Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications
Abstract
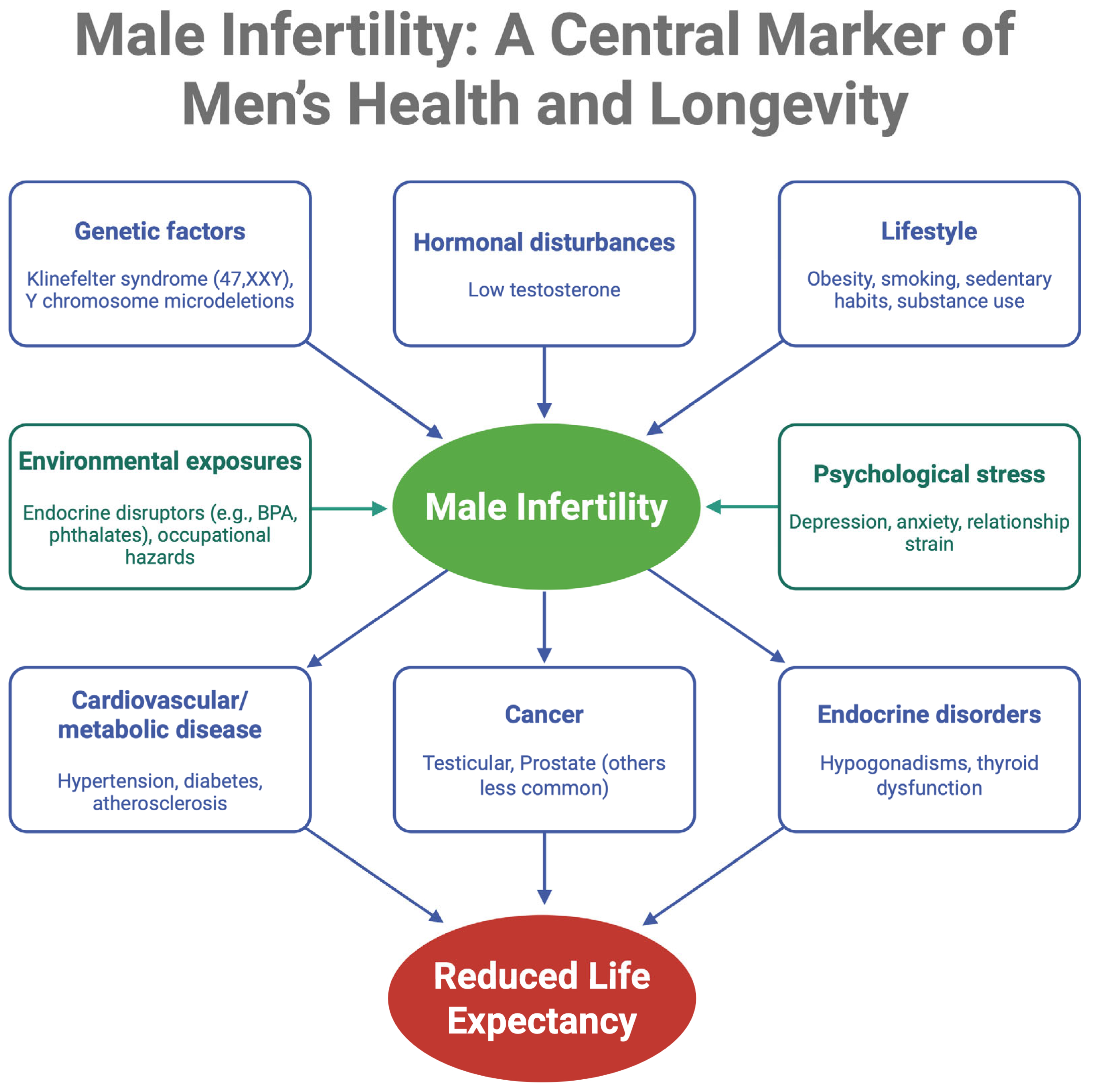
1. Introduction
2. Epidemiological Links Between Male Infertility and Mortality
3. Common Comorbidities in Infertile Men
3.1. Cardiovascular Disease and Metabolic Syndrome
3.2. Oncologic Concerns
3.3. Endocrine and Autoimmune Disorders
3.4. Genetic Syndromes
3.5. Other Health Issues
4. Biological and Hormonal Mechanisms Connecting Infertility with Systemic Disease
4.1. Genetic Pleiotropy and Developmental Pathways
4.2. Hormonal Imbalances
4.3. Shared Risk Factors and Organ Reserve
4.4. Molecular and Cellular Mechanisms
5. Psychosocial Impact of Infertility on Mental Health
6. Lifestyle, Socioeconomic Status, and Environmental Exposures
6.1. Lifestyle Behaviors
6.2. Socioeconomic Determinants and Health Inequalities
6.3. Environmental and Occupational Exposures
6.4. The Role of Social and Relationship Factors
7. Clinical Implications and Recommendations
7.1. Comprehensive Health Screeing
7.2. Hormonal Evalutation and Management Strategies
7.3. Cancer Surveillance
7.4. Intergrating Mental Health Support into Fertility Support
7.5. Lifestyle Interventions
7.6. Long-Term Follow-up
7.7. Patient Education and Counseling
7.8. Interdisciplinary Collaboration
7.9. Public Health Strategies and Awareness as a Window to Health
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L.R.; Bjorndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef]
- Kasman, A.M.; Del Giudice, F.; Eisenberg, M.L. New insights to guide patient care: The bidirectional relationship between male infertility and male health. Fertil. Steril. 2020, 113, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.M.; Eisenberg, M.L.; Hotaling, J.M. Male infertility: A biomarker of individual and familial cancer risk. Fertil. Steril. 2018, 109, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chen, Y.J.; Yang, C.C.; Lin, T.T.; Huang, C.C.; Chung, C.H.; Sun, C.A.; Chien, W.C. Male Infertility Increases the Risk of Cardiovascular Diseases: A Nationwide Population-Based Cohort Study in Taiwan. World J. Mens. Health 2022, 40, 490–500. [Google Scholar] [CrossRef]
- Tesarik, J. Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention. Int. J. Mol. Sci. 2025, 26, 2797. [Google Scholar] [CrossRef]
- Kaminski, P.; Baszynski, J.; Jerzak, I.; Kavanagh, B.P.; Nowacka-Chiari, E.; Polanin, M.; Szymanski, M.; Wozniak, A.; Kozera, W. External and Genetic Conditions Determining Male Infertility. Int. J. Mol. Sci. 2020, 21, 5274. [Google Scholar] [CrossRef]
- Roudbaraki, S.N.; Ramezani, M.; Saifi, B.; Salimi, M.; Cheshani, M.I. Assessing psychological health and reproductive function: Depression, anxiety, and stress in infertile men compared to controls: A case-control study. Int. J. Reprod. Biomed. 2024, 22, 1025–1034. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef]
- Jensen, T.K.; Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good semen quality and life expectancy: A cohort study of 43,277 men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Li, S.; Behr, B.; Cullen, M.R.; Galusha, D.; Lamb, D.J.; Lipshultz, L.I. Semen quality, infertility and mortality in the USA. Hum. Reprod. 2014, 29, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Priskorn, L.; Lindahl-Jacobsen, R.; Jensen, T.K.; Holmboe, S.A.; Hansen, L.S.; Kriegbaum, M.; Lind, B.S.; Siersma, V.; Andersen, C.L.; Jorgensen, N. Semen quality and lifespan: A study of 78 284 men followed for up to 50 years. Hum. Reprod. 2025, 40, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Kasman, A.M.; Chen, T.; De Berardinis, E.; Busetto, G.M.; Sciarra, A.; Ferro, M.; Lucarelli, G.; Belladelli, F.; Salonia, A.; et al. The Association between Mortality and Male Infertility: Systematic Review and Meta-analysis. Urology 2021, 154, 148–157. [Google Scholar] [CrossRef]
- Fallara, G.; Pozzi, E.; Belladelli, F.; Boeri, L.; Capogrosso, P.; Corona, G.; D’Arma, A.; Alfano, M.; Montorsi, F.; Salonia, A. A Systematic Review and Meta-analysis on the Impact of Infertility on Men’s General Health. Eur. Urol. Focus. 2024, 10, 98–106. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Cullen, M.R.; Baker, L.C. Increased risk of incident chronic medical conditions in infertile men: Analysis of United States claims data. Fertil. Steril. 2016, 105, 629–636. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Johansson, A.L.; Ludvigsson, J.F. Mortality in 43,598 men with infertility—A Swedish nationwide population-based cohort study. Clin. Epidemiol. 2019, 11, 645–657. [Google Scholar] [CrossRef]
