High-Sensitivity Cardiac Troponin as a Predictor of Atrial Fibrillation Detected After Stroke: Implications for Subsequent Cerebrocardiovascular Events
Abstract
1. Introduction
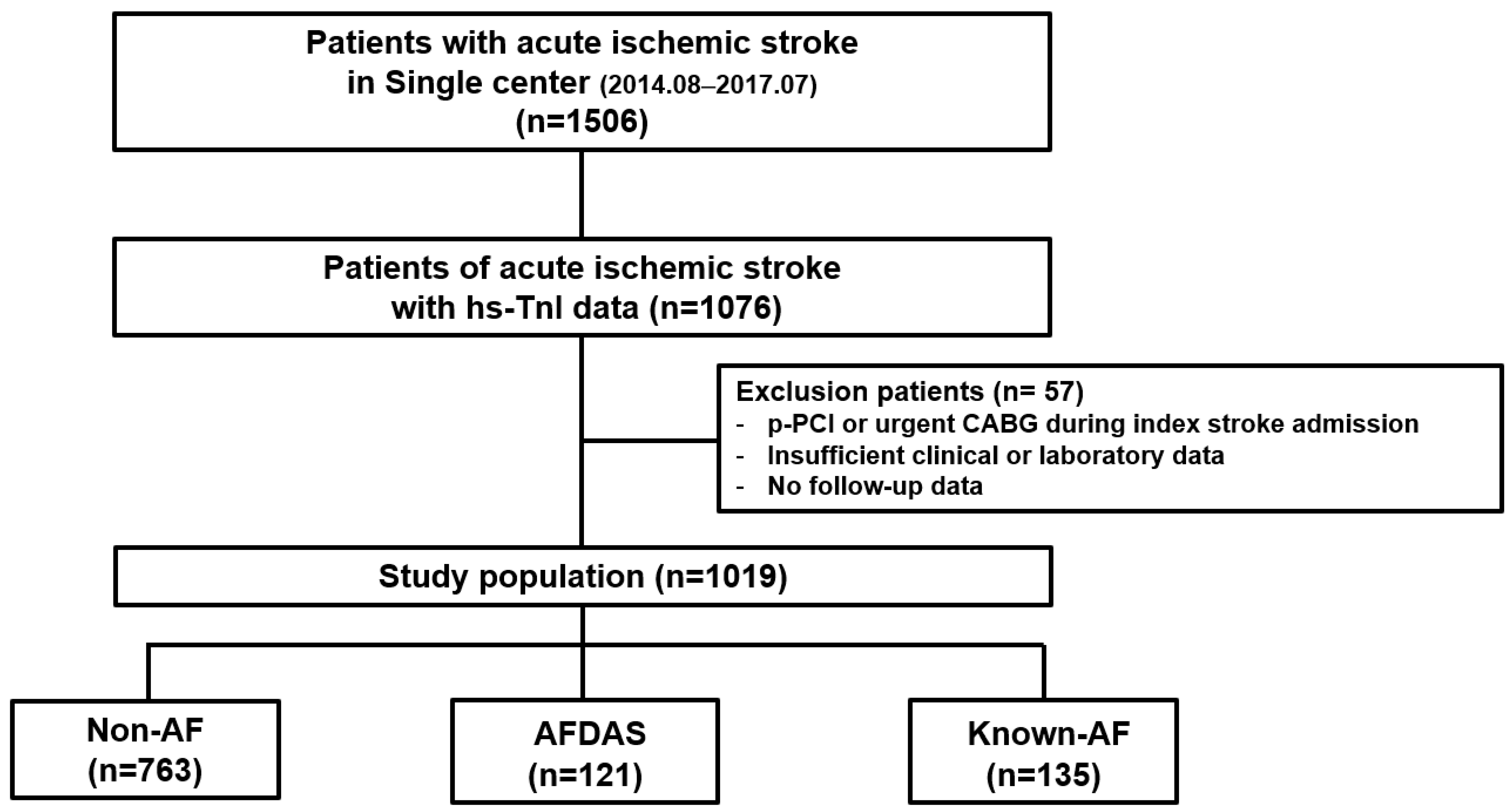
2. Methods
2.1. Study Population
2.2. Data Collection and High-Sensitive Cardiac Troponin I Assay
2.3. Definition of KAF and AFDAS and Study Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
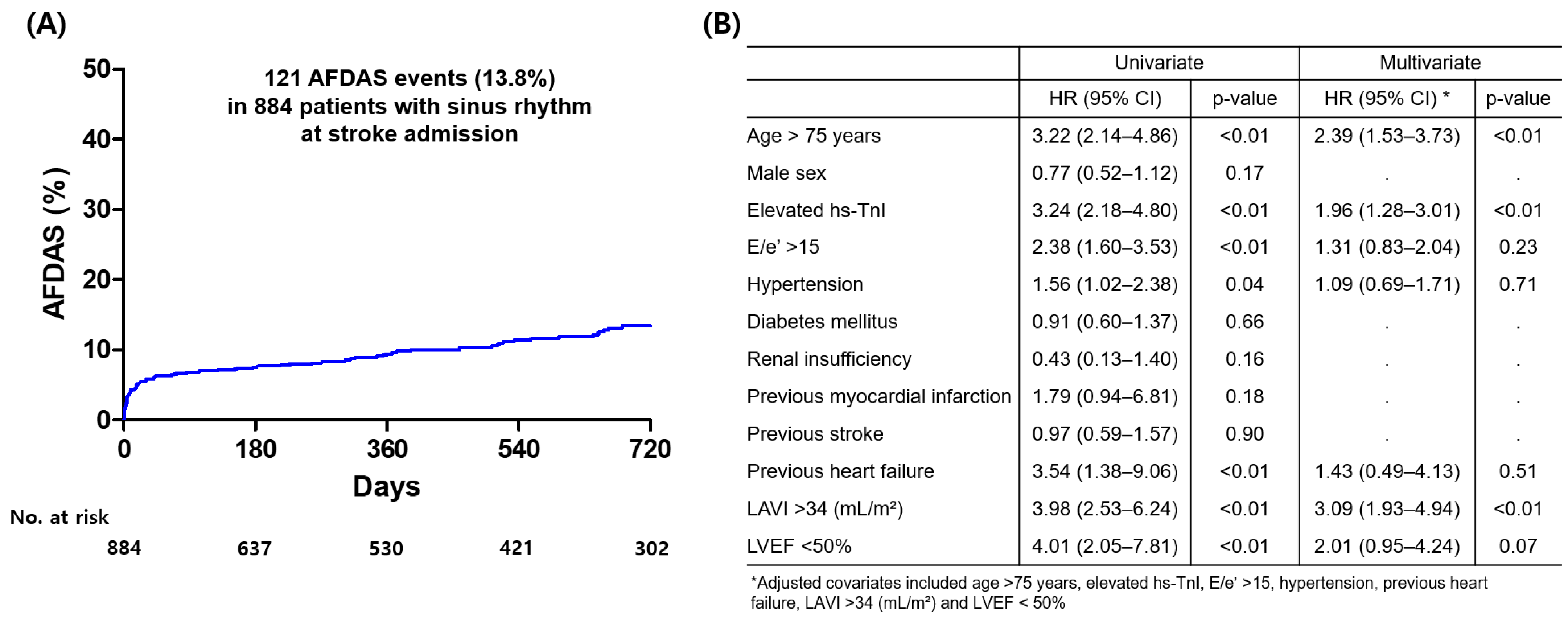
3.2. Occurrence of AFDAS During Follow-Up and Independent Predictors of AFDAS
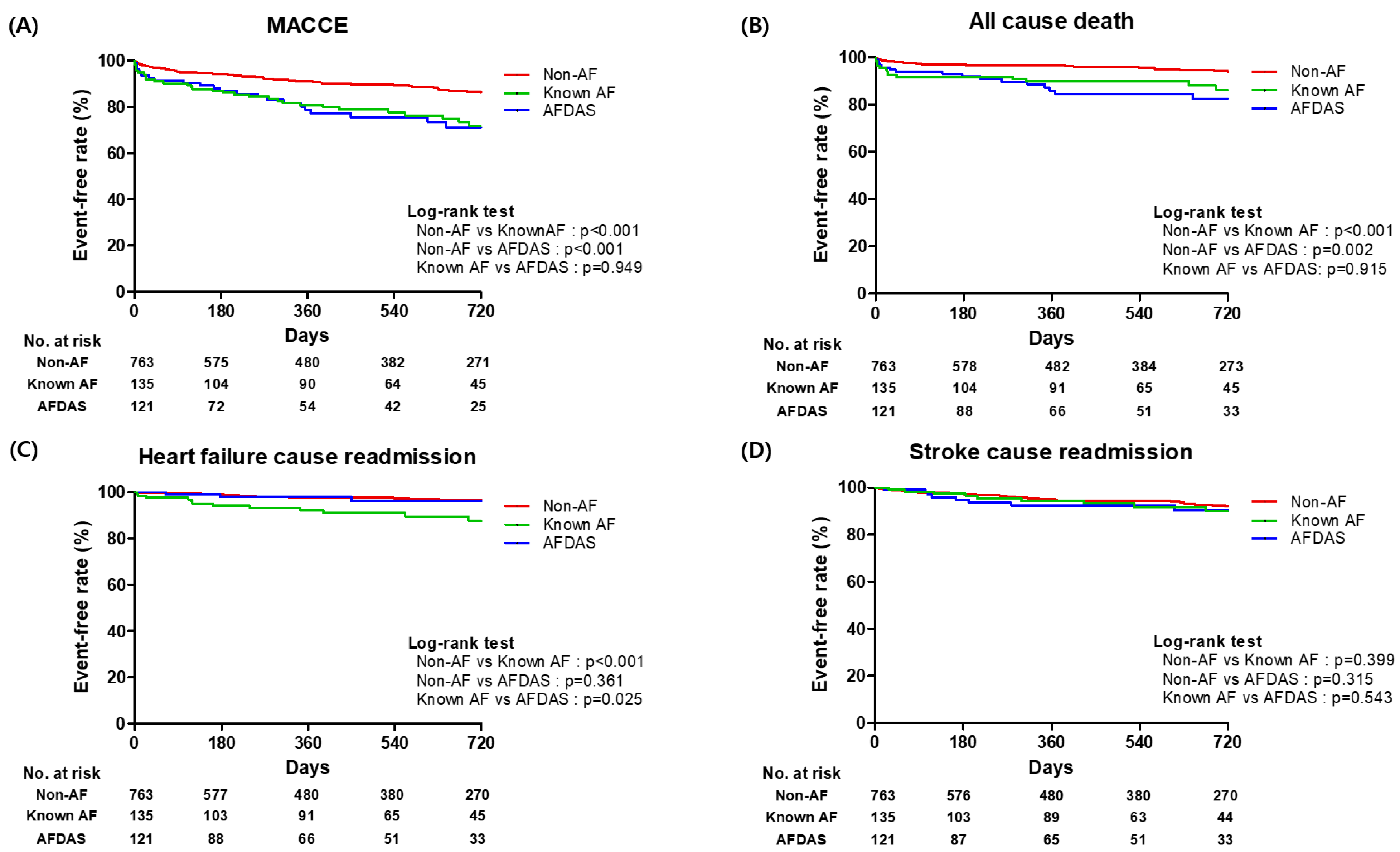
3.3. Clinical Outcomes According to Non-AF, AFDAS, and KAF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linz, D.; Gawalko, M.; Betz, K.; Hendriks, J.M.; Lip, G.Y.; Vinter, N.; Guo, Y.; Johnsen, S. Atrial fibrillation: Epidemiology, screening and digital health. Lancet Reg. Health Eur. 2024, 37, 100786. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cipriano, L.E.; Saposnik, G.; Vargas, E.R.; Riccio, P.M.; Hachinski, V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 377–387. [Google Scholar] [CrossRef]
- Sposato, L.A.; Field, T.S.; Schnabel, R.B.; Wachter, R.; Andrade, J.G.; Hill, M.D. Towards a new classification of atrial fibrillation detected after a stroke or a transient ischaemic attack. Lancet Neurol. 2024, 23, 110–122. [Google Scholar] [CrossRef]
- Sposato, L.A.; Cerasuolo, J.O.; Cipriano, L.E.; Fang, J.; Fridman, S.; Paquet, M.; Saposnik, G.; On behalf of the PARADISE Study Group; PARADISE Study Group; Whitehead, S.; et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology 2018, 90, e924–e931. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Urbinati, G.; Tudisco, V.; Toscano, A.; Eusebi, P.; Giammello, F.; D’ANna, L.; Palaiodimou, L.; Katsanos, A.H.; Diana, F.; et al. Risk of Recurrent Stroke, Mortality, and Intracerebral Hemorrhage in Patients With Atrial Fibrillation Detected Before or After a Stroke. Neurology 2025, 104, e213426. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-M.; Rao, Z.-Z.; Gu, H.-Q.; Zhao, X.-Q.; Wang, C.-J.; Liu, L.-P.; Liu, C.; Wang, Y.-L.; Li, Z.-X.; Xiao, R.-P.; et al. Atrial Fibrillation Known Before or Detected After Stroke Share Similar Risk of Ischemic Stroke Recurrence and Death. Stroke 2019, 50, 1124–1129. [Google Scholar] [CrossRef]
- Chang, Y.-K.; Hsu, C.-C.; Huang, C.-T.; Lien, C.-H.; Hsu, H.-Y. Differences between atrial fibrillation diagnosed before and after stroke: A large real-world cohort study. PLoS ONE 2024, 19, e0308507. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Drazner, M.H.; Omland, T.; Ayers, C.R.; Khera, A.; Rohatgi, A.; Hashim, I.; Berry, J.D.; Das, S.R.; Morrow, D.A.; et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 2010, 304, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Anegawa, T.; Kai, H.; Adachi, H.; Hirai, Y.; Enomoto, M.; Fukami, A.; Otsuka, M.; Kajimoto, H.; Yasuoka, S.; Iwamoto, Y.; et al. High-sensitive troponin T is associated with atrial fibrillation in a general population. Int. J. Cardiol. 2012, 156, 98–100. [Google Scholar] [CrossRef]
- Scheitz, J.F.; Pare, G.; Pearce, L.A.; Mundl, H.; Peacock, W.F.; Czlonkowska, A.; Sharma, M.; Nolte, C.H.; Shoamanesh, A.; Berkowitz, S.D.; et al. High-Sensitivity Cardiac Troponin T for Risk Stratification in Patients With Embolic Stroke of Undetermined Source. Stroke 2020, 51, 2386–2394. [Google Scholar] [CrossRef]
