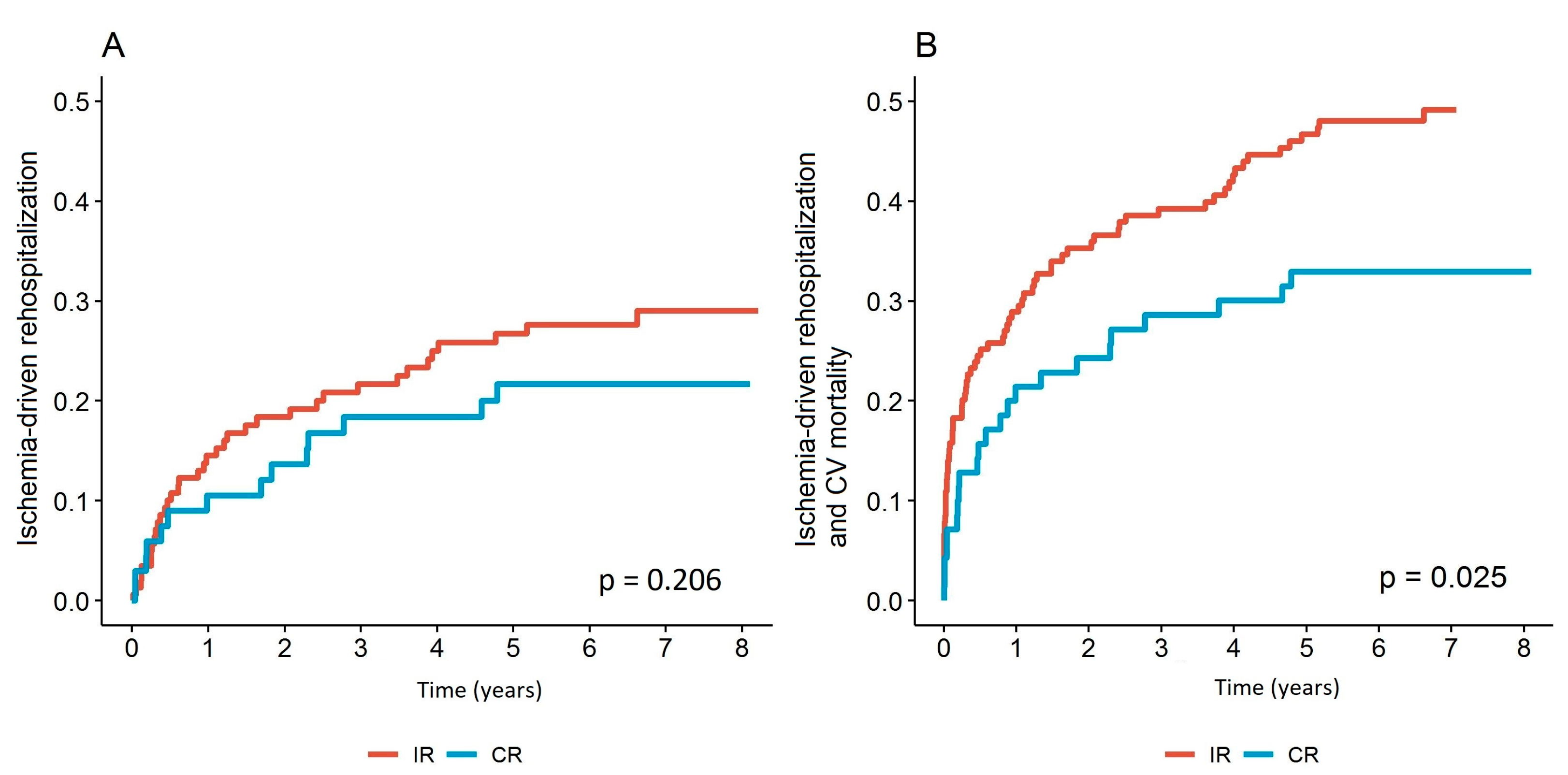
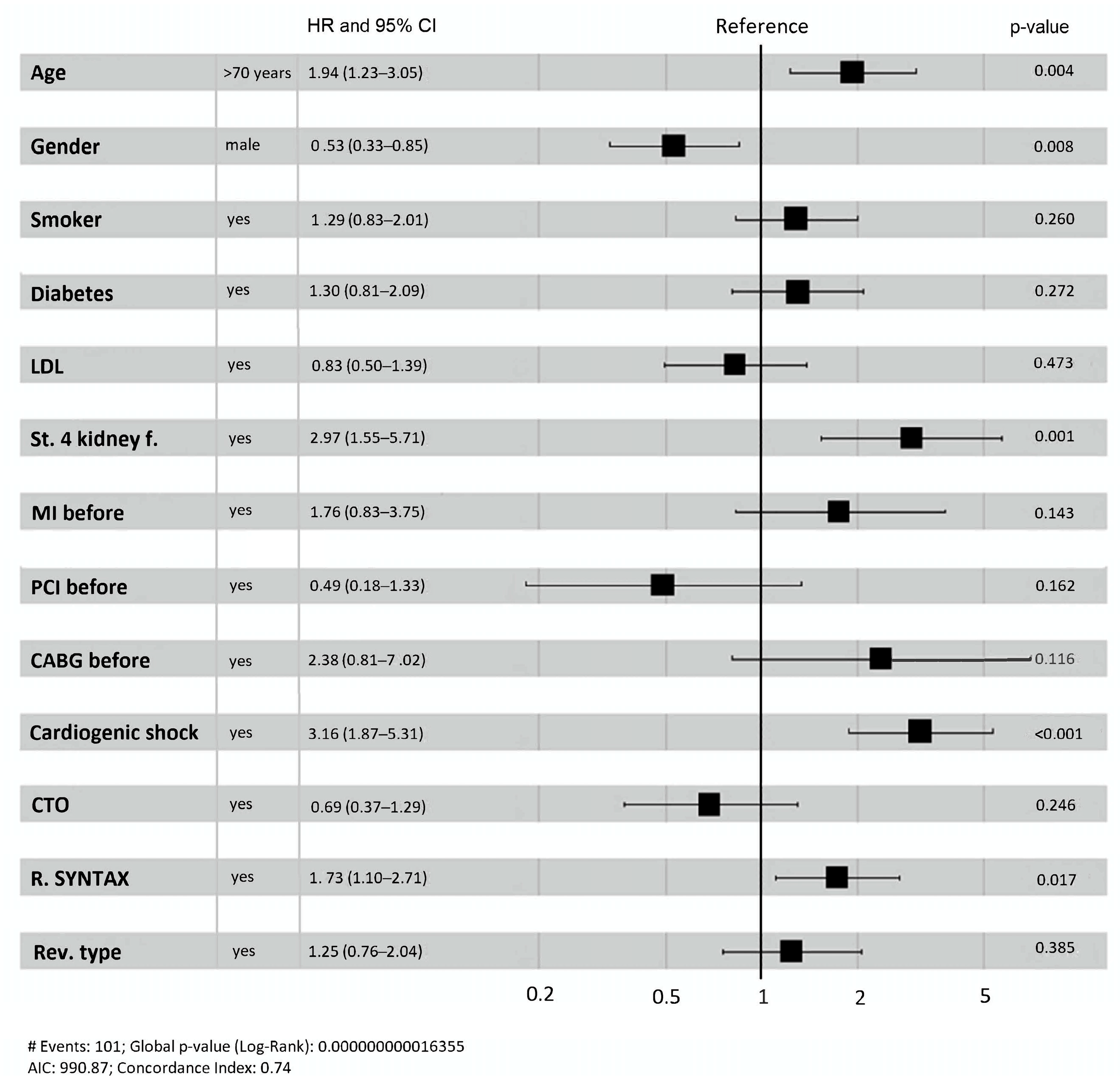
3.2. Ischemia-Driven Rehospitalization and Cardiovascular Mortality
3.3. Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sorajja, P.; Gersh, B.J.; Cox, D.A.; McLaughlin, M.G.; Zimetbaum, P.; Costantini, C.; Stuckey, T.; Tcheng, J.E.; Mehran, R.; Lansky, A.J.; et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur. Heart J. 2007, 28, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Milford-Beland, S.; Roe, M.T.; Peterson, E.D.; Weintraub, W.S.; Rao, S.V. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry). Am. J. Cardiol. 2009, 104, 507–513. [Google Scholar] [CrossRef]
- Hannan, E.L.; Samadashvili, Z.; Walford, G.; Holmes, D.R., Jr.; Jacobs, A.K.; Stamato, N.J.; Venditti, F.J.; Sharma, S.; King, S.B., 3rd. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc. Interv. 2010, 3, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.B.; Ilsley, C.; Kabir, T.; Smith, R.; Lane, R.; Mason, M.; Clifford, P.; Crake, T.; Firoozi, S.; Kalra, S.; et al. Culprit vessel versus multivessel intervention at the time of primary percutaneous coronary intervention in patients with ST-segment-elevation myocardial infarction and multivessel disease: Real-world analysis of 3984 patients in London. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.B.; Nadra, I.J.; Ding, L.; Fung, A.; Aymong, E.; Chan, A.W.; Hodge, S.; Della Siega, A.; Robinson, S.D.; British Columbia Cardiac Registry Investigators. Culprit Vessel Versus Multivessel Versus In-Hospital Staged Intervention for Patients With ST-Segment Elevation Myocardial Infarction and Multivessel Disease: Stratified Analyses in High-Risk Patient Groups and Anatomic Subsets of Nonculprit Disease. JACC Cardiovasc. Interv. 2017, 10, 11–23. [Google Scholar] [CrossRef]
- Wald, D.S.; Morris, J.K.; Wald, N.J.; Chase, A.J.; Edwards, R.J.; Hughes, L.O.; Berry, C.; Oldroyd, K.G.; PRAMI Investigators. Randomized trial of preventive angioplasty in myocardial infarction. N. Engl. J. Med. 2013, 369, 1115–1123. [Google Scholar] [CrossRef]