- Elenkov, A.; Giwercman, A.; Sogaard Tottenborg, S.; Bonde, J.P.E.; Glazer, C.H.; Haervig, K.K.; Bungum, A.B.; Nilsson, P.M. Male childlessness as independent predictor of risk of cardiovascular and all-cause mortality: A population-based cohort study with more than 30 years follow-up. PLoS ONE 2020, 15, e0237422. [Google Scholar] [CrossRef]
- Behboudi-Gandevani, S.; Bidhendi-Yarandi, R.; Panahi, M.H.; Vaismoradi, M. A Systematic Review and Meta-Analysis of Male Infertility and the Subsequent Risk of Cancer. Front. Oncol. 2021, 11, 696702. [Google Scholar] [CrossRef]
- Al-Jebari, Y.; Elenkov, A.; Wirestrand, E.; Schutz, I.; Giwercman, A.; Lundberg Giwercman, Y. Risk of prostate cancer for men fathering through assisted reproduction: Nationwide population based register study. BMJ 2019, 366, l5214. [Google Scholar] [CrossRef]
- Del Giudice, F.; Kasman, A.M.; Ferro, M.; Sciarra, A.; De Berardinis, E.; Belladelli, F.; Salonia, A.; Eisenberg, M.L. Clinical correlation among male infertility and overall male health: A systematic review of the literature. Investig. Clin. Urol. 2020, 61, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Latif, T.; Kold Jensen, T.; Mehlsen, J.; Holmboe, S.A.; Brinth, L.; Pors, K.; Skouby, S.O.; Jorgensen, N.; Lindahl-Jacobsen, R. Semen Quality as a Predictor of Subsequent Morbidity: A Danish Cohort Study of 4,712 Men With Long-Term Follow-up. Am. J. Epidemiol. 2017, 186, 910–917. [Google Scholar] [CrossRef]
- Latif, T.; Lindahl-Jacobsen, R.; Mehlsen, J.; Eisenberg, M.L.; Holmboe, S.A.; Pors, K.; Brinth, L.; Skouby, S.O.; Jorgensen, N.; Jensen, T.K. Semen quality associated with subsequent hospitalizations—Can the effect be explained by socio-economic status and lifestyle factors? Andrology 2018, 6, 428–435. [Google Scholar] [CrossRef]
- Bungum, A.B.; Glazer, C.H.; Bonde, J.P.; Nilsson, P.M.; Giwercman, A.; Sogaard Tottenborg, S. Risk of metabolic disorders in childless men: A population-based cohort study. BMJ Open 2018, 8, e020293. [Google Scholar] [CrossRef]
- Kaltsas, A.; Dimitriadis, F.; Zachariou, D.; Zikopoulos, A.; Symeonidis, E.N.; Markou, E.; Tien, D.M.B.; Takenaka, A.; Sofikitis, N.; Zachariou, A. From Diagnosis to Treatment: Comprehensive Care by Reproductive Urologists in Assisted Reproductive Technology. Medicina 2023, 59, 1835. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, E.; Chiu, P.K. Health risks associated with infertility and non-obstructive azoospermia. Asian J. Androl. 2025, 27, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Glazer, C.H.; Eisenberg, M.L.; Tottenborg, S.S.; Giwercman, A.; Flachs, E.M.; Brauner, E.V.; Vassard, D.; Pinborg, A.; Schmidt, L.; Bonde, J.P. Male factor infertility and risk of death: A nationwide record-linkage study. Hum. Reprod. 2019, 34, 2266–2273. [Google Scholar] [CrossRef]
- Choy, J.T.; Eisenberg, M.L. Comprehensive men’s health and male infertility. Transl. Androl. Urol. 2020, 9, S239–S243. [Google Scholar] [CrossRef]
- Elenkov, A.; Zaren, P.; Sundell, B.; Lundin, L.; Giwercman, A. Testosterone deficiency and metabolic disturbances in men who fathered a child by use of donated spermatozoa. Sci. Rep. 2022, 12, 14458. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zachariou, A.; Dimitriadis, F.; Chrisofos, M.; Sofikitis, N. Empirical Treatments for Male Infertility: A Focus on Lifestyle Modifications and Medicines. Diseases 2024, 12, 209. [Google Scholar] [CrossRef]
- Salvio, G.; Ciarloni, A.; Cutini, M.; Delli Muti, N.; Finocchi, F.; Perrone, M.; Rossi, S.; Balercia, G. Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. Int. J. Mol. Sci. 2022, 23, 5497. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jackson, G.; Jones, T.H.; Matsumoto, A.M.; Nehra, A.; Perelman, M.A.; Swerdloff, R.S.; Traish, A.; Zitzmann, M.; Cunningham, G. Low testosterone associated with obesity and the metabolic syndrome contributes to sexual dysfunction and cardiovascular disease risk in men with type 2 diabetes. Diabetes Care 2011, 34, 1669–1675. [Google Scholar] [CrossRef]