- Nolte, C.H.; von Rennenberg, R.; Litmeier, S.; Leistner, D.M.; Szabo, K.; Baumann, S.; Mengel, A.; Michalski, D.; Siepmann, T.; Blankenberg, S.; et al. Type 1 Myocardial Infarction in Patients With Acute Ischemic Stroke. JAMA Neurol. 2024, 81, 703–711. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Lee, J.-S.; Kim, Y.-H.; Kim, B.J.; Kim, Y.-J.; Kang, D.-W.; Kim, J.S.; Kwon, S.U. Prognostic Significance of Troponin Elevation for Long-Term Mortality after Ischemic Stroke. J. Stroke 2017, 19, 312–322. [Google Scholar] [CrossRef]
- Mochmann, H.C.; Scheitz, J.F.; Petzold, G.C.; Haeusler, K.G.; Audebert, H.J.; Laufs, U.; Schneider, C.; Landmesser, U.; Werner, N.; Endres, M.; et al. Coronary Angiographic Findings in Acute Ischemic Stroke Patients With Elevated Cardiac Troponin: The Troponin Elevation in Acute Ischemic Stroke (TRELAS) Study. Circulation 2016, 133, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.; Anderson, K.M.; Kannel, W.B.; Grossman, W.; Levy, D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993, 88, 107–115. [Google Scholar] [CrossRef]
- Wrigley, P.; Khoury, J.; Eckerle, B.; Alwell, K.; Moomaw, C.J.; Woo, D.; Flaherty, M.L.; Rosa, F.D.L.R.l.; Mackey, J.; Adeoye, O.; et al. Prevalence of Positive Troponin and Echocardiogram Findings and Association With Mortality in Acute Ischemic Stroke. Stroke 2017, 48, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Scheitz, J.F.; Nolte, C.H.; Doehner, W.; Hachinski, V.; Endres, M. Stroke-heart syndrome: Clinical presentation and underlying mechanisms. Lancet Neurol. 2018, 17, 1109–1120. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Yousuf, O.; Alonso, A.; Selvin, E.; Calkins, H.; McEvoy, J.W. High-Sensitivity Troponin as a Biomarker in Heart Rhythm Disease. Am. J. Cardiol. 2017, 119, 1407–1413. [Google Scholar] [CrossRef]
