Association Between Allostatic Load and Delirium in ICU Patients: A Retrospective Analysis of the MIMIC-IV Database
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
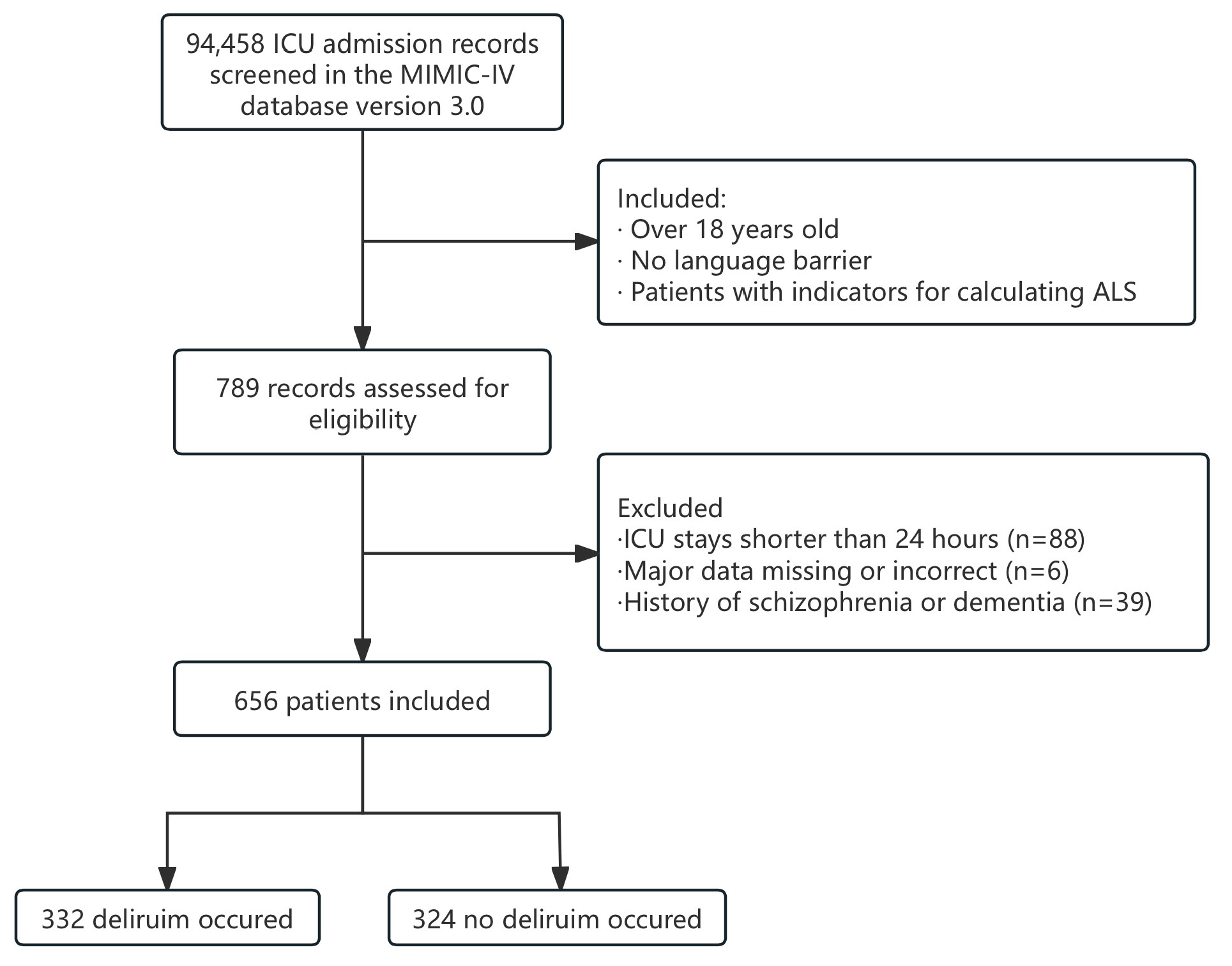
2.2. Cohort Selection
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population
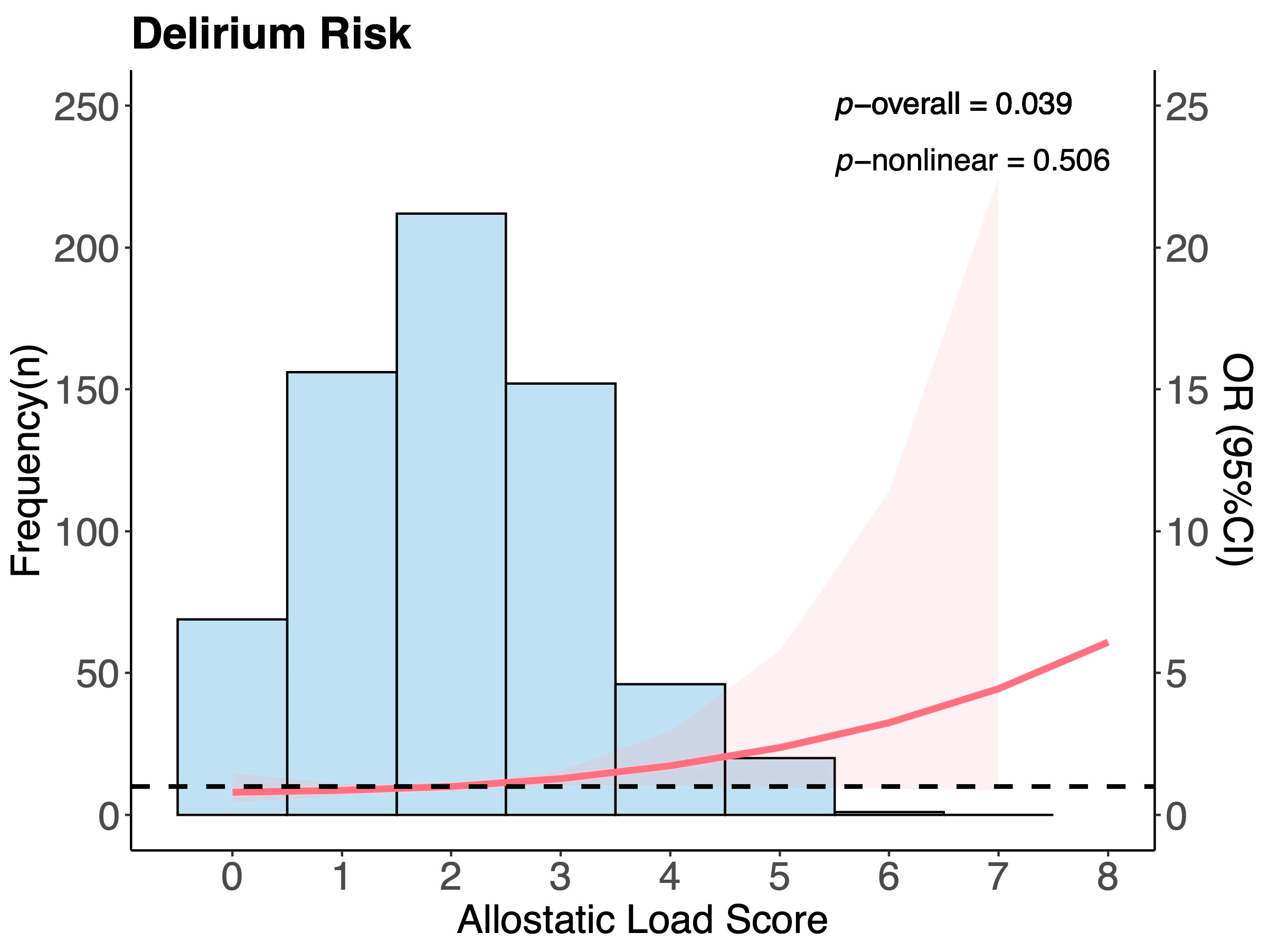
3.2. ALS and Other Risk Factors
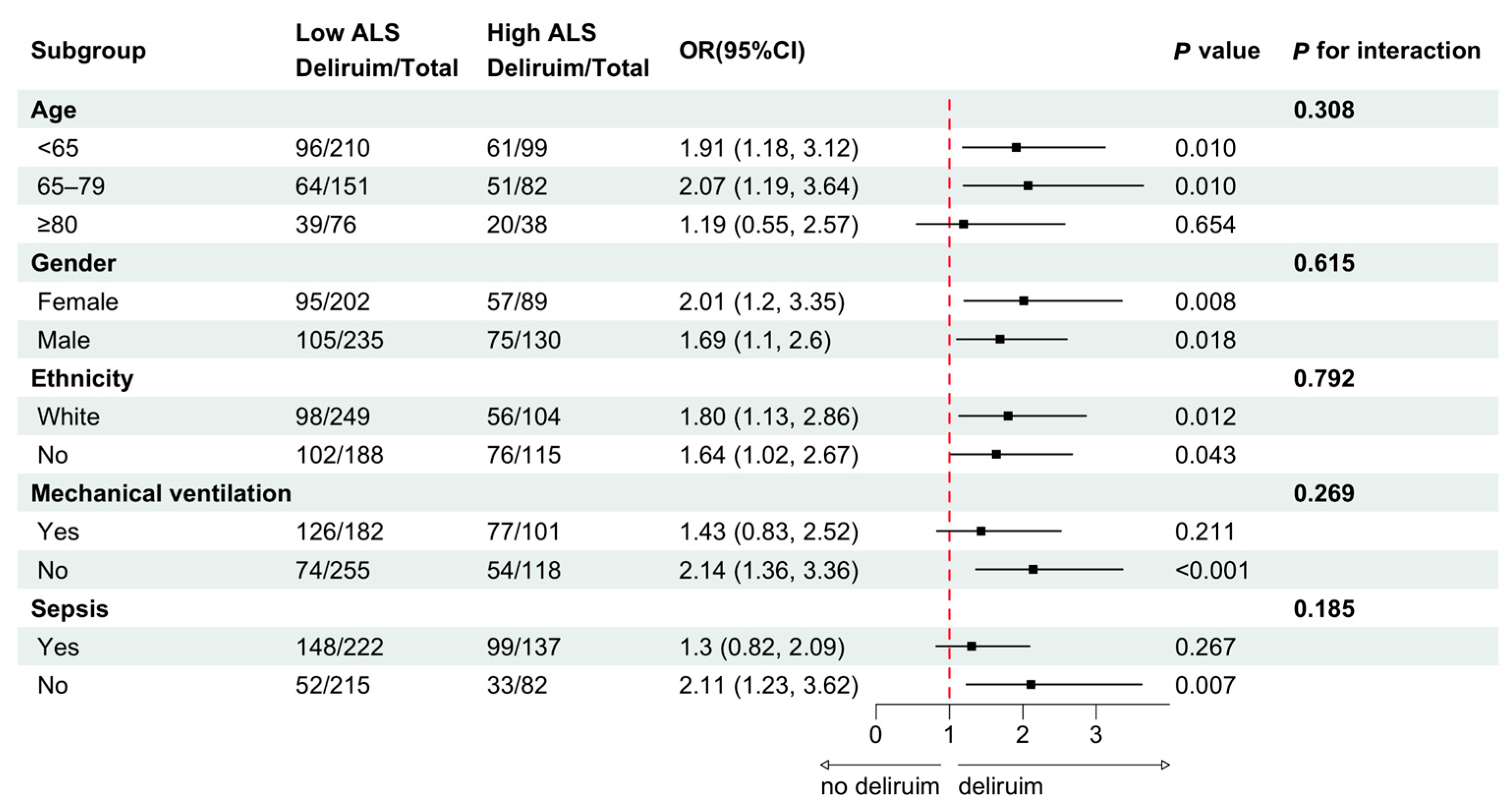
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mattison, M.L.P. Delirium. Ann. Intern. Med. 2020, 173, Itc49–Itc64. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.; Soares, M.; Teles, J.M.; Ceraso, D.; Raimondi, N.; Nava, V.S.; Blasquez, P.; Ugarte, S.; Ibanez-Guzman, C.; Centeno, J.V.; et al. Delirium epidemiology in critical care (DECCA): An international study. Crit. Care 2010, 14, R210. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Inouye, S.K.; Bernard, G.R.; Gordon, S.; Francis, J.; May, L.; Truman, B.; Speroff, T.; Gautam, S.; Margolin, R.; et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef]