- Walsh, T.J.; Croughan, M.S.; Schembri, M.; Chan, J.M.; Turek, P.J. Increased risk of testicular germ cell cancer among infertile men. Arch. Intern. Med. 2009, 169, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Valkna, A.; Juchnewitsch, A.G.; Polluaas, L.; Lillepea, K.; Tjagur, S.; Dutta, A.; Pomm, K.; Punab, M.; Laan, M. Significantly increased load of hereditary cancer-linked germline variants in infertile men. Hum. Reprod. Open 2025, 2025, hoaf008. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Karkada, I.R.; Chinni, S.V. Endocrinopathies and Male Infertility. Life 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, J.; Guo, B.; Jiang, C.; Sun, W. Diabetes-induced male infertility: Potential mechanisms and treatment options. Mol. Med. 2024, 30, 11. [Google Scholar] [CrossRef]
- Freeman, H.J. Reproductive changes associated with celiac disease. World J. Gastroenterol. 2010, 16, 5810–5814. [Google Scholar] [CrossRef]
- Sciorio, R.; De Paola, L.; Notari, T.; Ganduscio, S.; Amato, P.; Crifasi, L.; Marotto, D.; Billone, V.; Cucinella, G.; Perino, A.; et al. Decoding the Puzzle of Male Infertility: The Role of Infection, Inflammation, and Autoimmunity. Diagnostics 2025, 15, 547. [Google Scholar] [CrossRef]
- Hawksworth, D.J.; Szafran, A.A.; Jordan, P.W.; Dobs, A.S.; Herati, A.S. Infertility in Patients With Klinefelter Syndrome: Optimal Timing for Sperm and Testicular Tissue Cryopreservation. Rev. Urol. 2018, 20, 56–62. [Google Scholar] [CrossRef]
- Deebel, N.A.; Bradshaw, A.W.; Sadri-Ardekani, H. Infertility considerations in klinefelter syndrome: From origin to management. Best. Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101480. [Google Scholar] [CrossRef]
- Bojesen, A.; Gravholt, C.H. Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatr. 2011, 100, 807–813. [Google Scholar] [CrossRef]
- Bojesen, A.; Juul, S.; Birkebaek, N.; Gravholt, C.H. Increased mortality in Klinefelter syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 3830–3834. [Google Scholar] [CrossRef]
- Carto, C.; Loloi, J.; Campbell, K.; Ramasamy, R. Testosterone Therapy in Men with Klinefelter Syndrome: Analysis of a Global Federated Research Network. Androg. Clin. Res. Ther. 2022, 3, 208–212. [Google Scholar] [CrossRef]
- Ridder, L.O.; Berglund, A.; Stochholm, K.; Chang, S.; Gravholt, C.H. Morbidity, mortality, and socioeconomics in Klinefelter syndrome and 47,XYY syndrome: A comparative review. Endocr. Connect. 2023, 12, e230024. [Google Scholar] [CrossRef]
- Le, M.T.; Tran, N.Q.T.; Nguyen, N.D.; Nguyen, Q.H.V. The Prevalence and Components of Metabolic Syndrome in Men from Infertile Couples and Its Relation on Semen Analysis. Diabetes Metab. Syndr. Obes. 2021, 14, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Sachdev, D.; Lee, R.; Sauer, M.V.; Ananth, C.V. Infertility treatment is associated with increased risk of postpartum hospitalization due to heart disease. J. Intern. Med. 2024, 295, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, A.; Al-Jebari, Y.; Giwercman, A. More Prevalent Prescription of Medicine for Hypertension and Metabolic Syndrome in Males from Couples Undergoing Intracytoplasmic Sperm Injection. Sci. Rep. 2018, 8, 14521. [Google Scholar] [CrossRef] [PubMed]
- O’Flynn O’Brien, K.L.; Varghese, A.C.; Agarwal, A. The genetic causes of male factor infertility: A review. Fertil. Steril. 2010, 93, 1–12. [Google Scholar] [CrossRef]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders. Front. Cell Dev. Biol. 2021, 9, 634536. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Betts, P.; Herder, D.; Lamb, D.J.; Lipshultz, L.I. Increased risk of cancer among azoospermic men. Fertil. Steril. 2013, 100, 681–685. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health. Genes 2025, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Gravholt, C.H.; Chang, S.; Wallentin, M.; Fedder, J.; Moore, P.; Skakkebaek, A. Klinefelter Syndrome: Integrating Genetics, Neuropsychology, and Endocrinology. Endocr. Rev. 2018, 39, 389–423. [Google Scholar] [CrossRef]
- Wohlfahrt-Veje, C.; Main, K.M.; Skakkebaek, N.E. Testicular dysgenesis syndrome: Foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009, 71, 459–465. [Google Scholar] [CrossRef]
- Tsutida, C.A.; Veiga, A.C.B.; Martino-Andrade, A.J.; de Andrade, D.P.; Mello, R.G.; Muller, J.C. Association between Late Manifestations of Testicular Dysgenesis Syndrome and Anogenital Distance: A Systematic Review and Meta-analysis. J. Hum. Reprod. Sci. 2023, 16, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, W.; Wang, Z.; Huang, R.; Xia, H.; Li, Z.; Deng, J.; Ye, T.; Huang, Y.; Yang, Y. Hormone Regulation in Testicular Development and Function. Int. J. Mol. Sci. 2024, 25, 5805. [Google Scholar] [CrossRef]
