Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis
Abstract
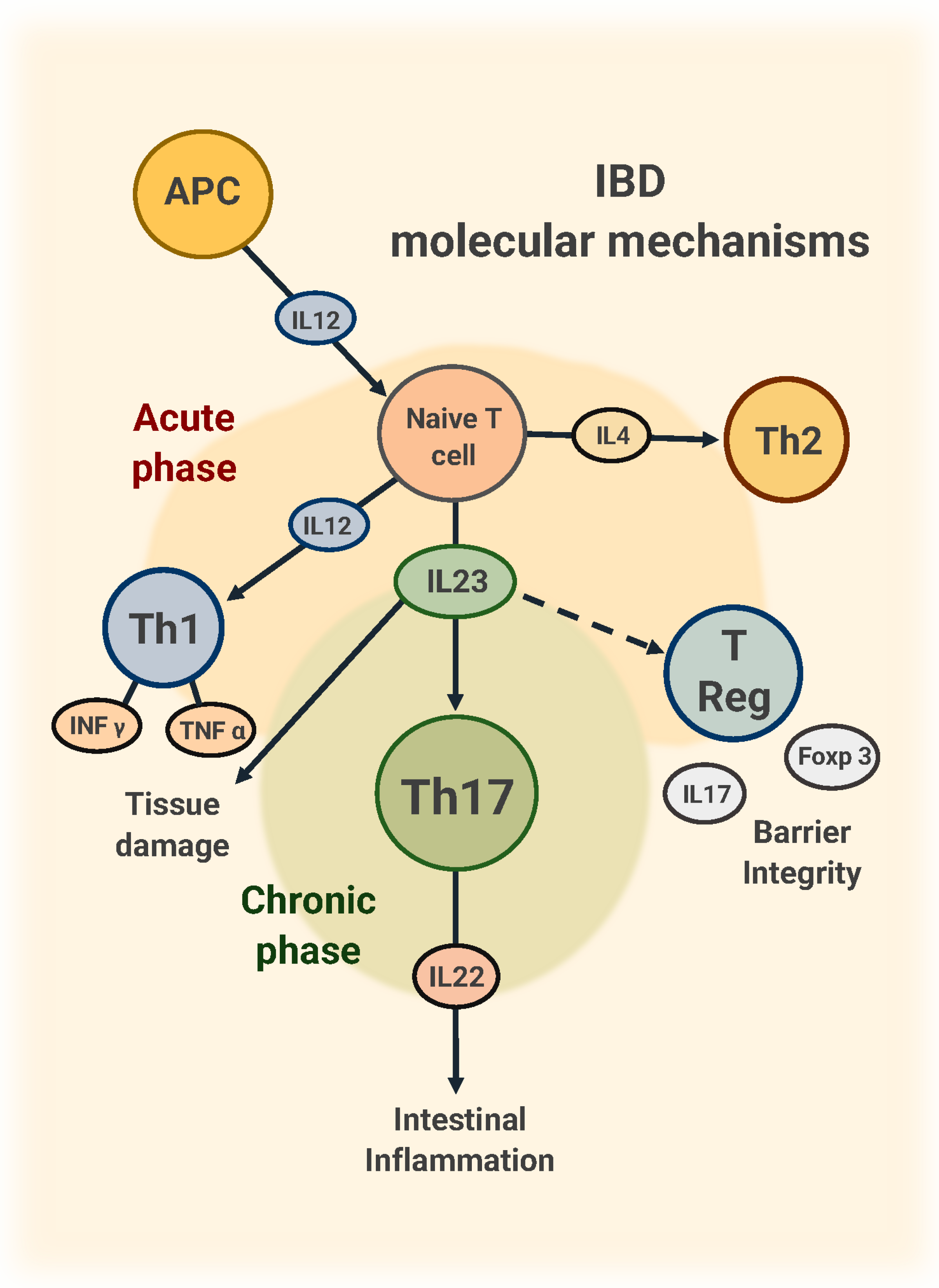
1. Introduction
2. Pathogenetic Role of IL-23
3. Efficacy and Safety of IL-23 Inhibitors: Randomized Controlled Trials (RCTs)
3.1. Mirikizumab
3.2. Risankizumab
3.3. Guselkumab
4. Effectiveness of IL-23 Inhibitors in Real-Life Studies
5. Safety of IL-23 Inhibitors
6. Positioning of IL-23 Inhibitors
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Armuzzi, A.; Liguori, G. Quality of Life in Patients with Moderate to Severe Ulcerative Colitis and the Impact of Treatment: A Narrative Review. Dig. Liver Dis. 2021, 53, 803–808. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Papi, C.; Orlando, A.; Festa, S.; Pugliese, D.; Bonovas, S.; Pansieri, C.; Piovani, D.; Fiorino, G.; Fantini, M.C.; et al. Use of Biologics for the Management of Crohn’s Disease: IG-IBD Clinical Guidelines Based on the GRADE Methodology. Dig. Liver Dis. 2023, 55, 442–453. [Google Scholar] [CrossRef]
- Neurath, M.F. Targeting Immune Cell Circuits and Trafficking in Inflammatory Bowel Disease. Nat. Immunol. 2019, 20, 970–979. [Google Scholar] [CrossRef]
- D’Haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef]
- Rubin, D.T.; Allegretti, J.R.; Panés, J.; Shipitofsky, N.; Yarandi, S.S.; Huang, K.-H.G.; Germinaro, M.; Wilson, R.; Zhang, H.; Johanns, J.; et al. Guselkumab in Patients with Moderately to Severely Active Ulcerative Colitis (QUASAR): Phase 3 Double-Blind, Randomised, Placebo-Controlled Induction and Maintenance Studies. Lancet 2025, 405, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.; Schreiber, S.; Panaccione, R.; Bossuyt, P.; Biedermann, L.; Colombel, J.-F.; Parkes, G.; Peyrin-Biroulet, L.; D’Haens, G.; Hisamatsu, T.; et al. Risankizumab for Ulcerative Colitis: Two Randomized Clinical Trials. JAMA 2024, 332, 881. [Google Scholar] [CrossRef]
- Daniele, S.G.; Eldirany, S.A.; Ho, M.; Bunick, C.G. Structural Basis for Differential P19 Targeting by IL-23 Biologics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Daniele, S.G.; Eldirany, S.A.; Damiani, G.; Ho, M.; Bunick, C.G. Structural Basis for P19 Targeting by Anti–IL-23 Biologics: Correlations with Short- and Long-Term Efficacy in Psoriasis. JID Innov. 2024, 4, 100261. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Omvoh: EPAR—Product Information 2025; European Medicines Agency: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Zhao, Q.; Duck, L.W.; Huang, F.; Alexander, K.L.; Maynard, C.L.; Mannon, P.J.; Elson, C.O. CD4+ T Cell Activation and Concomitant mTOR Metabolic Inhibition Can Ablate Microbiota-Specific Memory Cells and Prevent Colitis. Sci. Immunol. 2020, 5, eabc6373. [Google Scholar] [CrossRef] [PubMed]
- Smids, C.; Horjus Talabur Horje, C.S.; Drylewicz, J.; Roosenboom, B.; Groenen, M.J.M.; Van Koolwijk, E.; Van Lochem, E.G.; Wahab, P.J. Intestinal T Cell Profiling in Inflammatory Bowel Disease: Linking T Cell Subsets to Disease Activity and Disease Course. J. Crohn’s Colitis 2018, 12, 465–475. [Google Scholar] [CrossRef]
- Neurath, M.F. Strategies for Targeting Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2024, 24, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Wali, S.; Nurieva, R. T Helper 2 and T Follicular Helper Cells: Regulation and Function of Interleukin-4. Cytokine Growth Factor Rev. 2016, 30, 29–37. [Google Scholar] [CrossRef]