- Liu, S.B.; Wu, H.Y.; Duan, M.L.; Yang, R.L.; Ji, C.H.; Liu, J.J.; Zhao, H. Delirium in the ICU: How much do we know? A narrative review. Ann. Med. 2024, 56, 2405072. [Google Scholar] [CrossRef]
- Juster, R.P.; Misiak, B. Advancing the allostatic load model: From theory to therapy. Psychoneuroendocrinology 2023, 154, 106289. [Google Scholar] [CrossRef]
- Fava, G.A.; McEwen, B.S.; Guidi, J.; Gostoli, S.; Offidani, E.; Sonino, N. Clinical characterization of allostatic overload. Psychoneuroendocrinology 2019, 108, 94–101. [Google Scholar] [CrossRef]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- Guidi, J.; Lucente, M.; Sonino, N.; Fava, G.A. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother. Psychosom. 2021, 90, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Elsaid, M.I.; Handley, D.; Plascak, J.J.; Andersen, B.L.; Carson, W.E.; Pawlik, T.M.; Fareed, N.; Obeng-Gyasi, S. Association Between Neighborhood Opportunity, Allostatic Load, and All-Cause Mortality in Patients with Breast Cancer. J. Clin. Oncol. 2024, 42, 1788–1798. [Google Scholar] [CrossRef]