- Spaziani, M.; Carlomagno, F.; Tarantino, C.; Angelini, F.; Vincenzi, L.; Gianfrilli, D. New perspectives in functional hypogonadotropic hypogonadism: Beyond late onset hypogonadism. Front Endocrinol 2023, 14, 1184530. [Google Scholar] [CrossRef] [PubMed]
- Spaziani, M.; Carlomagno, F.; Tenuta, M.; Sesti, F.; Angelini, F.; Bonaventura, I.; Ferrari, D.; Tarantino, C.; Fiore, M.; Petrella, C.; et al. Extra-Gonadal and Non-Canonical Effects of FSH in Males. Pharmaceuticals 2023, 16, 813. [Google Scholar] [CrossRef]
- El Osta, R.; Almont, T.; Diligent, C.; Hubert, N.; Eschwege, P.; Hubert, J. Anabolic steroids abuse and male infertility. Basic. Clin. Androl. 2016, 26, 2. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, X.; Liu, Y.F.; Song, B.; Sun, Z.; Zhao, S. Immunoregulation and male reproductive function: Impacts and mechanistic insights into inflammation. Andrology 2024, 1–20. [Google Scholar] [CrossRef]
- Service, C.A.; Puri, D.; Al Azzawi, S.; Hsieh, T.C.; Patel, D.P. The impact of obesity and metabolic health on male fertility: A systematic review. Fertil. Steril. 2023, 120, 1098–1111. [Google Scholar] [CrossRef]
- Parekh, N.V.; Lundy, S.D.; Vij, S.C. Fertility considerations in men with testicular cancer. Transl. Androl. Urol. 2020, 9, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- van der Kaaij, M.A.; Heutte, N.; van Echten-Arends, J.; Raemaekers, J.M.; Carde, P.; Noordijk, E.M.; Ferme, C.; Thomas, J.; Eghbali, H.; Brice, P.; et al. Sperm quality before treatment in patients with early stage Hodgkin’s lymphoma enrolled in EORTC-GELA Lymphoma Group trials. Haematologica 2009, 94, 1691–1697. [Google Scholar] [CrossRef]
- Persily, J.B.; Vijay, V.; Najari, B.B. How do we counsel men with obstructive azoospermia due to CF mutations?-a review of treatment options and outcomes. Transl. Androl. Urol. 2021, 10, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G.; et al. The association of female and male infertility with telomere length (Review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The impact of mitochondrial impairments on sperm function and male fertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2021, 8, 799294. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Yan, H.; Han, P.; Zhang, J.; Liu, Y. Deciphering the Role of Oxidative Stress in Male Infertility: Insights from Reactive Oxygen Species to Antioxidant Therapeutics. Front Biosci (Landmark Ed) 2025, 30, 27046. [Google Scholar] [CrossRef]
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox Balance in Male Infertility: Excellence through Moderation-“Muepsilontaurhoomicronnu ἄrhoiotasigmatauomicronnu”. Antioxidants 2021, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Basile, U.; Vergani, E.; Napodano, C.; Oliva, A.; Gulli, F.; Meucci, E.; Silvestrini, A.; Orlando, P.; Silvestri, S.; et al. Inflammation and Oxidative Stress in Seminal Plasma: Search for Biomarkers in Diagnostic Approach to Male Infertility. J. Pers. Med. 2022, 12, 857. [Google Scholar] [CrossRef]
- Ayad, B.; Omolaoye, T.S.; Louw, N.; Ramsunder, Y.; Skosana, B.T.; Oyeipo, P.I.; Du Plessis, S.S. Oxidative Stress and Male Infertility: Evidence From a Research Perspective. Front. Reprod. Health 2022, 4, 822257. [Google Scholar] [CrossRef]
- Camargo, M.; Intasqui, P.; Bertolla, R.P. Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J. Androl. 2016, 18, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Zikopoulos, A.; Markou, E.; Zachariou, A.; Stavropoulos, M.; Kratiras, Z.; Symeonidis, E.N.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. Proteomics and Metabolomics in Varicocele-Associated Male Infertility: Advancing Precision Diagnostics and Therapy. J. Clin. Med. 2024, 13, 7390. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ridgeway, A.D.; Lamb, D.J. DNA mismatch repair and infertility. Curr. Opin. Urol. 2010, 20, 525–532. [Google Scholar] [CrossRef]
- Avila, D.M.; Zoppi, S.; McPhaul, M.J. The androgen receptor (AR) in syndromes of androgen insensitivity and in prostate cancer. J. Steroid Biochem. Mol. Biol. 2001, 76, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Vivacqua, A.; Peluso, G.; Castiglione, R.; D’Agata, R. New insight in human sperm pro-survival and pro-apoptotic pathways: Potential new therapeutical targets in male infertility. Biol. Reprod. 2025, ioaf078. [Google Scholar] [CrossRef] [PubMed]
