Etrasimod: Modulating Sphingosine-1-Phosphate Receptors to Treat Ulcerative Colitis
Abstract
1. Introduction
2. Modulating Sphigosine-1-Phosphate Receptors
3. The Small-Molecule Drug Etrasimod
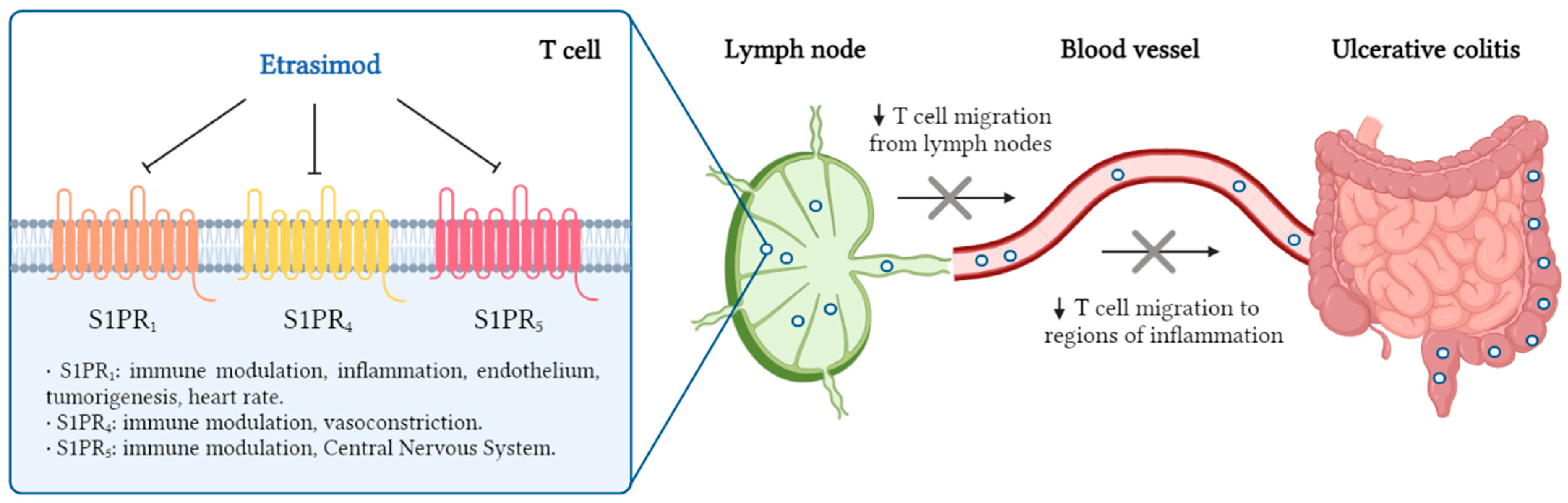
3.1. Pharmacodinamic Effects
3.2. Pharmacokinetic Properties
3.3. Clinical Efficacy and Safety
3.4. Current Clinical Trials
4. Etrasimod in the Treatment of Ulcerative Colitis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| TNF | Tumour necrosis factor |
| IL | Interleukin |
| JAK | Janus kinase |
| S1PR | Sphingosine-1-phosphate receptor |
| FDA | U.S. Food and Drug Administration |
| EMA | European Medicines Agency |
| PRES | Posterior reversible encephalopathy syndrome |
| CYP | Cytochrome P450 |
| UGTs | Uridine diphosphate glucuronosyltransferase |
| mMS | Modified Mayo score |
| ES | Endoscopic subscore |
| RBS | Rectal bleeding subscore |
| AE | Adverse event |
| COVID-19 | Coronavirus disease 2019 |
| AV | Atrioventricular |
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Lee, C.; Yu, Z.; Chen, C.; Liang, C. Ulcerative colitis: Molecular insights and intervention therapy. Mol. Biomed. 2024, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohns. Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Remsima (Infliximab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/remsima (accessed on 11 January 2025).
- European Medicines Agency (EMA). Amgevita (Adalimumab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/amgevita (accessed on 11 January 2025).
- European Medicines Agency (EMA). Simponi (Golimumab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/simponi (accessed on 11 January 2025).
- European Medicines Agency (EMA). Entyvio (Vedolizumab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/entyvio (accessed on 11 January 2025).
- European Medicines Agency (EMA). Stelara. 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/stelara (accessed on 11 January 2025).
- European Medicines Agency (EMA). Omvoh (Mirikizumab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/omvoh (accessed on 11 January 2025).
- European Medicines Agency (EMA). Skyrizi (Risankizumab). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/skyrizi (accessed on 11 January 2025).
- European Medicines Agency (EMA). Xeljanz (Tofacitinib). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/xeljanz (accessed on 11 January 2025).
- European Medicines Agency (EMA). Rinvoq (Upadacitinib). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq (accessed on 11 January 2025).
- European Medicines Agency (EMA). Jyseleca (Filgotinib). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jyseleca (accessed on 11 January 2025).
