Feasibility and Impact of Left Atrial Appendage Closure in Patients with Cardiac Implantable Electronic Devices: Insights from a Prospective Registry
Abstract
1. Introduction
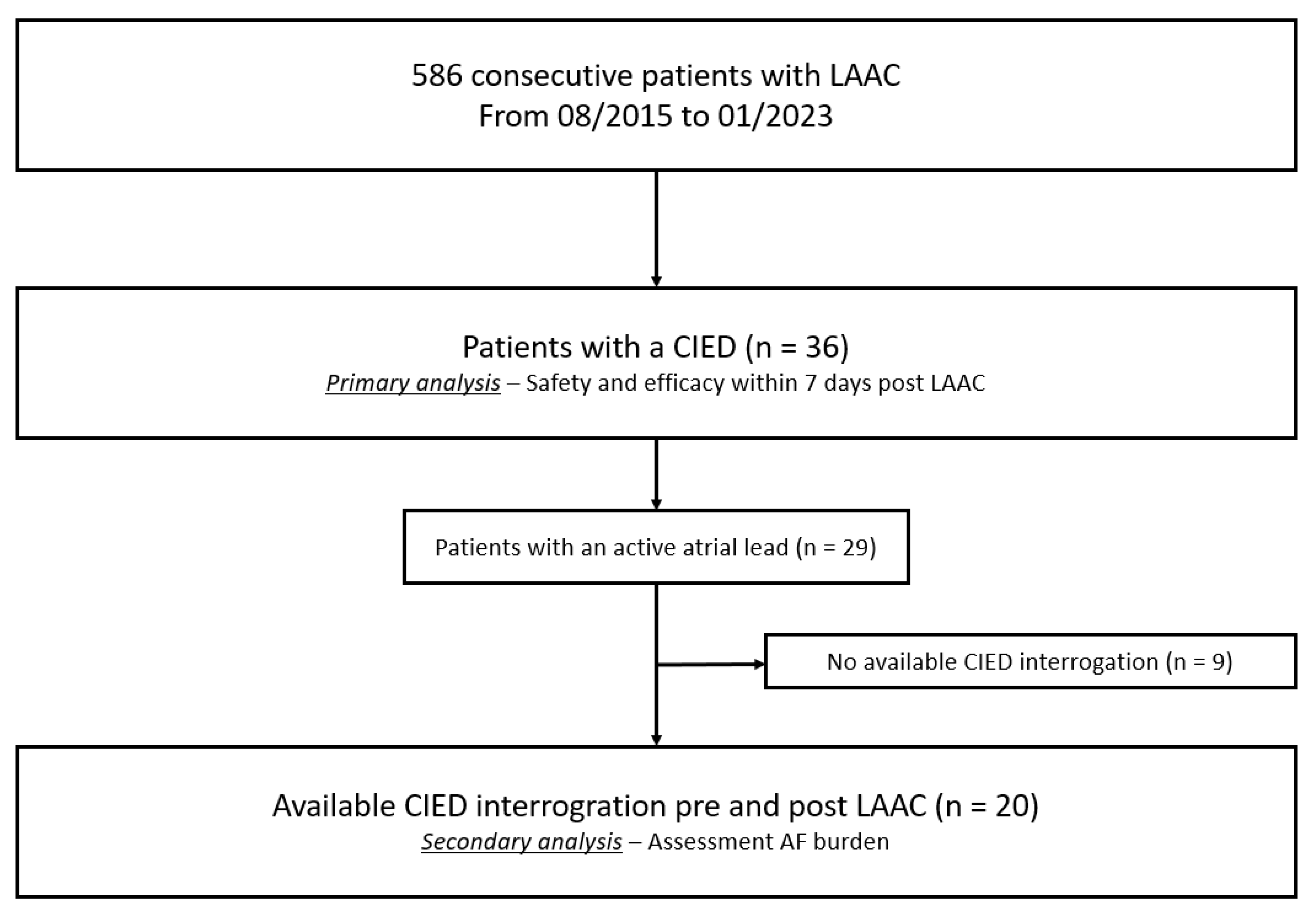
2. Methods
2.1. Follow-Up
2.2. Study Endpoints
2.3. Statistical Analysis
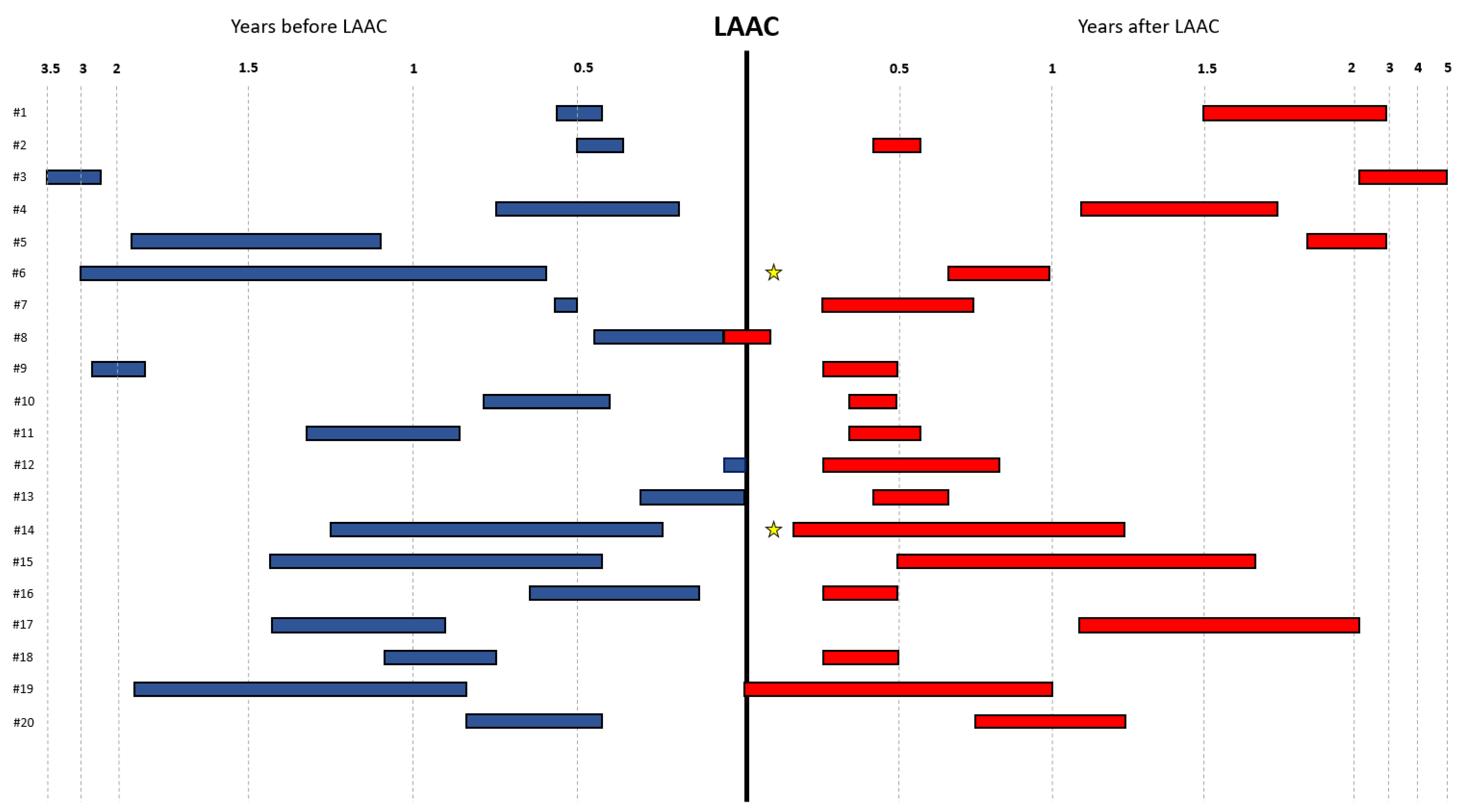
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Gunes, M.; Gokoglan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016, 68, 1929–1940. [Google Scholar] [CrossRef]
- Yorgun, H.; Canpolat, U.; Oksul, M.; Sener, Y.Z.; Ates, A.H.; Crijns, H.; Aytemir, K. Long-term outcomes of cryoballoon-based left atrial appendage isolation in addition to pulmonary vein isolation in persistent atrial fibrillation. Europace 2019, 21, 1653–1662. [Google Scholar] [CrossRef]
- Fink, T.; Schluter, M.; Heeger, C.H.; Lemes, C.; Maurer, T.; Reissmann, B.; Rottner, L.; Santoro, F.; Tilz, R.R.; Alessandrini, H.; et al. Combination of Left Atrial Appendage Isolation and Ligation to Treat Nonresponders of Pulmonary Vein Isolation. JACC Clin. Electrophysiol. 2018, 4, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Black-Maier, E.W.; Barnett, A.S.; Pokorney, S.D.; Al-Khatib, S.M.; Jackson, K.P.; Bahnson, T.D.; Ellis, C.R.; Atwater, B.D.; Lewis, R.K.; et al. Left Atrial Appendage Electrical Isolation for Treatment of Recurrent Atrial Fibrillation: A Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 112–120. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Sridhar Mahankali, A.; Kanmanthareddy, A.; Lee, R.; Badhwar, N.; Bartus, K.; Atkins, D.; Bommana, S.; Cheng, J.; Rasekh, A.; et al. Left Atrial Appendage Ligation and Ablation for Persistent Atrial Fibrillation: The LAALA-AF Registry. JACC Clin. Electrophysiol. 2015, 1, 153–160. [Google Scholar] [CrossRef]