- Stabellini, N.; Cullen, J.; Bittencourt, M.S.; Moore, J.X.; Cao, L.; Weintraub, N.L.; Harris, R.A.; Wang, X.; Datta, B.; Coughlin, S.S.; et al. Allostatic load and cardiovascular outcomes in males with prostate cancer. JNCI Cancer Spectr. 2023, 7, pkad005. [Google Scholar] [CrossRef]
- Sonino, N.; Fava, G.A.; Lucente, M.; Guidi, J. Allostatic Load and Endocrine Disorders. Psychother. Psychosom. 2023, 92, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.B.; Jones, E.M.; Ovbiagele, B.; Markovic, D.; Towfighi, A. Effects of Allostatic Load on Long-Term Survival After Stroke. Stroke 2025, 56, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shen, J.; Lu, J.; Fuemmeler, B.F.; Shock, L.S.; Zhao, H. Association between allostatic load and breast cancer risk: A cohort study. Breast Cancer Res. 2023, 25, 155. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Bingham, B.A.; Aldana, P.C.; Chung, S.T.; Sumner, A.E. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. J. Racial Ethn. Health Disparities 2017, 4, 455–461. [Google Scholar] [CrossRef]
- Carbone, J.T.; Clift, J.; Alexander, N. Measuring allostatic load: Approaches and limitations to algorithm creation. J. Psychosom. Res. 2022, 163, 111050. [Google Scholar] [CrossRef]
- Maldonado, J.R. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am. J. Geriatr. Psychiatry 2013, 21, 1190–1222. [Google Scholar] [CrossRef]
- Dilmen, O.K.; Meco, B.C.; Evered, L.A.; Radtke, F.M. Postoperative neurocognitive disorders: A clinical guide. J. Clin. Anesth. 2024, 92, 111320. [Google Scholar] [CrossRef]
- Thomson, E.M. Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. J. Alzheimers Dis. 2019, 69, 597–614. [Google Scholar] [CrossRef]
- Johnson, A.E.W.; Bulgarelli, L.; Shen, L.; Gayles, A.; Shammout, A.; Horng, S.; Pollard, T.J.; Hao, S.; Moody, B.; Gow, B.; et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 2023, 10, 1. [Google Scholar] [CrossRef]
- Johnson, A.; Bulgarelli, L.; Pollard, T.; Gow, B.; Moody, B.; Horng, S.; Celi, L.A.; Mark, R. MIMIC-IV (Version 3.0). PhysioNet. Available online: https://physionet.org/content/mimiciv/3.0/ (accessed on 22 March 2025).