- Gomez-Bris, R.; Saez, A.; Herrero-Fernandez, B.; Rius, C.; Sanchez-Martinez, H.; Gonzalez-Granado, J.M. CD4 T-Cell Subsets and the Pathophysiology of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 2696. [Google Scholar] [CrossRef]
- Monteleone, I.; Sarra, M.; Pallone, F.; Monteleone, G. Th17-Related Cytokines in Inflammatory Bowel Diseases: Friends or Foes? Curr. Mol. Med. 2012, 12, 592–597. [Google Scholar] [CrossRef]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.-Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical Gastrointestinal Effects of Interleukin-17 Blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel P19 Protein Engages IL-12p40 to Form a Cytokine, IL-23, with Biological Activities Similar as Well as Distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Riol-Blanco, L.; Jäger, A.; Korn, T.; Pot, C.; Galileos, G.; Bettelli, E.; Kuchroo, V.K.; Oukka, M. Cutting Edge: IL-23 Receptor GFP Reporter Mice Reveal Distinct Populations of IL-17-Producing Cells. J. Immunol. 2009, 182, 5904–5908. [Google Scholar] [CrossRef]
- Jacobse, J.; Brown, R.E.; Li, J.; Pilat, J.M.; Pham, L.; Short, S.P.; Peek, C.T.; Rolong, A.; Washington, M.K.; Martinez-Barricarte, R.; et al. Interleukin-23 Receptor Signaling Impairs the Stability and Function of Colonic Regulatory T Cells. Cell Rep. 2023, 42, 112128. [Google Scholar] [CrossRef]
- Uhlig, H.H.; McKenzie, B.S.; Hue, S.; Thompson, C.; Joyce-Shaikh, B.; Stepankova, R.; Robinson, N.; Buonocore, S.; Tlaskalova-Hogenova, H.; Cua, D.J.; et al. Differential Activity of IL-12 and IL-23 in Mucosal and Systemic Innate Immune Pathology. Immunity 2006, 25, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, M.C.; Jankovic, D.; Feng, C.G.; Hue, S.; Gorelick, P.L.; McKenzie, B.S.; Cua, D.J.; Powrie, F.; Cheever, A.W.; Maloy, K.J.; et al. IL-23 Plays a Key Role in Helicobacter hepaticus–Induced T Cell–Dependent Colitis. J. Exp. Med. 2006, 203, 2485–2494. [Google Scholar] [CrossRef]
- Sun, R.; Hedl, M.; Abraham, C. IL23 Induces IL23R Recycling and Amplifies Innate Receptor-Induced Signalling and Cytokines in Human Macrophages, and the IBD-Protective IL23R R381Q Variant Modulates These Outcomes. Gut 2020, 69, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Ahern, P.P.; Schiering, C.; Buonocore, S.; McGeachy, M.J.; Cua, D.J.; Maloy, K.J.; Powrie, F. Interleukin-23 Drives Intestinal Inflammation through Direct Activity on T Cells. Immunity 2010, 33, 279–288. [Google Scholar] [CrossRef]
- Peng, L.-L.; Wang, Y.; Zhu, F.-L.; Xu, W.-D.; Ji, X.-L.; Ni, J. IL-23R Mutation Is Associated with Ulcerative Colitis: A Systemic Review and Meta-Analysis. Oncotarget 2017, 8, 4849–4863. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, W.; Wang, J.; Zhang, J.; Guo, X.; Wang, J.; Song, J.; Dong, W. Interleukin-23 Receptor Genetic Polymorphisms and Ulcerative Colitis Susceptibility: A Meta-Analysis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 516–525. [Google Scholar] [CrossRef]
- Sewell, G.W.; Kaser, A. Interleukin-23 in the Pathogenesis of Inflammatory Bowel Disease and Implications for Therapeutic Intervention. J. Crohn’s Colitis 2022, 16, ii3–ii19. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14+ Intestinal Macrophages Contribute to the Pathogenesis of Crohn Disease via IL-23/IFN-γ Axis. J. Clin. Investig. 2008, 118, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, S.; Ahern, P.P.; Uhlig, H.H.; Ivanov, I.I.; Littman, D.R.; Maloy, K.J.; Powrie, F. Innate Lymphoid Cells Drive Interleukin-23-Dependent Innate Intestinal Pathology. Nature 2010, 464, 1371–1375. [Google Scholar] [CrossRef]