- Engstrom, T.; Kelbaek, H.; Helqvist, S.; Hofsten, D.E.; Klovgaard, L.; Holmvang, L.; Jorgensen, E.; Pedersen, F.; Saunamaki, K.; Clemmensen, P.; et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): An open-label, randomised controlled trial. Lancet 2015, 386, 665–671. [Google Scholar] [CrossRef]
- Gershlick, A.H.; Khan, J.N.; Kelly, D.J.; Greenwood, J.P.; Sasikaran, T.; Curzen, N.; Blackman, D.J.; Dalby, M.; Fairbrother, K.L.; Banya, W.; et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: The CvLPRIT trial. J. Am. Coll. Cardiol. 2015, 65, 963–972. [Google Scholar] [CrossRef]
- Smits, P.C.; Abdel-Wahab, M.; Neumann, F.J.; Boxma-de Klerk, B.M.; Lunde, K.; Schotborgh, C.E.; Piroth, Z.; Horak, D.; Wlodarczak, A.; Ong, P.J.; et al. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N. Engl. J. Med. 2017, 376, 1234–1244. [Google Scholar] [CrossRef]
- Mehta, S.R.; Wood, D.A.; Storey, R.F.; Mehran, R.; Bainey, K.R.; Nguyen, H.; Meeks, B.; Di Pasquale, G.; Lopez-Sendon, J.; Faxon, D.P.; et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N. Engl. J. Med. 2019, 381, 1411–1421. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Boden, W.E.; O’Rourke, R.A.; Teo, K.K.; Hartigan, P.M.; Maron, D.J.; Kostuk, W.J.; Knudtson, M.; Dada, M.; Casperson, P.; Harris, C.L.; et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N. Engl. J. Med. 2007, 356, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef]
- Kuku, K.O.; Singh, M.; Ozaki, Y.; Dan, K.; Chezar-Azerrad, C.; Waksman, R.; Garcia-Garcia, H.M. Near-Infrared Spectroscopy Intravascular Ultrasound Imaging: State of the Art. Front. Cardiovasc. Med. 2020, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Echeverri, N.; Mehta, S.R.; Wang, J.; Lavi, S.; Schampaert, E.; Cantor, W.J.; Bainey, K.R.; Welsh, R.C.; Kassam, S.; Mehran, R.; et al. Nonculprit Lesion Plaque Morphology in Patients With ST-Segment-Elevation Myocardial Infarction: Results From the COMPLETE Trial Optical Coherence Tomography Substudys. Circ. Cardiovasc. Interv. 2020, 13, e008768. [Google Scholar] [CrossRef]