- Puzuka, A.; Alksere, B.; Gailite, L.; Erenpreiss, J. Idiopathic Infertility as a Feature of Genome Instability. Life 2021, 11, 628. [Google Scholar] [CrossRef]
- Ho, S.M.; Johnson, A.; Tarapore, P.; Janakiram, V.; Zhang, X.; Leung, Y.K. Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 2012, 53, 289–305. [Google Scholar] [CrossRef]
- Martens, M.; Schroer, M.; Williams, T.; Kennedy, B. Identifying Mental Health Issues Associated With Infertility. J. Nurse Pract. 2023, 19, 104471. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Wang, Y.; Liu, C.; Sun, J.; Zhang, Z.; Guan, L.; Xiao, K.; Zhu, Z.; Luo, J. Association between mental health and male fertility: Depression, rather than anxiety, is linked to decreased semen quality. Front Endocrinol (Lausanne) 2024, 15, 1478848. [Google Scholar] [CrossRef]
- Rogers, E.M.; Banks, N.F.; Jenkins, N.D.M. The effects of sleep disruption on metabolism, hunger, and satiety, and the influence of psychosocial stress and exercise: A narrative review. Diabetes Metab. Res. Rev. 2024, 40, e3667. [Google Scholar] [CrossRef]
- Kiani, Z.; Fakari, F.R.; Hakimzadeh, A.; Hajian, S.; Fakari, F.R.; Nasiri, M. Prevalence of depression in infertile men: A systematic review and meta-analysis. BMC Public Health 2023, 23, 1972. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.G.; Forthofer, M.S. Associations of psychosocial factors with the stress of infertility treatment. Health Soc. Work. 2005, 30, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Persily, J.; Stair, S.; Najari, B.B. Access to infertility services: Characterizing potentially infertile men in the United States with the use of the National Survey for Family Growth. Fertil. Steril. 2020, 114, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Horns, J.J.; Fendereski, K.; Ramsay, J.M.; Halpern, J.; Iko, I.N.; Ferlic, E.; Emery, B.R.; Aston, K.; Hotaling, J. The impact of socioeconomic status on bulk semen parameters, fertility treatment, and fertility outcomes in a cohort of subfertile men. Fertil. Steril. 2023, 120, 72–79. [Google Scholar] [CrossRef]
- Zhao, C.C.; Scott, M.; Eisenberg, M.L. Male Fertility as a Proxy for Health. J. Clin. Med. 2024, 13, 5559. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef]
- Minguez-Alarcon, L.; Gaskins, A.J.; Meeker, J.D.; Braun, J.M.; Chavarro, J.E. Endocrine-disrupting chemicals and male reproductive health. Fertil. Steril. 2023, 120, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Sousa, A.C.A. The Impact of Endocrine-Disrupting Chemicals in Male Fertility: Focus on the Action of Obesogens. J. Xenobiot. 2021, 11, 163–196. [Google Scholar] [CrossRef]
- Torkel, S.; Moran, L.; Wang, R.; Villani, A.; Mantzioris, E.; Norman, R.J.; Cowan, S. Barriers and enablers to a healthy lifestyle in people with infertility: A qualitative descriptive study. Reprod. Biol. Endocrinol. 2025, 23, 52. [Google Scholar] [CrossRef]
- Greenberg, D.R.; Panken, E.J.; Keeter, M.K.; Bennett, N.E.; Brannigan, R.E.; Halpern, J.A. Reproductive Urology Consultation Is an Opportunity to Evaluate for Medical Comorbidity: The Prevalence of Elevated Blood Pressure and Obesity at Fertility Evaluation. Cureus 2024, 16, e57071. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Li, S.; Behr, B.; Pera, R.R.; Cullen, M.R. Relationship between semen production and medical comorbidity. Fertil. Steril. 2015, 103, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, W.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Boeri, L.; Frego, N.; Abbate, C.; Alfano, M.; Vigano, P.; Montorsi, F.; et al. High Blood Pressure Is a Highly Prevalent but Unrecognised Condition in Primary Infertile Men: Results of a Cross-sectional Study. Eur. Urol. Focus. 2020, 6, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Minhas, S.; Boeri, L.; Capogrosso, P.; Cocci, A.; Corona, G.; Dinkelman-Smit, M.; Falcone, M.; Jensen, C.F.; Gul, M.; Kalkanli, A.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2025 Update on Male Infertility. Eur. Urol. 2025, 87, 601–616. [Google Scholar] [CrossRef]
- Bassil, N.; Alkaade, S.; Morley, J.E. The benefits and risks of testosterone replacement therapy: A review. Ther. Clin. Risk Manag. 2009, 5, 427–448. [Google Scholar] [CrossRef]