- Izcue, A.; Hue, S.; Buonocore, S.; Arancibia-Cárcamo, C.V.; Ahern, P.P.; Iwakura, Y.; Maloy, K.J.; Powrie, F. Interleukin-23 Restrains Regulatory T Cell Activity to Drive T Cell-Dependent Colitis. Immunity 2008, 28, 559–570. [Google Scholar] [CrossRef]
- Geremia, A.; Jewell, D.P. The IL-23/IL-17 Pathway in Inflammatory Bowel Disease. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 223–237. [Google Scholar] [CrossRef]
- Mangan, P.R.; Harrington, L.E.; O’Quinn, D.B.; Helms, W.S.; Bullard, D.C.; Elson, C.O.; Hatton, R.D.; Wahl, S.M.; Schoeb, T.R.; Weaver, C.T. Transforming Growth Factor-β Induces Development of the TH17 Lineage. Nature 2006, 441, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, J.; D’Amico, F.; Lusetti, F.; Fasulo, E.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Radice, S.; Peyrin-Biroulet, L.; et al. The Role of IL-23 Inhibitors in Crohn’s Disease. J. Clin. Med. 2023, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Mirikizumab: First Approval. Drugs 2023, 83, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; Van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD Scores and General Principles and Technical Aspects. J. Crohn’s Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Irving, P.M.; Panaccione, R.; Naegeli, A.N.; Potts-Bleakman, A.; Arora, V.; Shan, M.; Travis, S. Incorporating Patient Experience into Drug Development for Ulcerative Colitis: Development of the Urgency Numeric Rating Scale, a Patient-Reported Outcome Measure to Assess Bowel Urgency in Adults. J. Patient Rep. Outcomes 2022, 6, 31. [Google Scholar] [CrossRef]
- Li, K.; Marano, C.; Zhang, H.; Yang, F.; Sandborn, W.J.; Sands, B.E.; Feagan, B.G.; Rubin, D.T.; Peyrin-Biroulet, L.; Friedman, J.R.; et al. Relationship Between Combined Histologic and Endoscopic Endpoints and Efficacy of Ustekinumab Treatment in Patients With Ulcerative Colitis. Gastroenterology 2020, 159, 2052–2064. [Google Scholar] [CrossRef]
- Bryant, R.V.; Burger, D.C.; Delo, J.; Walsh, A.J.; Thomas, S.; Von Herbay, A.; Buchel, O.C.; White, L.; Brain, O.; Keshav, S.; et al. Beyond Endoscopic Mucosal Healing in UC: Histological Remission Better Predicts Corticosteroid Use and Hospitalisation over 6 Years of Follow-Up. Gut 2016, 65, 408–414. [Google Scholar] [CrossRef]
- Christensen, B.; Hanauer, S.B.; Erlich, J.; Kassim, O.; Gibson, P.R.; Turner, J.R.; Hart, J.; Rubin, D.T. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated With Improved Clinical Outcomes. Clin. Gastroenterol. Hepatol. 2017, 15, 1557–1564.e1. [Google Scholar] [CrossRef]
- Sands, B.E.; D’Haens, G.; Clemow, D.B.; Irving, P.M.; Johns, J.T.; Gibble, T.H.; Abreu, M.T.; Lee, S.D.; Hisamatsu, T.; Kobayashi, T.; et al. Three-Year Efficacy and Safety of Mirikizumab Following 152 Weeks of Continuous Treatment for Ulcerative Colitis: Results From the LUCENT-3 Open-Label Extension Study. Inflamm. Bowel Dis. 2024, izae253. [Google Scholar] [CrossRef]