- Chen, T.J.; Chung, Y.W.; Chang, H.R.; Chen, P.Y.; Wu, C.R.; Hsieh, S.H.; Chiu, H.Y. Diagnostic accuracy of the CAM-ICU and ICDSC in detecting intensive care unit delirium: A bivariate meta-analysis. Int. J. Nurs. Stud. 2021, 113, 103782. [Google Scholar] [CrossRef]
- International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available online: https://archive.cdc.gov/www_cdc_gov/nchs/icd/icd9cm.htm (accessed on 21 March 2025).
- International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Available online: https://www.cdc.gov/nchs/icd/icd-10-cm/index.html (accessed on 22 May 2025).
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed]
- McEwen, B.S. Interacting mediators of allostasis and allostatic load: Towards an understanding of resilience in aging. Metabolism 2003, 52 (Suppl. S2), 10–16. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Du, E.Y.; Jiang, K.; Carlson, M.C.; Reed, N.S.; Deal, J.A. Hearing Impairment and Allostatic Load in Older Adults. JAMA Otolaryngol. Head. Neck Surg. 2023, 149, 597–606. [Google Scholar] [CrossRef]
- Wilcox, M.E.; Girard, T.D.; Hough, C.L. Delirium and long term cognition in critically ill patients. BMJ 2021, 373, n1007. [Google Scholar] [CrossRef]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Primers 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Dunne, S.S.; Coffey, J.C.; Konje, S.; Gasior, S.; Clancy, C.C.; Gulati, G.; Meagher, D.; Dunne, C.P. Biomarkers in delirium: A systematic review. J. Psychosom. Res. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.G.; Inouye, S.K. The inter-relationship between delirium and dementia: The importance of delirium prevention. Nat. Rev. Neurol. 2022, 18, 579–596. [Google Scholar] [CrossRef]
- Smith, P.J.; Attix, D.K.; Weldon, B.C.; Greene, N.H.; Monk, T.G. Executive function and depression as independent risk factors for postoperative delirium. Anesthesiology 2009, 110, 781–787. [Google Scholar] [CrossRef]
- Engel, G.L.; Romano, J. Delirium, a syndrome of cerebral insufficiency. J. Chronic Dis. 1959, 9, 260–277. [Google Scholar] [CrossRef]
- Maclullich, A.M.; Ferguson, K.J.; Miller, T.; de Rooij, S.E.; Cunningham, C. Unravelling the pathophysiology of delirium: A focus on the role of aberrant stress responses. J. Psychosom. Res. 2008, 65, 229–238. [Google Scholar] [CrossRef] [PubMed]
- de Pablos, R.M.; Villarán, R.F.; Argüelles, S.; Herrera, A.J.; Venero, J.L.; Ayala, A.; Cano, J.; Machado, A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J. Neurosci. 2006, 26, 5709–5719. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J.A.; Hough, C.L. How We Prevent and Treat Delirium in the ICU. Chest 2021, 160, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Rigney, T. Allostatic load and delirium in the hospitalized older adult. Nurs. Res. 2010, 59, 322–330. [Google Scholar] [CrossRef]