- Lakkireddy, D.R.; Wilber, D.J.; Mittal, S.; Tschopp, D.; Ellis, C.R.; Rasekh, A.; Hounshell, T.; Evonich, R.; Chandhok, S.; Berger, R.D.; et al. Pulmonary Vein Isolation With or Without Left Atrial Appendage Ligation in Atrial Fibrillation: The aMAZE Randomized Clinical Trial. JAMA 2024, 331, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Kanmanthareddy, A.; Earnest, M.; Reddy, M.; Atkins, D.; Bommana, S.; Bartus, K.; Rasekh, A.; Han, F.; Badhwar, N.; et al. Impact of left atrial appendage exclusion using an epicardial ligation system (LARIAT) on atrial fibrillation burden in patients with cardiac implantable electronic devices. Heart Rhythm 2015, 12, 52–59. [Google Scholar] [CrossRef]
- Toquica, C.; Jazayeri, M.A.; Noheria, A.; Berenbom, L.; Emert, M.; Pimentel, R.; Dendi, R.; Reddy, Y.M.; Sheldon, S.H. Safety of catheter ablation in patients with recently implanted cardiac implantable electronic device: A 5-year experience. Pacing Clin. Electrophysiol. 2024, 47, 878–884. [Google Scholar] [CrossRef]
- Iqbal, A.M.; Li, K.Y.; Mahmood, M.; Gautam, S. Safety of fluoroless radiofrequency catheter ablation for atrial fibrillation in patients with pre-existing cardiac implantable electronic device: A single-center study. Pacing Clin. Electrophysiol. 2023, 46, 1387–1392. [Google Scholar] [CrossRef]
- Dinshaw, L.; Schaffer, B.; Akbulak, O.; Jularic, M.; Hartmann, J.; Klatt, N.; Dickow, J.; Gunawardene, M.; Munkler, P.; Hakmi, S.; et al. Long-term efficacy and safety of radiofrequency catheter ablation of atrial fibrillation in patients with cardiac implantable electronic devices and transvenous leads. J. Cardiovasc. Electrophysiol. 2019, 30, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone, J., Jr.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Primary Outcome Evaluation of a Next-Generation Left Atrial Appendage Closure Device: Results From the PINNACLE FLX Trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left Atrial Appendage Closure Versus Direct Oral Anticoagulants in High-Risk Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef]
- Naik, H.; Price, M.J.; Kapadia, S.; Whisenant, B.K.; Tadros, P.; Makkar, R.; Asgar, A.W.; Fam, N.; Tang, G.H.L.; Mehta, S.R.; et al. Tricuspid Transcatheter Edge-to-Edge Repair in Patients With Transvalvular CIED Leads: The TRILUMINATE Pivotal Trial. JACC Clin. Electrophysiol. 2025, 11, 1012–1020. [Google Scholar] [CrossRef]