- Thim, T.; van der Hoeven, N.W.; Musto, C.; Nijveldt, R.; Götberg, M.; Engstrøm, T.; Smits, P.C.; Oldroyd, K.G.; Gershlick, A.H.; Escaned, J.; et al. Evaluation and Management of Nonculprit Lesions in STEMI. JACC Cardiovasc. Interv. 2020, 13, 1145–1154. [Google Scholar] [CrossRef]
- Sustersic, M.; Mrak, M.; Svegl, P.; Kodre, A.R.; Kranjec, I.; Fras, Z.; Bunc, M. Complete Revascularization and Survival in STEMI. Glob. Heart 2021, 16, 64. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Dimitriu-Leen, A.C.; Hermans, M.P.; Veltman, C.E.; van der Hoeven, B.L.; van Rosendael, A.R.; van Zwet, E.W.; Schalij, M.J.; Delgado, V.; Bax, J.J.; Scholte, A.J. Prognosis of complete versus incomplete revascularisation of patients with STEMI with multivessel coronary artery disease: An observational study. Open Heart 2017, 4, e000541. [Google Scholar] [CrossRef] [PubMed]
- Galvao Braga, C.; Cid-Alvarez, A.B.; Redondo Dieguez, A.; Trillo-Nouche, R.; Alvarez Alvarez, B.; Lopez Otero, D.; Ocaranza Sanchez, R.; Gestal Romani, S.; Gonzalez Ferreiro, R.; Gonzalez-Juanatey, J.R. Multivessel Versus Culprit-only Percutaneous Coronary Intervention in ST-segment Elevation Acute Myocardial Infarction: Analysis of an 8-year Registry. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Hlinomaz, O.; Groch, L.; Poloková, K.; Lehar, F.; Vekov, T.; Petkov, R.; Stojnev, M.; Gřiva, M.; Sitár, J.; Rezek, M.; et al. Multivessel coronary disease diagnosed at the time of primary PCI for STEMI: Complete revascularisation versus conservative strategy. Prague-13 trial. Kardiol. Rev. Int. Med. 2015, 17, 214–220. [Google Scholar]
- Bravo, C.A.; Hirji, S.A.; Bhatt, D.L.; Kataria, R.; Faxon, D.P.; Ohman, E.M.; Anderson, K.L.; Sidi, A.I.; Sketch, M.H., Jr.; Zarich, S.W.; et al. Complete versus culprit-only revascularisation in ST elevation myocardial infarction with multi-vessel disease. Cochrane Database Syst. Rev. 2017, 5, CD011986. [Google Scholar] [CrossRef]