- Kim, B.S.; Park, J.-J.; Chang, H.; Kim, S.H.; Kwon, C.H.; Chung, S.-M.; Kim, H.Y.; Kim, H.-J. Association of High-Sensitivity Troponin I with Cardiac and Cerebrovascular Events in Patient after Ischemic Stroke. Cerebrovasc. Dis. 2023, 52, 153–159. [Google Scholar] [CrossRef]
- Ogawa, H.; An, Y.; Ikeda, S.; Aono, Y.; Doi, K.; Ishii, M.; Iguchi, M.; Masunaga, N.; Esato, M.; Tsuji, H.; et al. Progression From Paroxysmal to Sustained Atrial Fibrillation Is Associated With Increased Adverse Events. Stroke 2018, 49, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.C.; Arnold, M.; Katsas, G.; Yang, J.; Quinn, T.J.; Abdul-Rahim, A.H.; Campbell, R.; Docherty, K.; Marchis, G.M.D.; Arnold, M.; et al. Natriuretic Peptides to Classify Risk of Atrial Fibrillation Detection After Stroke: Analysis of the BIOSIGNAL and PRECISE Cohort Studies. Neurology 2024, 103, e209625. [Google Scholar] [CrossRef]
- Cameron, A.; Cheng, H.K.; Lee, R.-P.; Doherty, D.; Hall, M.; Khashayar, P.; Lip, G.; Quinn, T.; Abdul-Rahim, A.; Dawson, J. Biomarkers for Atrial Fibrillation Detection After Stroke: Systematic Review and Meta-analysis. Neurology 2021, 97, e1775–e1789. [Google Scholar] [CrossRef]
- Palà, E.; Bustamante, A.; Pagola, J.; Juega, J.; Francisco-Pascual, J.; Penalba, A.; Rodriguez, M.; Alfonso, M.D.L.; Arenillas, J.F.; Cabezas, J.A.; et al. Blood-Based Biomarkers to Search for Atrial Fibrillation in High-Risk Asymptomatic Individuals and Cryptogenic Stroke Patients. Front. Cardiovasc. Med. 2022, 9, 908053. [Google Scholar] [CrossRef]
- Papakonstantinou, P.E.; Rivera-Caravaca, J.M.; Chiarito, M.; Ehrlinder, H.; Iliakis, P.; Gąsecka, A.; Romiti, G.F.; Parker, W.A.; Lip, G.Y. Atrial fibrillation versus atrial myopathy in thrombogenesis: Two sides of the same coin? Trends Cardiovasc. Med. 2025, 35, 271–281. [Google Scholar] [CrossRef]