- Braun, D.; Orban, M.; Nabauer, M.; Orban, M.; Gross, L.; Englmaier, A.; Rosler, D.; Mehilli, J.; Bauer, A.; Hagl, C.; et al. Transcatheter Treatment of Severe Tricuspid Regurgitation Using the Edge-to-Edge Repair Technique in the Presence and Absence of Pacemaker Leads. JACC Cardiovasc. Interv. 2017, 10, 2014–2016. [Google Scholar] [CrossRef]
- Benito-Gonzalez, T.; Estevez-Loureiro, R.; Garrote-Coloma, C.; Arellano-Serrano, C.; Tundidor-Sanz, E.; Fernandez-Lozano, I.; Toquero, J.; Perez de Prado, A.; Goicolea, J.; Fernandez-Vazquez, F. Effect of Successful Edge-to-Edge Mitral Valve Repair on Ventricular Arrhythmic Burden in Patients With Functional Mitral Regurgitation and Implantable Cardiac Devices. Am. J. Cardiol. 2019, 124, 1113–1119. [Google Scholar] [CrossRef]
- Kuck, K.H.; Lebedev, D.S.; Mikhaylov, E.N.; Romanov, A.; Geller, L.; Kalejs, O.; Neumann, T.; Davtyan, K.; On, Y.K.; Popov, S.; et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: The randomized controlled atrial fibrillation progression trial (ATTEST). Europace 2021, 23, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left Atrial Appendage Closure after Ablation for Atrial Fibrillation. N. Engl. J. Med. 2025, 392, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Romanov, A.; Pokushalov, E.; Artemenko, S.; Yakubov, A.; Stenin, I.; Kretov, E.; Krestianinov, O.; Grazhdankin, I.; Risteski, D.; Karaskov, A.; et al. Does left atrial appendage closure improve the success of pulmonary vein isolation? Results of a randomized clinical trial. J. Interv. Card. Electrophysiol. 2015, 44, 9–16. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 36) | |
|---|---|
| Age (years), median ± IQR | 73.5 (66; 78) |
| Sex, female n (%) | 9 (25.0%) |
| Body mass index (kg/m2), median ± IQR | 26.6 (24.1; 30.4) |
| Arterial hypertension, n (%) | 29 (80.6%) |
| Diabetes mellitus, n (%) | 14 (38.9%) |
| CHA2DS2-VASc score, median ± IQR | 4.0 (3.0; 5.0) |