- Xu, B.; Yang, Y.; Han, Y.-l.; Lu, S.-Z.; Li, B.; Liu, Q.; Zhu, G.-Y.; Cui, J.-Y.; Li, L.; Zhao, Y.-L.; et al. Validation of residual SYNTAX score with second-generation drug-eluting stents: One-year results from the prospective multicentre SEEDS study. EuroIntervention 2014, 10, 65–73. [Google Scholar] [CrossRef]
- Ahmed, T.A.N.; Othman, A.A.A.; Demitry, S.R.; Elmaghraby, K.M. Impact of residual coronary lesions on outcomes of myocardial infarction patients with multi-vessel disease. BMC Cardiovasc. Disord. 2024, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Altekin, R.E.; Kilinc, A.Y.; Onac, M.; Cicekcibasi, O. Prognostic Value of the Residual SYNTAX Score on In-Hospital and Follow-Up Clinical Outcomes in ST Elevation Myocardial Infarction Patients Undergoing Percutaneous Coronary Interventions. Cardiol. Res. Pract. 2020, 2020, 9245431. [Google Scholar] [CrossRef]
- Kim, L.K.; Yeo, I.; Cheung, J.W.; Swaminathan, R.V.; Wong, S.C.; Charitakis, K.; Adejumo, O.; Chae, J.; Minutello, R.M.; Bergman, G.; et al. Thirty-Day Readmission Rates, Timing, Causes, and Costs after ST-Segment-Elevation Myocardial Infarction in the United States: A National Readmission Database Analysis 2010–2014. J. Am. Heart Assoc. 2018, 7, e009863. [Google Scholar] [CrossRef]
- Spitzer, E.; Frei, M.; Zaugg, S.; Hadorn, S.; Kelbaek, H.; Ostojic, M.; Baumbach, A.; Tüller, D.; Roffi, M.; Engstrom, T.; et al. Rehospitalizations Following Primary Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction: Results From a Multi-Center Randomized Trial. J. Am. Heart Assoc. 2017, 6, e005926. [Google Scholar] [CrossRef]
- Brown, J.R.; Conley, S.M.; Niles, N.W., 2nd. Predicting readmission or death after acute ST-elevation myocardial infarction. Clin. Cardiol. 2013, 36, 570–575. [Google Scholar] [CrossRef]
- Laudani, C.; Occhipinti, G.; Greco, A.; Spagnolo, M.; Giacoppo, D.; Capodanno, D. Completeness, timing, and guidance of percutaneous coronary intervention for myocardial infarction and multivessel disease: A systematic review and network meta-analysis. EuroIntervention 2025, 21, e203–e216. [Google Scholar] [CrossRef] [PubMed]