- Steere, B.; Schmitz, J.; Powell, N.; Higgs, R.; Gottlieb, K.; Liu, Y.; Jia, B.; Tuttle, J.L.; Sandborn, W.J.; Sands, B.E.; et al. Mirikizumab Regulates Genes Involved in Ulcerative Colitis Disease Activity and Anti-TNF Resistance: Results From a Phase 2 Study. Clin. Transl. Gastroenterol. 2023, 14, e00578. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; D’Cunha, R.; Winzenborg, I.; Veldman, G.; Pivorunas, V.; Wallace, K. Risankizumab: Mechanism of Action, Clinical and Translational Science. Clin. Transl. Sci. 2024, 17, e13706. [Google Scholar] [CrossRef]
- Panaccione, R.; Melmed, G.; Drobne, D.; Kaur, M.; Danese, S.; Hisamatsu, T.; Levine, P.; Neimark, E.; Chen, S.; Cheng, L.; et al. Additional Risankizumab Therapy Is Effective in Patients with Moderately to Severely Active Ulcerative Colitis Who Did Not Achieve Clinical Response to Initial 12-Week Inducation Therapy: An Analysis of Phase 3 INSPIRE and COMMAND Studies. Presented at: Digestive Disease Week. 2024. Available online: https://ddw.digitellinc.com/p/s/additional-risankizumab-therapy-is-effective-in-patients-with-moderately-to-severely-active-ulcerative-colitis-who-did-not-achieve-clinical-response-to-initial-12-week-induction-therapy-an-analysis-of-6654 (accessed on 20 May 2024).
- Panaccione, R.; Louis, E.; Colombel, J.-F.; D’Haens, G.; Peyrin-Biroulet, L.; Dubinsky, M.; Takeuchi, K.; Rubin, D.T.; Kalabic, J.; Chien, K.B.; et al. Risankizumab Efficacy and Safety Based on Prior Inadequate Response or Intolerance to Advanced Therapy: Post Hoc Analysis of the INSPIRE and COMMAND Phase 3 Studies. J. Crohn’s Colitis 2025, 19, jjaf005. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Peyrin-Biroulet, L.; Higgins, P.D.; Hirai, F.; Jairath, V.; D’Haens, G.; Abreu, M.T.; Belin, R.; Valderas, E.G.; et al. 132 Efficacy And Safety of Mirikizumab After 52-Weeks Maintenance Treatment in Patients with Moderate-to-Severe Crohn’s Disease. Gastroenterology 2021, 160, S-37. [Google Scholar] [CrossRef]
- Gordon, K.B.; Armstrong, A.W.; Foley, P.; Song, M.; Shen, Y.-K.; Li, S.; Muñoz-Elías, E.J.; Branigan, P.; Liu, X.; Reich, K. Guselkumab Efficacy after Withdrawal Is Associated with Suppression of Serum IL-23-Regulated IL-17 and IL-22 in Psoriasis: VOYAGE 2 Study. J. Investig. Dermatol. 2019, 139, 2437–2446. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; D’Haens, G.; Panés, J.; Kaser, A.; Ferrante, M.; Louis, E.; Franchimont, D.; Dewit, O.; Seidler, U.; et al. Induction Therapy with the Selective Interleukin-23 Inhibitor Risankizumab in Patients with Moderate-to-Severe Crohn’s Disease: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Study. Lancet 2017, 389, 1699–1709. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Thangaswamy, A.; Thangaraju, P.; Varthya, S.B. Efficacy and Safety of Risankizumab in Moderate to Severe Psoriasis: A Systematic Review and Meta-analysis. Dermatol. Ther. 2021, 34, e14487. [Google Scholar] [CrossRef]
- Atreya, R.; Abreu, M.T.; Krueger, J.G.; Eyerich, K.; Sachen, K.; Greving, C.; Hammaker, D.; Bao, P.; Lacy, E.; Sarabia, I.; et al. P504 Guselkumab, an IL-23p19 Subunit–Specific Monoclonal Antibody, Binds CD64+ Myeloid Cells and Potently Neutralises IL-23 Produced from the Same Cells. J. Crohn’s Colitis 2023, 17, i634–i635. [Google Scholar] [CrossRef]