- Patel, R.B.; Alenezi, F.; Sun, J.-L.; Alhanti, B.; Vaduganathan, M.; Oh, J.K.; Redfield, M.M.; Butler, J.; Hernandez, A.F.; Velazquez, E.J.; et al. Biomarker Profile of Left Atrial Myopathy in Heart Failure With Preserved Ejection Fraction: Insights From the RELAX Trial. J. Card Fail. 2020, 26, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, A.; Borovskiy, Y.; Katz, R.; Hyman, M.C.; Patel, P.J.; Arkles, J.; Callans, D.J.; Chokshi, N.; Dixit, S.; Epstein, A.E.; et al. Stroke, Timing of Atrial Fibrillation Diagnosis, and Risk of Death. Neurology 2021, 96, e1655–e1662. [Google Scholar] [CrossRef]
- Induruwa, I.; Bhakta, S.; Herlekar, R.; Roy, A.S.; Hajiev, S.; Warburton, E.A.; Khadjooi, K.; McCabe, J.J. Recurrent vascular events and mortality outcomes in patients with known atrial fibrillation, compared to atrial fibrillation detected early after stroke. Eur. Stroke J. 2025, 10, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S.; Jimenez-Ruiz, A.; Vargas-Gonzalez, J.C.; Sposato, L.A. Differences between Atrial Fibrillation Detected before and after Stroke and TIA: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2022, 51, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Bolaños, A.; Ayan, D.; Khaw, A.V.; Mai, L.M.; Mandzia, J.L.; Bogiatzi, C.; Mrkobrada, M.; Bres-Bullrich, M.; Fleming, L.A.; Lippert, C.; et al. Differences in Stroke Recurrence Risk Between Atrial Fibrillation Detected on ECG and 14-Day Cardiac Monitoring. Stroke 2023, 54, 2022–2030. [Google Scholar] [CrossRef]
- Rubiera, M.; Aires, A.; Antonenko, K.; Lémeret, S.; Nolte, C.H.; Putaala, J.; Schnabel, R.B.; Tuladhar, A.M.; Werring, D.J.; Zeraatkar, D.; et al. European Stroke Organisation (ESO) guideline on screening for subclinical atrial fibrillation after stroke or transient ischaemic attack of undetermined origin. Eur. Stroke J. 2022, 7, Vi. [Google Scholar] [CrossRef]