| Variable | CR N = 70 | IR N = 165 | p-Value |
|---|---|---|---|
| Age ≥ 61 years, n (%) | 41 (59) | 114 (69) | 0.160 |
| Men, n (%) | 49 (70) | 120 (73) | 0.790 |
| Arterial hypertension, n (%) | 43 (61) | 116 (70) | 0.239 |
| Diabetes, n (%) | 11 (16) | 42 (25) | 0.143 |
| Current smoker, n (%) | 26 (37) | 57 (35) | 0.817 |
| Hyperlipidemia, n (%) | 46 (66) | 104 (63) | 0.808 |
| Family history of cardiovascular disease *, n (%) | 11 (16) | 30 (18) | 0.789 |
| Chronic kidney disease, n (%) | 6 (9) | 23 (14) | 0.354 |
| Previous myocardial infarction, n (%) | 4 (6) | 23 (14) | 0.077 |
| Previous PCI, n (%) | 5 (7) | 13 (8) | 1.000 |
| Previous CABG, n (%) | 0 (0) | 6 (4) | 0.183 |
| Coronary intervention | |||
| Culprit artery | |||
| Left descending coronary | 26 (37) | 57 (35) | 0.766 |
| Right coronary | 36 (51) | 81 (49) | 0.424 |
| Left circumflex coronary | 8 (11) | 27 (16) | 0.777 |
| Number of significant stenoses of non-culprit artery | 0.005 | ||
| 1 | 38 (54) | 56 (34) | |
| >1 | 32 (46) | 109 (66) | |
| CTO | 1 (1) | 29 (18) | <0.001 |
| After CABG | 4 (2) | 0.321 | |
| Number of PCI procedures † | |||
| 1 | 32 (46) | 130 (79) | <0.001 |
| >1 | 38 (54) | 35 (21) | <0.001 |
| Integrilin use | 13 (19) | 34 (21) | 0.859 |
| Intra-aortic balloon pump | 5 (7) | 26 (16) | 0.092 |
| Transfusion due to coronary intervention complication | 4 (6) | 8 (5) | 0.754 |
| LVEF after PCI † | 0.871 | ||
| ˃55% | 20 (29) | 40 (24) | |
| 45 to ≤54% | 7 (10) | 21 (13) | |
| 30 to ˂45% | 7 (10) | 13 (8) | |
| ˂30% | 3 (4) | 10 (6) |
| CR (N = 70) | IR (N = 165) | Fisher Test | |
|---|---|---|---|
| Reason for Rehospitalization | N (%) | N (%) | p-Value |
| AP | 6 (8.6) | 11 (6.7) | 0.519 |
| NAP | 3 (4.3) | 5 (3.0) | |
| STEMI | 1 (1.4) | 8 (4.8) | |
| NSTEMI | 1 (1.4) | 5 (3.0) | |
| Heart failure | 1 (1.4) | 6 (3.6) | |
| Other | 3 (4.3) | 10 (6.1) | |
| With rehospitalization | 15 (21.4) | 45 (27.3) | |
| Without rehospitalization | 55 (78.6) | 120 (72.7) | |
| Method of revascularization | |||
| CABG | 1 (1.4) | 5 (3.0) | 0.672 |