| Variables | Overall | Non-Delirium | Delirium | p Value |
|---|---|---|---|---|
| Total | n = 656 | n = 324 | n = 332 | |
| Age (year) | 66 (54, 76) | 66 (53, 76) | 66 (55, 75) | 0.696 |
| Female Sex (n, %) | 291 (44.4) | 139 (42.9) | 152 (45.8) | 0.458 |
| Race (n, %) | <0.001 * | |||
| White | 374 (57) | 209 (64.5) | 165 (49.7) | |
| Others | 282 (43) | 115 (35.5) | 167 (50.3) | |
| BMI (kg/m2) | 27.29 (23.48, 31.73) | 27.3 (23.51, 31.76) | 27.1 (23.29, 31.73) | 0.615 |
| ALS (score) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | <0.001 * |
| ALS ≥ 3 (n, %) | 219 (33.4) | 87 (26.9) | 132 (39.8) | <0.001 * |
| SOFA (score) | 4(2, 6) | 3(1, 5) | 4(3, 7) | <0.001 * |
| Heart rate (beats/min) | 83 (73, 93) | 80 (72, 92) | 86 (73, 96) | 0.012 * |
| Respiratory rate (times/min) | 19 (17, 22) | 19 (17, 21) | 20 (18, 22) | <0.001 * |
| Temperature (°C) | 36.9 (36.7, 37.2) | 36.9 (36.7, 37.1) | 37 (36.7, 37.3) | <0.001 * |
| SBP (mmHg) | 129.7 (101.65, 155.76) | 128.59 (100.84, 156.34) | 130.78 (102.44, 159.12) | 0.316 |
| DBP (mmHg) | 74 (63, 85) | 73 (63, 83.75) | 74 (63, 87) | 0.331 |
| MBP (mmHg) | 83 (75, 92) | 83 (75.25, 92.75) | 82 (75, 92) | 0.580 |
| SpO2 (%) | 97 (96, 98) | 97 (96, 98) | 98 (96, 99) | 0.001 * |
| Laboratory tests | ||||
| HbA1c (%) | 5.7 (5.3, 6.5) | 5.6 (5.3, 6.4) | 5.8 (5.3, 6.58) | 0.528 |
| Serum albumin (g/dL) | 3.4 (2.9, 3.8) | 3.5 (3.1, 3.9) | 3.3 (2.73, 3.7) | <0.001 * |
| C-reactive protein (mg/dL) | 34.65 (6.5, 99.5) | 35.7 (6.75, 91.4) | 33.25 (6.25, 107.25) | 0.870 |
| Cholesterol (mg/dL) | 143.5 (113, 180.75) | 143 (113, 173.75) | 146 (113, 188.75) | 0.248 |
| HDL(mg/dL) | 41 (29, 55) | 40.5 (28, 53) | 42 (30, 57) | 0.105 |
| WBC (109/L) | 10.65 (7.96, 14.49) | 9.85 (7.52, 13.63) | 11.43 (8.81, 15.25) | <0.001 * |
| Hemoglobin(g/dL) | 11.1 (9.15, 12.95) | 11.4 (9.45, 13.1) | 10.7 (8.81, 12.65) | 0.034 * |
| Platelet (109/L) | 207 (156, 273) | 206.5 (162.5, 269) | 207.5 (151, 278.38) | 0.569 |
| Creatinine (mg/dL) | 1 (0.75, 1.5) | 0.95 (0.7, 1.4) | 1.05 (0.75, 1.75) | 0.014 * |
| Blood urea nitrogen (mg/dL) | 18 (12.5, 30) | 17 (12, 27) | 20 (13, 35) | 0.003 * |
| AG (mEq/L) | 14 (12, 16.5) | 14 (12, 16) | 14.5 (12, 17) | 0.044 * |
| INR | 1.2 (1.1, 1.45) | 1.2 (1.1, 1.4) | 1.2 (1.1, 1.5) | 0.043 * |
| Comorbidities | ||||
| Myocardial infarct (n, %) | 144 (22) | 69 (21.3) | 75 (22.5) | 0.689 |
| Congestive heart failure (n, %) | 234 (35.7) | 109 (33.6) | 125 (37.7) | 0.284 |
| Peripheral vascular disease (n, %) | 80 (12.2) | 44 (13.6) | 36 (10.8) | 0.341 |
| Cerebrovascular disease (n, %) | 423 (64.5) | 206 (63.6) | 217 (65.4) | 0.693 |
| Chronic pulmonary disease (n, %) | 115 (17.5) | 54 (16.7) | 61 (18.4) | 0.565 |
| Rheumatic disease (n, %) | 25 (3.8) | 19 (5.9) | 6 (1.8) | 0.012 * |
| Peptic ulcer disease (n, %) | 19 (2.9) | 8 (2.5) | 11 (3.3) | 0.681 |