| Overall Population | Non-AF | AFDAS | p-Value (Non-AF vs. AFDAS) | Known-AF | p-Value (Non-AF vs. KAF) |
|---|---|---|---|---|---|
| (n = 1019) | (n = 763) | (n = 121) | (n = 135) | ||
| Age (years) | 69.2 ± 13.7 | 77.2 ± 10.7 | <0.01 | 77.5 ± 8.9 | <0.01 |
| Age > 75 years old | 308 (40.4) | 83 (68.6) | <0.01 | 95 (70.4) | <0.01 |
| Male sex | 432 (56.6) | 60 (49.6) | 0.15 | 73 (45.1) | 0.58 |
| Medical history | |||||
| Hypertension | 474 (62.1) | 87 (71.9) | 0.04 | 98 (72.6) | 0.02 |
| Diabetes mellitus | 235 (30.8) | 36 (29.8) | 0.82 | 50 (37.0) | 0.15 |
| Current smoking | 182 (23.9) | 15 (12.4) | <0.01 | 12 (8.9) | <0.01 |
| Renal insufficiency | 79 (10.4) | 8 (6.6) | 0.19 | 19 (14.1) | 0.21 |
| Dyslipidemia | 154 (20.2) | 14 (11.6) | 0.03 | 29 (21.5) | 0.73 |
| Previous myocardial infarction | 18 (2.4) | 10 (8.3) | <0.01 | 10 (7.4) | <0.01 |
| Previous PCI | 29 (3.8) | 7 (5.8) | 0.31 | 11 (8.1) | 0.02 |
| Previous CABG | 13 (1.7) | 4 (3.3) | 0.23 | 3 (2.2) | 0.67 |
| Previous heart failure | 13 (1.7) | 7 (5.8) | <0.01 | 26 (19.3) | <0.01 |
| Previous stroke | 134 (17.6) | 23 (19.0) | 0.69 | 41 (30.4) | <0.01 |
| Echocardiographic parameters | |||||
| LVEF (%) | 66.9 ± 8.3 | 63.2 ± 12.4 | <0.01 | 60.5 ± 12.5 | <0.01 |
| LVEF < 50% | 26 (3.4) | 15 (12.4) | <0.01 | 20 (14.8) | <0.01 |
| LAVI > 34 (mL/m2) | 356 (46.7) | 94 (77.7) | <0.01 | 124 (91.9) | <0.01 |
| E/e’ >15 | 80 (10.5) | 31 (25.6) | <0.01 | 36 (26.7) | <0.01 |
| Laboratory parameters | |||||
| hs-TnI (ng/L) | 49.06 ± 30.07 | 56.50 ± 32.04 | 0.03 | 140.86 ± 105.87 | <0.01 |
| >99 percentile hs-TnI | 188 (24.6) | 53 (43.8) | <0.01 | 70 (51.9) | <0.01 |
| Hemoglobin (g/dL) | 13.6 ± 2.0 | 13.5 ± 2.0 | 0.45 | 13.1 ± 1.8 | 0.01 |