| HAS-BLED score, median ± IQR | 3.0 (2.0; 3.0) |
| Paroxysmal AF, n (%) | 22 (61.1%) |
| Persistent AF, n (%) | 7 (19.4%) |
| Permanent AF, n (%) | 3 (8.3%) |
| Unknown AF pattern, n (%) | 1 (2.8%) |
| Atrial flutter, n (%) | 3 (8.3%) |
| History of coronary artery disease, n (%) | 17 (47.2%) |
| History of congestive heart failure, n (%) | 22 (61.1%) |
| History of TIA or stroke, n (%) | 6 (16.7%) |
| History of bleeding, n (%) | 26 (72.2%) |
| History of kidney disease 1, n (%) | 6 (16.7%) |
| History of liver disease 2, n (%) | 0 (0.0%) |
| LVEF (%), median ± IQR | 58.8 (37.5; 60.0) |
| LAVI (ml/m2), median ± IQR | 44.6 (35.1; 54.2) |
| LA diameter (mm), median ± IQR | 41.5 (37.8; 45.1) |
| Prior ECV, n (%) | 9 (25%) |
| Prior PVI, n (%) | 4 (11%) |
| CIED | |
| Single chamber ICD, n (%) | 2 (5.5%) |
| Single chamber pacemaker, n (%) | 5 (13.9%) |
| Dual chamber ICD, n (%) | 4 (11.2%) |
| Dual chamber pacemaker, n (%) | 19 (52.8%) |
| CRT-P, n (%) | 1 (2.8%) |
| CRT-D, n (%) | 5 (13.8%) |
| All Patients (n = 36) | |
|---|---|
| Procedure time (min), median (IQR) | 36.5 (29.5; 45.0) |
| Fluoroscopy time (min), median (IQR) | 11.7 (8.9; 15.7) |
| Contrast medium used (mL), median (IQR) | 65.0 (46.0; 82.0) |
| General anesthesia, n (%) | 4 (11%) |
| Conscious sedation, n (%) | 32 (89%) |
| Fluoroscopic guidance | 3 (8%) |
| TEE guidance, n (%) | 33 (92%) |
| Diameter of LAA ostium by TEE (mm), median (IQR) | 19 (18; 23) |
| Diameter of LZ by TEE (mm), median (IQR) | 16 (14.5; 19) |
| LAA depth by TEE (mm), median (IQR) | 21 (18; 24.5) |
| Chicken wing shape of LAA, n (%) | 7 (19%) |
| Aborted procedure, n (%) | None |
| LAAC-Device used | |
| Watchman 2.5, n (%) | 7 (19%) |
| Watchman FLX, n (%) | 11 (31%) |
| Amulet/ACP, n (%) | 18 (50%) |
| Procedural complications within 7 days | |
| Composite of death, cerebrovascular accident, systemic or pulmonary embolism and major bleeding, n (%) | 1 (3%) |
| Death, n (%) | 0 (0.0%) |
| Stroke or TIA, n (%) | 0 (0.0%) |
| Systemic or pulmonary embolism, n (%) | 0 (0.0%) |