- Elenkov, A.; Al-Jebari, Y.; Giwercman, Y.L.; Giwercman, A. Testosterone replacement therapy in men who conceived with intracytoplasmic sperm injection: Nationwide register study. Eur. J. Endocrinol. 2020, 182, 423–428. [Google Scholar] [CrossRef]
- Stanworth, R.; Jones, T. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front. Horm. Res. 2009, 37, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Garolla, A.; Ghezzi, M.; Selice, R.; Palego, P.; Caretta, N.; Di Mambro, A.; Valente, U.; De Rocco Ponce, M.; Dipresa, S.; et al. Sperm Count and Hypogonadism as Markers of General Male Health. Eur. Urol. Focus. 2021, 7, 205–213. [Google Scholar] [CrossRef]
- Achermann, A.P.P.; Esteves, S.C. Prevalence and clinical implications of biochemical hypogonadism in patients with nonobstructive azoospermia undergoing infertility evaluation. F S Rep. 2024, 5, 14–22. [Google Scholar] [CrossRef]
- McBride, J.A.; Coward, R.M. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian J. Androl. 2016, 18, 373–380. [Google Scholar] [CrossRef]
- Fink, J.; Ide, H.; Horie, S. Management of Male Fertility in Hypogonadal Patients on Testosterone Replacement Therapy. Medicina 2024, 60, 275. [Google Scholar] [CrossRef]
- Maiolino, G.; Fernandez-Pascual, E.; Ochoa Arvizo, M.A.; Vishwakarma, R.; Martinez-Salamanca, J.I. Male Infertility and the Risk of Developing Testicular Cancer: A Critical Contemporary Literature Review. Medicina 2023, 59, 1305. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Boeri, L.; Capogrosso, P.; Cazzaniga, W.; Ventimiglia, E.; Pozzi, E.; Belladelli, F.; Schifano, N.; Candela, L.; Alfano, M.; Pederzoli, F.; et al. Infertile Men Have Higher Prostate-specific Antigen Values than Fertile Individuals of Comparable Age. Eur. Urol. 2021, 79, 234–240. [Google Scholar] [CrossRef]
- Vezyraki, P.; Vlachaki, A.; Baltogiannis, D.; Batistatou, A.; Tsampalas, S.; Simos, Y.V.; Kaltsas, A.; Pappas, P.; Dounousi, E.; Ragos, V.; et al. Impact of total PSA and percent free PSA in the differentiation of prostate disease: A retrospective comparative study implicating neoplastic and non-neoplastic entities. J. BUON 2019, 24, 2107–2113. [Google Scholar]
- Tiwari, P.; Yadav, A.; Kaushik, M.; Dada, R. Cancer risk and male Infertility: Unravelling predictive biomarkers and prognostic indicators. Clin. Chim. Acta 2024, 558, 119670. [Google Scholar] [CrossRef]
- Del Giudice, F.; Kasman, A.M.; De Berardinis, E.; Busetto, G.M.; Belladelli, F.; Eisenberg, M.L. Association between male infertility and male-specific malignancies: Systematic review and meta-analysis of population-based retrospective cohort studies. Fertil. Steril. 2020, 114, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Sharma, P.; Kumar, P. Role of Mental Health Practitioner in Infertility Clinics: A Review on Past, Present and Future Directions. J. Hum. Reprod. Sci. 2018, 11, 219–228. [Google Scholar] [CrossRef]
- Read, S.C.; Carrier, M.E.; Boucher, M.E.; Whitley, R.; Bond, S.; Zelkowitz, P. Psychosocial services for couples in infertility treatment: What do couples really want? Patient Educ. Couns. 2014, 94, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Greco, C.; Barbonetti, A.; Simoni, M.; Maggi, M.; Corona, G. Weight Loss as Therapeutic Option to Restore Fertility in Obese Men: A Meta-Analytic Study. World J. Mens. Health 2025, 43, 333–343. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Zachariou, A.; Dimitriadis, F.; Mamoulakis, C.; Andreadakis, S.; Giannakis, I.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. Varicoceles in Men With Non-obstructive Azoospermia: The Dilemma to Operate or Not. Front. Reprod. Health 2022, 4, 811487. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.; Harlev, A.; Esteves, S.C. Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: A systematic review and meta-analysis. Asian J. Androl. 2016, 18, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Vancsa, S.; Hegyi, P.; Koi, T.; Acs, J.; Hermanne, R.J.; Acs, N.; Szarvas, T.; Nyirady, P.; Kopa, Z. Assessing the efficacy of varicocelectomy, antioxidants, FSH treatment, and lifestyle modifications on sperm DNA fragmentation: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 10118. [Google Scholar] [CrossRef] [PubMed]