- European Medicines Agency (EMA). Zeposia (Ozanimod). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zeposia (accessed on 11 January 2025).
- European Medicines Agency (EMA). Velsipity (Etrasimod). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/velsipity (accessed on 11 January 2025).
- U.S. Food & Drug Administration (FDA). Velsipity (Etrasimod). 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216956s000lbl.pdf#page=24 (accessed on 11 January 2025).
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): Two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023, 401, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Kitsou, K.; Kokkotis, G.; Rivera-Nieves, J.; Bamias, G. Targeting the Sphingosine-1-Phosphate Pathway: New Opportunities in Inflammatory Bowel Disease Management. Drugs 2024, 84, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76 (Suppl. S3), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Wils, P.; Peyrin-Biroulet, L. Etrasimod for the treatment of ulcerative colitis. Immunotherapy 2023, 15, 311–321. [Google Scholar] [CrossRef] [PubMed]
- McGinley, M.P.; Cohen, J.A. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 2021, 398, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Karuppuchamy, T.; Behrens, E.H.; González-Cabrera, P.; Sarkisyan, G.; Gima, L.; Boyer, J.D.; Bamias, G.; Jedlicka, P.; Veny, M.; Clark, D.; et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 2017, 10, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Montrose, D.C.; Scherl, E.J.; Bosworth, B.P.; Zhou, X.K.; Jung, B.; Dannenberg, A.J.; Hla, T. S1P₁ localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J. Lipid Res. 2013, 54, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Christopher, R.; Behan, D.; Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 2017, 16, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Argollo, M.; Furfaro, F.; Gilardi, D.; Roda, G.; Allocca, M.; Peyrin-Biroulet, L.; Danese, S. Modulation of sphingosine-1-phosphate in ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Parigi, T.L.; Roda, G.; Argollo, M.; Gilardi, D.; Danese, S. Is there a role for therapeutic sphingolipids in inflammatory bowel disease? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 47–54. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (FDA). Gilenya (Fingolimod). 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/022527s042lbl.pdf#page=26 (accessed on 12 January 2025).
- European Medicines Agency (EMA). Gilenya (Fingolimod). 2025. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/gilenya (accessed on 12 January 2025).
- Calabresi, P.A.; Radue, E.W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Im, D.S. Sphingosine 1-Phosphate Receptor Modulators and Drug Discovery. Biomol. Ther. 2017, 25, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.E.; Im, D.S. Blocking the Sphingosine-1-Phosphate Receptor 2 (S1P2) Reduces the Severity of Collagen-Induced Arthritis in DBA-1J Mice. Int. J. Mol. Sci. 2024, 25, 13393. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration (FDA). Zeposia (Ozanimod). 2025. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/209899s013s014lbl.pdf#page=27 (accessed on 12 January 2025).
- Cohen, J.A.; Arnold, D.L.; Comi, G.; Bar-Or, A.; Gujrathi, S.; Hartung, J.P.; Cravets, M.; Olson, A.; Frohna, P.A.; Selmaj, K.W.; et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamma, H.; Lehmann-Bruinsma, K.; Carroll, C.; Solomon, M.; Komori, H.K.; Peyrin-Biroulet, L.; Adams, J. The Selective Sphingosine 1-Phosphate Receptor Modulator Etrasimod Regulates Lymphocyte Trafficking and Alleviates Experimental Colitis. J. Pharmacol. Exp. Ther. 2019, 369, 311–317. [Google Scholar] [CrossRef] [PubMed]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. PubChem Compound Summary for CID 44623998, Etrasimod. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Etrasimod (accessed on 12 January 2025).
- Clinical Trials [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. Etrasimod. 2004. Available online: https://clinicaltrials.gov/search?intr=etrasimod&viewType=Table&limit=100&page=1 (accessed on 22 January 2025).
- AdisInsight [Internet]. Springer Nature: Springer Nature Switzerland AG. Etrasimod. Available online: https://adisinsight.springer.com/drugs/800037849 (accessed on 22 January 2025).
- European Medicines Agency (EMA). Janus Kinase Inhibitors (JAKi)—Referral [Internet]. The Netherlands. 2024. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki (accessed on 28 January 2025).