| Major bleeding 1, n (%) | 1 (3%) |
| All Patients (n = 36) | |
|---|---|
| Days between LAAC and TEE follow-up, median (IQR) | 52.0 (48; 97) |
| TEE follow-up, n (%) | 31 (86%) |
| DRT, n (%) | None |
| Non-relevant PDL < 5 mm, n (%) | 7 (22.6%) |
| Relevant PDL ≥ 5 mm, n (%) | None |
| Before LAAC | All Patients (n = 36) |
|---|---|
| Beta blockade, n (%) | 23 (63.9%) |
| CCA, n (%) | 1 (2.8%) |
| Amiodarone, n (%) | 8 (22.2%) |
| Digoxin, n (%) | 1 (2.8%) |
| After LAAC | |
| Beta blockade, n (%) | 30 (83.3%) |
| CCA, n (%) | 1 (2.8%) |
| Amiodarone, n (%) | 4 (11.1%) |
| Digoxin, n (%) | none |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, T.; Ledwoch, S.; Galea, R.; Gasys, A.; Gamardella, M.; Siontis, G.C.M.; Räber, L.; Roten, L. Feasibility and Impact of Left Atrial Appendage Closure in Patients with Cardiac Implantable Electronic Devices: Insights from a Prospective Registry. J. Clin. Med. 2025, 14, 3857. https://doi.org/10.3390/jcm14113857
Bini T, Ledwoch S, Galea R, Gasys A, Gamardella M, Siontis GCM, Räber L, Roten L. Feasibility and Impact of Left Atrial Appendage Closure in Patients with Cardiac Implantable Electronic Devices: Insights from a Prospective Registry. Journal of Clinical Medicine. 2025; 14(11):3857. https://doi.org/10.3390/jcm14113857
Chicago/Turabian StyleBini, Tommaso, Sven Ledwoch, Roberto Galea, Antanas Gasys, Marco Gamardella, George C. M. Siontis, Lorenz Räber, and Laurent Roten. 2025. "Feasibility and Impact of Left Atrial Appendage Closure in Patients with Cardiac Implantable Electronic Devices: Insights from a Prospective Registry" Journal of Clinical Medicine 14, no. 11: 3857. https://doi.org/10.3390/jcm14113857
APA StyleBini, T., Ledwoch, S., Galea, R., Gasys, A., Gamardella, M., Siontis, G. C. M., Räber, L., & Roten, L. (2025). Feasibility and Impact of Left Atrial Appendage Closure in Patients with Cardiac Implantable Electronic Devices: Insights from a Prospective Registry. Journal of Clinical Medicine, 14(11), 3857. https://doi.org/10.3390/jcm14113857