- Markham, A. Guselkumab: First Global Approval. Drugs 2017, 77, 1487–1492. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Allegretti, J.R.; Rubin, D.T.; Bressler, B.; Germinaro, M.; Huang, K.-H. (Gary); Shipitofsky, N.; Zhang, H.; Wilson, R.; Han, C.; et al. Guselkumab in Patients with Moderately to Severely Active Ulcerative Colitis: QUASAR Phase 2b Induction Study. Gastroenterology 2023, 165, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Allegretti, J.R.; Rubin, D.T.; Feagan, B.G.; Bressler, B.; Panés, J.; Afif, W.; Samaan, M.A.; Ye, B.D.; Yarandi, S.; et al. Efficacy and Safety of Guselkumab for Ulcerative Colitis through Week 92 of the QUASAR Long-Term Extension Study. Poster Presentation at Digestive Disease Week. 2025. Available online: https://www.jnjmedicalconnect.com/media/attestation/congresses/immunology/2025/ddw/efficacy-and-safety-of-guselkumab-for-ulcerative-colitis-through-week-92-of-the-quasar-longterm-exte.pdf (accessed on 6 May 2025).
- Peyrin-Biroulet, L.; Allegretti, J.R.; Danese, S.; Germinaro, M.; Baker, T.; Alvarez, Y.; Jörgens, S.; Jiang, L.; Zhang, H.; Hisamatsu, T.; et al. OP10 Efficacy and Safety of Subcutaneous Guselkumab Induction Therapy in Patients with Ulcerative Colitis: Results through Week 12 from the Phase 3 ASTRO Study. J. Crohn’s Colitis 2025, 19, i19–i20. [Google Scholar] [CrossRef]
- Long, M.; Allegretti, J.R.; Danese, S.; Germinaro, M.; Baker, T.; Alvarez, Y.; Jorgens, S.; Jiang, L.; Zhang, H.; Hisamatsu, T.; et al. Efficacy and Safety of Subcutaneous Guselkumab Induction Therapy in Patients with Ulcerative Colitis: Results through Week 24 from the Phase 3 ASTRO Study. 2025, Poster Presentation at Digestive Disease Week. Available online: https://www.jnjmedicalconnect.com/media/attestation/congresses/immunology/2025/ddw/efficacy-and-safety-of-subcutaneous-guselkumab-induction-therapy-in-patients-with-ulcerative-colitis.pdf (accessed on 6 May 2025).
- Takagi, Y.; Sato, T.; Nishiguchi, T.; Nogami, A.; Igeta, M.; Yagi, S.; Ikenouchi, M.; Kawai, M.; Kamikozuru, K.; Yokoyama, Y.; et al. Real-World Effectiveness and Safety of Mirikizumab Induction Therapy in Patients with Ulcerative Colitis: A Multicentre Retrospective Observational Study. Aliment. Pharmacol. Ther. 2025, 61, 1923–1934. [Google Scholar] [CrossRef]
- St-Pierre, J.; Choi, D.; Fear, E.; Choi, N.K.; Mathew, A.J.; Cohen, R.D.; Dalal, S.R.; Pekow, J.; Krugliak Cleveland, N.; Rubin, D.T. Mirikizumab in the Treatment of Ulcerative Colitis: Initial Real-World Data in a Population from a Large Tertiary Center. Dig. Dis. Sci. 2025, 70, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, R.S.; Ayres, R.C.S.; Pounder, R.E.; Allan, R.N. A Simple Clinical Colitis Activity Index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Sawada, T.; Nakamura, M.; Yamamura, T.; Maeda, K.; Ishikawa, E.; Murate, K.; Kawashima, H. Efficacy of Mirikizumab in Patients with Prior Ustekinumab Exposure: A Case Series. Inflamm. Bowel Dis. 2025, Apr 8, izaf018. [Google Scholar] [CrossRef]
- Colwill, M.; Baillie, S.; Clough, J.; Pollok, R.; Poullis, A.; Patel, K.; Honap, S. Role of Mirikizumab in the Treatment of Inflammatory Bowel Disease—From Bench to Bedside. J. Clin. Med. 2025, 14, 1001. [Google Scholar] [CrossRef]
- Singh, S.; Loftus, E.V.; Limketkai, B.N.; Haydek, J.P.; Agrawal, M.; Scott, F.I.; Ananthakrishnan, A.N. AGA Living Clinical Practice Guideline on Pharmacological Management of Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2024, 167, 1307–1343. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alsayegh, A.; Aldallal, U.; Ma, C.; Narula, N.; Jairath, V.; Singh, S.; Bessissow, T. Comparative Efficacy of Biologics and Small Molecule in Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Clin. Gastroenterol. Hepatol. 2025, 23, 250–262. [Google Scholar] [CrossRef]