- Nahata, L.; Caltabellotta, N.M.; Yeager, N.D.; Lehmann, V.; Whiteside, S.L.; O’Brien, S.H.; Quinn, G.P.; Gerhardt, C.A. Fertility perspectives and priorities among male adolescents and young adults in cancer survivorship. Pediatr. Blood Cancer 2018, 65, e27019. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Park, Y.; Hollenbeck, A.R.; Lipshultz, L.I.; Schatzkin, A.; Pletcher, M.J. Fatherhood and the risk of cardiovascular mortality in the NIH-AARP Diet and Health Study. Hum. Reprod. 2011, 26, 3479–3485. [Google Scholar] [CrossRef]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Belladelli, F.; Muncey, W.; Eisenberg, M.L. Reproduction as a window for health in men. Fertil. Steril. 2023, 120, 429–437. [Google Scholar] [CrossRef]
- Daumler, D.; Chan, P.; Lo, K.C.; Takefman, J.; Zelkowitz, P. Men’s knowledge of their own fertility: A population-based survey examining the awareness of factors that are associated with male infertility. Hum. Reprod. 2016, 31, 2781–2790. [Google Scholar] [CrossRef]
- Montano, L.; Iannuzzi, L.; Rubes, J.; Avolio, C.; Pistos, C.; Gatti, A.; Raimondo, S.; Notari, T. EcoFoodFertility—Environmental and food impact assessment on male reproductive function. Andrology 2014, 2 (Suppl. S2), 69–70. [Google Scholar] [CrossRef]
- Barratt, C.L.R.; De Jonge, C.J.; Sharpe, R.M. ‘Man Up’: The importance and strategy for placing male reproductive health centre stage in the political and research agenda. Hum. Reprod. 2018, 33, 541–545. [Google Scholar] [CrossRef]
- Kruglova, K.; Gelgoot, E.N.; Chan, P.; Lo, K.; Rosberger, Z.; Belanger, E.; Kazdan, J.; Robins, S.; Zelkowitz, P. Risky Business: Increasing Fertility Knowledge of Men in the General Public Using the Mobile Health Application Infotility XY. Am. J. Mens. Health 2021, 15, 15579883211049027. [Google Scholar] [CrossRef]
| Study | Population Size (N) | Fertility Status | Mortality Risk (HR/RR) | Key Findings |
|---|---|---|---|---|
| Fallara et al. (2024) [15] | 3,173,122 fertile vs. 212,791 infertile men | Infertile vs. fertile men | All-cause mortality: HR = 1.37 (95% CI: 1.04–1.81); Testicular cancer: RR = 1.86 (95% CI: 1.41–2.45); Prostate cancer: RR = 1.66 (95% CI: 1.06–2.61); Melanoma: RR = 1.30 (95% CI: 1.08–1.56); Diabetes: HR = 1.39 (95% CI: 1.09–1.71); Cardiovascular events: HR = 1.20 (95% CI: 1.00–1.44) | Infertile men had significantly increased risks of all-cause mortality, specific cancers (testicular, prostate, melanoma), diabetes, and cardiovascular events compared to fertile men, supporting infertility as a marker for general health risks. |
| Del Giudice et al. (2021) [14] | Total: 202,456 (infertility cohorts); 59,291 (semen parameters cohorts) | Infertile vs. fertile men; Oligo/Azoospermic vs. Normospermic | HR = 1.26 (95% CI: 1.01–1.59); RR = 1.67 (95% CI: 1.26–2.21) for Oligo/Azoospermic vs. Normospermic | Infertile men had a 26% higher all-cause mortality compared to fertile men. Those with combined oligo- and azoospermia had a 67% higher risk of death compared to normospermic men. Azoospermic men specifically had over twofold risk (HR = 2.17, 95% CI: 1.55–3.04) compared to normospermic controls. |
| Behboudi-Gandevani et al. (2021) [19] | 168,327 infertile men; 2,252,806 controls | Infertility vs. Fertility | OR = 1.43 (95% CI 1.25–1.64) | Infertile men had approximately 43% higher risk of developing any cancer compared to fertile men. Specifically elevated risks were noted for testicular cancer (OR = 1.91, 95% CI: 1.52–2.42), prostate cancer (OR = 1.48, 95% CI: 1.05–2.08), and melanoma (OR = 1.31, 95% CI: 1.06–1.62). Male infertility was concluded to be a significant independent risk factor for future cancer development. |
| Elenkov et al. (2020) [18] | 22,444 men from Malmö Preventive Project | Childless vs. Fathers (proxy for infertility) | HR (CVD mortality) = 1.33 (95% CI: 1.18–1.49); HR (all-cause mortality) = 1.23 (95% CI: 1.14–1.33) | Childless men exhibited significantly higher cardiovascular mortality (33% increased risk) and all-cause mortality (23% increased risk) compared to fathers. Childless men also had a worse baseline metabolic profile with increased odds of high triglycerides (OR 1.24), high fasting glucose (OR 1.23), and hypertension (OR 1.28). Suggests childlessness (likely related to infertility) independently predicts increased cardiovascular and metabolic risks. |