| PCI LM | 0 (0.0) | 1 (0.6) | 1.000 |
| PCI LAD | 4 (5.7) | 11 (6.7) | 1.000 |
| PCI LCX | 1 (1.4) | 10 (6.1) | 0.181 |
| PCI RCA | 2 (2.9) | 8 (4.8) | 0.727 |
| Adjustment of treatment with medications | 7 (10.0) | 10 (6.1) | 0.257 |
| Comparators and Risk Factors | Ischemia-Driven Rehospitalization and CV Mortality | ||
|---|---|---|---|
| Coefficient | HR (95% CI) | p -Value | |
| IR | −0.19 | 0.83 (0.39–1.80) | 0.622 |
| CR | 0.19 | 1.21 (0.56–2.54) | 0.622 |
| Age | 0.04 | 1.04 (1.00–1.08) | 0.014 |
| Smoker | −0.26 | 0.77 (0.35–1.67) | 0.507 |
| Diabetes | 1.13 | 3.08 (1.36–6.78) | 0.006 |
| LDL | −0.03 | 0.97 (0.66–1.39) | 0.860 |
| Creatinine value at inclusion (natural logarithm) | 0.01 | 1.01 (1.00–1.01) | 0.001 |
| Previous MI | −0.08 | 0.92 (0.21–3.31) | 0.909 |
| Previous PCI | 0.43 | 1.54 (0.27–8.26) | 0.612 |
| Previous CABG | 1.16 | 3.18 (0.15–22.58) | 0.319 |
| Cardiogenic shock | 1.38 | 3.97 (1.57–9.66) | 0.003 |
| CTO | 0.99 | 2.70 (0.96–6.94) | 0.046 |
| Residual SYNTAX I score | 0.01 | 1.01 (0.95–1.07) | 0.692 |
| Rehospitalization † | 2.08 | 8.03 (3.85–17.52) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sustersic, M.; Bunc, M. Effect of Complete Revascularization in STEMI: Ischemia-Driven Rehospitalization and Cardiovascular Mortality. J. Clin. Med. 2025, 14, 4793. https://doi.org/10.3390/jcm14134793
Sustersic M, Bunc M. Effect of Complete Revascularization in STEMI: Ischemia-Driven Rehospitalization and Cardiovascular Mortality. Journal of Clinical Medicine. 2025; 14(13):4793. https://doi.org/10.3390/jcm14134793
Chicago/Turabian StyleSustersic, Miha, and Matjaz Bunc. 2025. "Effect of Complete Revascularization in STEMI: Ischemia-Driven Rehospitalization and Cardiovascular Mortality" Journal of Clinical Medicine 14, no. 13: 4793. https://doi.org/10.3390/jcm14134793
APA StyleSustersic, M., & Bunc, M. (2025). Effect of Complete Revascularization in STEMI: Ischemia-Driven Rehospitalization and Cardiovascular Mortality. Journal of Clinical Medicine, 14(13), 4793. https://doi.org/10.3390/jcm14134793