- Fudman, D.I.; McConnell, R.A.; Ha, C.; Singh, S. Modern Advanced Therapies for Inflammatory Bowel Diseases: Practical Considerations and Positioning. Clin. Gastroenterol. Hepatol. 2025, 23, 454–468. [Google Scholar] [CrossRef]
- Bezzio, C.; Cavalli, C.A.M.; Franchellucci, G.; Dal Buono, A.; Gabbiadini, R.; Scalvini, D.; Manara, S.; Narcisi, A.; Armuzzi, A.; Saibeni, S. Psoriasis and Inflammatory Bowel Disease: Concomitant IMID or Paradoxical Therapeutic Effect? A Scoping Review on Anti-IL-12/23 and Anti-IL-23 Antibodies. Therap. Adv. Gastroenterol. 2024, 17, 17562848241299564. [Google Scholar] [CrossRef]
- Huang, X.; Shentu, H.; He, Y.; Lai, H.; Xu, C.; Chen, M.; Zhu, H. Efficacy and Safety of IL-23 Inhibitors in the Treatment of Psoriatic Arthritis: A Meta-Analysis Based on Randomized Controlled Trials. Immunol. Res. 2023, 71, 505–515. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Palumbo, A.; Susca, S.; Merli, M.; Dapavo, P.; Venero, M. Anti-IL23 in Inflammatory Bowel Disease Patients with Dermatological Indication: The Shared Gastroenterological-Dermatological Clinic Experience. Dig. Liver Dis. 2024, 56, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Colwill, M.; Clough, J.; Baillie, S.; Patel, K.; Peyrin-Biroulet, L.; Honap, S. Landscape of Anti-IL-23 Therapy in Inflammatory Bowel Disease: Recent Advances. Frontline Gastroenterol. 2025, 16, 227–238. [Google Scholar] [CrossRef]
- Baeten, D.; Østergaard, M.; Wei, J.C.-C.; Sieper, J.; Järvinen, P.; Tam, L.-S.; Salvarani, C.; Kim, T.-H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 Inhibitor, for Ankylosing Spondylitis: Results of a Randomised, Double-Blind, Placebo-Controlled, Proof-of-Concept, Dose-Finding Phase 2 Study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef]
- Torres, J.; Chaparro, M.; Julsgaard, M.; Katsanos, K.; Zelinkova, Z.; Agrawal, M.; Ardizzone, S.; Campmans-Kuijpers, M.; Dragoni, G.; Ferrante, M.; et al. European Crohn’s and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J. Crohn’s Colitis 2023, 17, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Onali, S.; Pugliese, D.; Renna, S.; Savarino, E.; Viola, A.; Ribaldone, D.G.; Buda, A.; Bezzio, C.; Fiorino, G.; et al. Dual Targeted Therapy: A Possible Option for the Management of Refractory Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 335–339. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sands, B.E.; Sandborn, W.J.; Germinaro, M.; Vetter, M.; Shao, J.; Sheng, S.; Johanns, J.; Panés, J.; Tkachev, A.; et al. Guselkumab plus Golimumab Combination Therapy versus Guselkumab or Golimumab Monotherapy in Patients with Ulcerative Colitis (VEGA): A Randomised, Double-Blind, Controlled, Phase 2, Proof-of-Concept Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 307–320. [Google Scholar] [CrossRef]
- Sarra, M.; Pallone, F.; Macdonald, T.T.; Monteleone, G. IL-23/IL-17 axis in IBD. Inflamm. Bowel Dis. 2010, 16, 1808–1813. [Google Scholar] [CrossRef]
- Murgiano, M.; Bartocci, B.; Puca, P.; di Vincenzo, F.; Del Gaudio, A.; Papa, A.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Lopetuso, L.R. Gut Microbiota Modulation in IBD: From the Old Paradigm to Revolutionary Tools. Int. J. Mol. Sci. 2025, 26, 3059. [Google Scholar] [CrossRef]