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; da Silva, B.C.; Hanauer, S.B. The role of immunogenicity in optimizing biological therapies for inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2025, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]

| Therapeutic Indications | Mechanism of Action | Method of Administration | Posology | Safety Considerations | |
|---|---|---|---|---|---|
| Ozanimod | Multiple sclerosis. Ulcerative colitis. | Selective S1PR1,5 modulator. | Oral use. Can be taken with or without food. | Days 1–4: 0.23 mg once daily. Days 5–7: 0.46 mg once daily. Days 8 and thereafter: 0.92 mg once daily. | Bradyarrythmia and atrioventricular conduction delays. Liver injury. Infections. Hypertension. Macular oedema. Respiratory effects. Malignancies. PRES. |
| Etrasimod | Ulcerative colitis. | Selective S1PR1,4,5 modulator. | Oral use. Can be taken with or without food (co-administration with food is recommended for the first 3 days). | 2 mg once daily. |
| Primary Endpoint | Secondary Endpoints | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical Remission n (%) | Endoscopic Improvement n (%) | Symptomatic Remission n (%) | Endoscopic Improvement with Histologic Remission n (%) | ||||||
| Week 12 | Week 52 | Week 12 | Week 52 | Week 12 | Week 52 | Week 12 | Week 52 | ||
| ELEVATE UC 52 (n = 433) | Placebo (n = 135) | 10 (7%) | 9 (7%) | 19 (14%) | 11 (8%) | 29 (21%) | 19 (13%) | 6 (4%) | 28 (19%) |
| Etrasimod 2 mg (n = 274) | 74 (27%) | 88 (32%) | 96 (35%) | 94 (33%) | 126 (46%) | 113 (39%) | 58 (21%) | 127 (44%) | |
| p-Value | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| ELEVATE UC 12 (n = 354) | Placebo (n = 112) | 17 (15%) | 21 (19%) | 33 (29%) | 10 (9%) | ||||
| Etrasimod 2 mg (n = 222) | 55 (25%) | 68 (31%) | 104 (47%) | 36 (16%) | |||||
| p-Value | p = 0.026 | p = 0.0092 | p = 0.0013 | p = 0.036 | |||||
| ELEVATE UC 52 | ELEVATE UC 12 | |||
|---|---|---|---|---|
| Etrasimod Group (n = 289) n (%) | Placebo Group (n = 144) n (%) | Etrasimod Group (n = 238) n (%) | Placebo Group (n = 116) n (%) | |
| Any AE | 206 (71%) | 81 (56%) | 112 (47%) | 54 (47%) |
| Any serious AE | 20 (7%) | 9 (6%) | 6 (3%) | 2 (2%) |
| Any AE leading to treatment discontinuation | 12 (4%) | 7 (5%) | 13 (5%) | 1 (1%) |
| AE leading to death | 0 | 0 | 0 | 0 |
| Most common AE | ||||
| Worsening of UC or flare | 22 (8%) | 13 (9%) | 9 (4%) | 1 (1%) |
| Anaemia | 24 (8%) | 14 (10%) | 14 (6%) | 8 (7%) |
| Headache | 24 (8%) | 7 (5%) | 11 (5%) | 2 (2%) |
| Nausea | 9 (3%) | 2 (1%) | 10 (4%) | 2 (2%) |
| COVID-19 | 20 (7%) | 9 (6%) | 3 (1%) | 3 (3%) |
| Dizziness | 15 (5%) | 1 (1%) | 3 (1%) | 0 |
| Pyrexia | 14 (5%) | 6 (4%) | 8 (3%) | 3 (3%) |
| Arthralgia | 13 (4%) | 3 (2%) | 4 (2%) | 3 (3%) |
| Abdominal pain | 11 (4%) | 5 (3%) | 3 (1%) | 3 (3%) |
| AE of special interest | ||||
| Serious infections | 3 (1%) | 5 (3%) | 0 | 0 |
| Herpes zoster | 2 (1%) | 0 | 0 | 2 (2%) |
| Opportunistic infections | 0 | 1 (1%) | 1 (<1%) | 0 |
| Hypertension | 8 (3%) | 1 (1%) | 3 (1%) | 1 (1%) |
| Sinus bradycardia | 0 | 0 | 4 (2%) | 0 |
| Bradycardia | 4 (1%) | 0 | 1 (<1%) | 0 |
| AV block, 1st degree | 1 (<1%) | 0 | 1 (<1%) | 0 |
| AV block, 2nd degree (Mobitz type I) | 1 (<1%) | 0 | 0 | 0 |
| Macular oedema | 1 (<1%) | 0 | 1 (<1%) | 1 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Molina, C.; González-Suárez, B. Etrasimod: Modulating Sphingosine-1-Phosphate Receptors to Treat Ulcerative Colitis. J. Clin. Med. 2025, 14, 3890. https://doi.org/10.3390/jcm14113890
Martinez-Molina C, González-Suárez B. Etrasimod: Modulating Sphingosine-1-Phosphate Receptors to Treat Ulcerative Colitis. Journal of Clinical Medicine. 2025; 14(11):3890. https://doi.org/10.3390/jcm14113890
Chicago/Turabian StyleMartinez-Molina, Cristina, and Begoña González-Suárez. 2025. "Etrasimod: Modulating Sphingosine-1-Phosphate Receptors to Treat Ulcerative Colitis" Journal of Clinical Medicine 14, no. 11: 3890. https://doi.org/10.3390/jcm14113890
APA StyleMartinez-Molina, C., & González-Suárez, B. (2025). Etrasimod: Modulating Sphingosine-1-Phosphate Receptors to Treat Ulcerative Colitis. Journal of Clinical Medicine, 14(11), 3890. https://doi.org/10.3390/jcm14113890