| Liver disease (n, %) | 78 (11.9) | 33 (10.2) | 45 (13.6) | 0.183 |
| Diabetes (n, %) | 232 (35.4) | 104 (32.1) | 128 (38.6) | 0.084 |
| Renal disease (n, %) | 148 (22.6) | 66 (20.4) | 82 (24.7) | 0.185 |
| Malignant tumor (n, %) | 58 (8.8) | 34 (10.5) | 24 (7.2) | 0.141 |
| Sepsis (n, %) | 359 (54.7) | 112 (34.6) | 247 (74.4) | <0.001 * |
| Events | ||||
| Mechanical ventilation (n, %) | 283 (43.1) | 80 (24.7) | 203 (61.1) | <0.001 * |
| Reintubation within 48 h (n, %) | 23 (3.5) | 7 (2.2) | 16 (4.8) | 0.064 |
| ICU LOS (day) | 5 (2, 10) | 3 (2, 5) | 8 (4, 15) | <0.001 * |
| Hospital LOS (day) | 16 (8, 28) | 11 (5, 19) | 22 (12, 37) | <0.001 * |
| In-hospital death (n, %) | 82 (12.5) | 29 (9.0) | 53 (16.0) | 0.009 * |
| Variable | OR (95% CI) | p Value |
|---|---|---|
| Allostatic load score | 1.20 (1.03, 1.40) | 0.016 * |
| Temperature (°C) | 1.05 (1.01, 1.09) | 0.021 * |
| Respiratory rate (times/min) | 1.04 (0.99, 1.09) | 0.087 |
| Race (n, %) | 0.61 (0.42, 0.87) | 0.007 * |
| AG (mEq/L) | 1.06 (1.01, 1.11) | 0.029 * |
| Sepsis (n, %) | 3.22 (2.21, 4.69) | <0.001 * |
| Rheumatic disease (n, %) | 0.32 (0.11, 0.91) | 0.032 * |
| Mechanical ventilation (n, %) | 3.48 (2.38, 5.08) | <0.001 * |
| Variable | Overall | ALS ≤ 2 | ALS ≥ 3 | p Value |
|---|---|---|---|---|
| n = 656 | n = 437 | n = 219 | ||
| Delirium (n, %) | 332 (50.6) | 200 (45.8) | 132 (60.3) | <0.001 * |
| Reintubation within 48 h (n, %) | 23 (3.51) | 15 (3.43) | 8 (3.65) | 0.885 |
| ICU LOS (day) | 5 (2, 10) | 5 (2, 10) | 5 (2, 11) | 0.546 |
| Hospital LOS (day) | 16 (8, 28) | 15 (7, 27) | 18 (10, 33) | 0.001 * |
| In-hospital death (n, %) | 82 (12.5) | 42 (9.6) | 40 (18.3) | 0.002 * |
| Mechanical ventilation (n, %) | 283 (43.1) | 182 (41.6) | 101 (46.1) | 0.276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Ni, Y.; Lan, L.; Wan, H.; Luo, F. Association Between Allostatic Load and Delirium in ICU Patients: A Retrospective Analysis of the MIMIC-IV Database. J. Clin. Med. 2025, 14, 3916. https://doi.org/10.3390/jcm14113916
Zhou Y, Ni Y, Lan L, Wan H, Luo F. Association Between Allostatic Load and Delirium in ICU Patients: A Retrospective Analysis of the MIMIC-IV Database. Journal of Clinical Medicine. 2025; 14(11):3916. https://doi.org/10.3390/jcm14113916
Chicago/Turabian StyleZhou, Yubei, Yuenan Ni, Lan Lan, Huajing Wan, and Fengming Luo. 2025. "Association Between Allostatic Load and Delirium in ICU Patients: A Retrospective Analysis of the MIMIC-IV Database" Journal of Clinical Medicine 14, no. 11: 3916. https://doi.org/10.3390/jcm14113916
APA StyleZhou, Y., Ni, Y., Lan, L., Wan, H., & Luo, F. (2025). Association Between Allostatic Load and Delirium in ICU Patients: A Retrospective Analysis of the MIMIC-IV Database. Journal of Clinical Medicine, 14(11), 3916. https://doi.org/10.3390/jcm14113916