- Xia, X.; Huang, Z.; Xu, C.; Fu, H.; Wang, S.; Tian, J.; Rui, K. Regulation of intestinal tissue-resident memory T cells: A potential target for inflammatory bowel disease. Cell Commun Signal. 2024, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Ungaro, R.C.; Mehandru, S.; Colombel, J.F. Targeting the Interleukin 23 Pathway in Inflammatory Bowel Disease. Gastroenterology 2025, 168, 29–52.e3. [Google Scholar] [CrossRef] [PubMed]
- Fourie, A.M.; Cheng, X.; Chang, L.; Greving, C.; Li, X.; Knight, B.; Polidori, D.; Patrick, A.; Bains, T.; Steele, R.; et al. JNJ-77242113, a Highly Potent, Selective Peptide Targeting the IL-23 Receptor, Provides Robust IL-23 Pathway Inhibition upon Oral Dosing in Rats and Humans. Sci. Rep. 2024, 14, 17515. [Google Scholar] [CrossRef] [PubMed]
- Jairath, V.; Danese, S.; D’Haens, G.; Feagan, B.; Peyrin-Biroulet, L.; Sands, B.E.; Wedel, P.; Barbat, S.; Sattler, C. OP31 Phase 1b Study of SOR102, a Novel, Orally Delivered Bispecific Anti-TNF/Anti-IL-23 Domain Antibody in Patients with Mild to Severe Ulcerative Colitis. J. Crohn’s Colitis 2025, 19, i62–i63. [Google Scholar] [CrossRef]
| RCT | Phase | Type | Design | Weeks | Year of Publication | Intervention (Randomization) | N^ of Patients (a) | Primary Endpoints | Results (Drug vs. PBO; p < 0.001) |
|---|---|---|---|---|---|---|---|---|---|
| LUCENT-1 [6] | 3 | Induction | Double blind | 12 | 2023 | MIR IV 300 mg vs. PBO (3:1) | 868 | Clinical remission | 24.2% vs. 13.3% |
| LUCENT-2 [6] | Maintenance | 40 | 2023 | MIRSC 200 mg SC vs. PBO (2:1) | 816 (responders to LUCENT-1) | 49.9% vs. 25.1% | |||
| INSPIRE (b) [8] | 2b/3 | Induction | 12 | 2025 | RIS IV 1200 mg vs. PBO q4w (2:1) | 977 | 20.3 vs. 6.2% | ||
| COMMAND (b) [8] | 3 | Maintenance | 52 | 2025 | RIS SC: 180 mg q8w vs. 360 mg q8w vs. PBO (1:1:1) | 584 (clinical responders to INSPIRE) | 40.2% vs. 37.6% vs. 25.1% | ||
| QUASAR [7] | 3 | Induction | 12 | 2025 | GUS IV 200 mg vs. PBO q4w (3:2) | 701 | 23% vs. 8% | ||
| Maintenance | 44 | 2025 | GUS SC: 200 mg qw4 vs. 100 q8w vs. PBO | 568 (GUS induction responders and PBO to GUS crossover week 24 responders) | 50% vs. 45% vs. 19% |
| RCT | Phase | Type | Design | Weeks | Year of Publication | Intervention (Randomization) | N^ of Patients (a) | Secondary Endpoints (Major) | Results (Drug vs. PBO; p < 0.001) |
|---|---|---|---|---|---|---|---|---|---|
| LUCENT-1 [6] | 3 | Induction | Double blind | 12 | 2023 | MIR IV 300 mg vs. PBO (3:1) | 868 | Clinical response Endoscopic remission HEMI Change in bowel urgency from baseline (at week 12) | 63.5% vs. 42.2% 36.3% vs. 21.1% 27.1% vs. 13.9% −2.6 vs. −1.6 |
| LUCENT-2 [6] | Maintenance | 40 | 2023 | MIRSC 200 mg SC vs. PBO (2:1) | 816 (responders to LUCENT-1) | Maintenance of clinical remission Endoscopic remission HEMR Change in bowel urgency from baseline of LUCENT-1 (at week 40) | 63.6% vs. 36.9% 58.6% vs. 29.1% 43.3% vs. 21.8% −3.8 vs. −2.7 | ||
| INSPIRE (b) [8] | 2b/3 | Induction | Double blind | 12 | 2025 | RIS IV 1200 mg vs. PBO q4w (2:1) | 977 | Clinical response Endoscopic remission Endoscopic improvement HEMI | 51.7% vs. 18.3% 10.6% vs. 3.4% 36.5% vs. 12.1% 24.5% vs. 7.7% |