| Al-Jebari et al. (2019) [20] | 1,181,490 fathers (20,618 IVF; 14,882 ICSI; 1,145,990 natural conception) | ICSI/IVF vs. Natural conception | HR = 1.64 (95% CI: 1.25–2.15) for ICSI; HR = 1.33 (95% CI: 1.06–1.66) for IVF; HR = 1.86 (95% CI: 1.25–2.77) for early-onset prostate cancer (ICSI) | Men fathering via ICSI had a 64% higher overall risk of prostate cancer compared with natural conception. ICSI-treated men had an 86% increased risk of early-onset prostate cancer (diagnosed before age 55). Fathers conceiving via IVF had a 33% higher risk of prostate cancer compared to natural conception. Severe male-factor infertility thus appears strongly associated with elevated long-term prostate cancer risks, particularly for early-onset disease. |
| Lundberg et al. (2019) [17] | 43,598 men with infertility diagnosis; 57,733 men with infertility-related diagnosis; 2,762,254 controls | Infertility and infertility-related diagnoses vs. controls | HR = 0.98 (95% CI: 0.89–1.08) for infertility diagnosis; HR = 1.23 (95% CI: 1.17–1.30) for infertility-related diagnoses; HR = 3.26 (95% CI: 2.42–4.41) for mortality before age 30 in infertile men | Overall, men diagnosed with infertility did not have significantly higher mortality compared to controls. However, significantly higher mortality occurred among those with infertility-related diagnoses and particularly among infertile men under 30, largely due to cancers diagnosed before infertility evaluation. A slightly elevated suicide risk (HR = 1.18; 95% CI: 1.01–1.37) was also observed, indicating possible psychosocial implications. |
| Eisenberg et al. (2016) [16] | 13,027 infertile men; 23,860 fertility-tested controls; 79,099 vasectomized men | Male infertility vs. fertility-tested and vasectomized men | Increased risk: Diabetes (HR 1.30, 95% CI 1.10–1.53), Ischemic heart disease (HR 1.48, 95% CI 1.19–1.84), Hypertension (HR 1.09, 95% CI 1.02–1.17), Renal disease (HR 1.60, 95% CI 1.14–2.24), Liver disease (HR 1.53, 95% CI 1.31–1.80), Peripheral vascular disease (HR 1.52, 95% CI 1.12–2.07) | Infertile men had significantly increased risks of developing chronic medical conditions including diabetes, ischemic heart disease, hypertension, renal and liver diseases, and peripheral vascular disease compared to fertile (vasectomized) men. Findings suggest infertility evaluation may identify men at increased risk for chronic health conditions later in life. |
| Eisenberg et al. (2014) [12] | 11,935 infertile men | Normal vs. Abnormal semen parameters | HR = 2.29 (95% CI: 1.12–4.65) for men with ≥2 semen abnormalities | Over ~8 years follow-up, men with two or more abnormal semen parameters had more than double the risk of death compared to those with normal semen parameters. Study confirms that more severe sperm abnormalities predict higher risks of premature mortality. |
| Jensen et al. (2009) [11] | 43,277 men | Normospermic vs. Oligo-/Azoospermic | Dose-response decrease in mortality with increasing sperm concentration (up to 40 million/mL), motility, and normal morphology (p < 0.05) | Men with higher sperm counts and greater percentages of motile and morphologically normal spermatozoa had significantly lower mortality rates. Mortality decreased steadily with improving semen quality parameters up to a concentration of 40 million/mL, supporting the concept of semen quality as a fundamental biomarker of overall male health and life expectancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsas, A.; Koumenis, A.; Stavropoulos, M.; Kratiras, Z.; Deligiannis, D.; Adamos, K.; Chrisofos, M. Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications. J. Clin. Med. 2025, 14, 3930. https://doi.org/10.3390/jcm14113930
Kaltsas A, Koumenis A, Stavropoulos M, Kratiras Z, Deligiannis D, Adamos K, Chrisofos M. Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications. Journal of Clinical Medicine. 2025; 14(11):3930. https://doi.org/10.3390/jcm14113930
Chicago/Turabian StyleKaltsas, Aris, Andreas Koumenis, Marios Stavropoulos, Zisis Kratiras, Dimitrios Deligiannis, Konstantinos Adamos, and Michael Chrisofos. 2025. "Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications" Journal of Clinical Medicine 14, no. 11: 3930. https://doi.org/10.3390/jcm14113930
APA StyleKaltsas, A., Koumenis, A., Stavropoulos, M., Kratiras, Z., Deligiannis, D., Adamos, K., & Chrisofos, M. (2025). Male Infertility and Reduced Life Expectancy: Epidemiology, Mechanisms, and Clinical Implications. Journal of Clinical Medicine, 14(11), 3930. https://doi.org/10.3390/jcm14113930