| COMMAND (b) [8] | 3 | Maintenance | Double blind | 52 | 2025 | RIS SC: 180 mg q8w vs. 360 mg q8w vs. PBO (1:1:1) | 584 (clinical responders to INSPIRE) | Clinical response Endoscopic improvement Endoscopic remission HEMI Steroid-free clinical remission | 68.2% vs. 62.3% vs. 51.9% 50.8% vs. 48.3% vs. 31.7% 23.2% vs. 24.3% vs. 14.8% (p 0.01) 42.8% vs. 42.2% vs. 23.5% 40% vs. 37% vs. 25% (p < 0.01) |
| QUASAR [7] | 3 | Induction | Double blind | 12 | 2025 | GUS IV 200 mg vs. PBO q4w (3:2) | 701 | Clinical response Endoscopic improvement Endoscopic remission HEMI | 62% vs. 28% 27% vs. 11% 15% vs. 5% 24% vs. 8% |
| Maintenance | 44 | 2025 | GUS SC: 200 mg qw4 vs. 100 q8w vs. PBO | 568 (GUS induction responders and PBO to GUS crossover, week 24 responders) | Maintenance of clinical remission Endoscopic improvement Endoscopic remission HEMI | 72% vs. 61% vs. 34% 52% vs. 49% vs. 19% 34% vs. 35% vs. 15% 48% vs. 44% vs. 17% |
| LUCENT-3 Open-Label Extension Study-152 Weeks [42] | QUASAR Phase 3 Double-Blind Study-44 Weeks [7] | COMMAND Phase 3 Double-Blind Study-52 Weeks [8] | |||||
|---|---|---|---|---|---|---|---|
| Drug | Mirikizumab | Guselkumab | Risankizumab | ||||
| Dose | 200 mg Q4W | Placebo | 100 mg Q8W | 200 mg Q4W | Placebo | 180 mg Q8W | 360 mg Q8W |
| Patients (N) | 339 | 192 | 186 | 190 | 196 | 193 | 195 |
| AE Total | 250 (73.7%) | 131 (68%) | 120 (65%) | 133 (70%) | 150 (76.5%) | 140 (72.5%) | 138 (70.6%) |
| Serious AE (%) * | 30 (8.8%) | 1 (1%) | 5 (3%) | 12 (6%) | 16 (8.1%) | 10 (5.1%) | 10 (5.1%) |
| AE leading to study drug discontinuation | 18 (5.3%) | 13 (7%) | 7 (4%) | 5 (3%) | 3 (1.5%) | 3 (1.5%) | 5 (2.5%) |
| Death | 1 (0.3%) | 0 | 0 | 0 | 0 | 0 | 1 (0.5%) |
| COVID-19 infections | 76 (22.4%) | 27 (14%) | 24 (13%) | 18 (9%) | 27 (13.7%) | 20 (10.3%) | 26 (13.3%) |
| Serious infections | 8 (2.4%) | 0 | 1 (1%) | 2 (1%) | 4 (2%) | 2 (1%) | 1 (0.5%) |
| Opportunistic infection | 6 (1.8%) | 0 | 0 | 0 | 0 | 0 | 1 (0.5%) |
| Hypersensitivity | 4 (1.2%) | 0 | 0 | 0 | 10 (5.1%) | 20 (10.3%) | 10 (5.1%) |
| Injection site reactions | 19 (5.6%) | 0 | 0 | 0 | 2 (1%) | 7 (3.6%) | 5 (2.5%) |
| Hepatic events | 11 (3.2%) | 0 | 0 | 0 | 1 (0.5%) | 3 (1.5%) | 13 (6.6%) |
| MACE | 1 (0.3%) | 0 | 0 | 1 (1%) | 0 | 0 | 0 |
| Malignancies and NMSC | 1 (0.3%) | 4 (2%) | 0 | 1 (1%) | 2 (1%) | 0 | 2 (1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisio, L.; Cuccia, G.; Giudice, A.; Carrabetta, F.; Del Gaudio, A.; Privitera, G.; Carbone, L.; Spagnuolo, R.; Pugliese, D. Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis. J. Clin. Med. 2025, 14, 4590. https://doi.org/10.3390/jcm14134590
Parisio L, Cuccia G, Giudice A, Carrabetta F, Del Gaudio A, Privitera G, Carbone L, Spagnuolo R, Pugliese D. Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis. Journal of Clinical Medicine. 2025; 14(13):4590. https://doi.org/10.3390/jcm14134590
Chicago/Turabian StyleParisio, Laura, Giuseppe Cuccia, Anna Giudice, Federico Carrabetta, Angelo Del Gaudio, Giuseppe Privitera, Luigi Carbone, Rocco Spagnuolo, and Daniela Pugliese. 2025. "Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis" Journal of Clinical Medicine 14, no. 13: 4590. https://doi.org/10.3390/jcm14134590
APA StyleParisio, L., Cuccia, G., Giudice, A., Carrabetta, F., Del Gaudio, A., Privitera, G., Carbone, L., Spagnuolo, R., & Pugliese, D. (2025). Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis. Journal of Clinical Medicine, 14(13), 4590. https://doi.org/10.3390/jcm14134590