| Creatinine (mg/dL) | 1.1 ± 0.58 | 1.0 ± 0.89 | 0.39 | 1.1 ± 0.46 | 0.69 |
| LDL cholesterol (mg/dL) | 103.3 ± 44.1 | 98.3 ± 29.8 | 0.23 | 94.1 ± 33.3 | 0.04 |
| Total cholesterol (mg/dL) | 174.6 ± 48.4 | 164.9 ± 36.6 | 0.03 | 154.1 ± 40.9 | <0.01 |
| Initial NIHSS | 4.3 ± 3.3 | 8.1 ± 5.3 | <0.01 | 8.5 ± 4.5 | <0.01 |
| CHA2DS2-VASc Score | 4.4 ± 1.4 | 5.3 ± 1.3 | <0.01 | 5.6 ± 1.4 | <0.01 |
| Reperfusion therapy | |||||
| Intravenous thrombolysis | 71 (9.3) | 27 (22.3) | <0.01 | 34 (25.2) | <0.01 |
| Endovascular treatment | 33 (4.3) | 11 (9.1) | 0.03 | 24 (17.8) | <0.01 |
| Medications | |||||
| Aspirin | 579 (75.9) | 46 (38.0) | <0.01 | 27 (20.0) | <0.01 |
| Clopidogrel | 329 (43.1) | 28 (23.1) | <0.01 | 22 (16.3) | <0.01 |
| Warfarin | 12 (1.6) | 19 (15.7) | <0.01 | 35 (25.9) | <0.01 |
| DOAC | 9 (1.2) | 37 (30.6) | <0.01 | 60 (44.4) | <0.01 |
| Statin | 663 (86.9) | 99 (81.8) | 0.13 | 105 (77.8) | <0.01 |
| Non-AF | AFDAS | Known-AF | |
|---|---|---|---|
| (n = 763) | (n = 121) | (n = 135) | |
| MACCE | 116 (15.2) | 33 (27.3) | 38 (28.1) |
| Unadjusted HR (95% CI) | 1 [Reference] | 2.21 (1.52–3.20) | 2.28 (1.57–3.32) |
| p-value | <0.01 | <0.01 | |
| Adjusted HR (95% CI) * | 1 [Reference] | 1.85 (1.23–2.79) | 1.76 (1.17–2.64) |
| p-value | <0.01 | 0.01 | |
| All-cause death | 49 (6.4) | 19 (15.7) | 19 (14.1) |
| Unadjusted HR (95% CI) | 1 [Reference] | 2.32 (1.35–3.99) | 2.77(1.61–4.78) |
| p-value | <0.01 | <0.01 | |
| Adjusted HR (95% CI) * | 1 [Reference] | 1.73 (0.96–3.12) | 2.05 (1.15–3.62) |
| p-value | 0.07 | 0.02 | |
| Heart failure-caused readmission | 25 (3.3) | 6 (5.0) | 14 (10.4) |
| Unadjusted HR (95% CI) | 1 [Reference] | 1.51 (0.62–3.71) | 3.69 (1.89–7.20) |
| p-value | 0.36 | <0.01 | |
| Adjusted HR (95% CI) * | 1 [Reference] | 0.89 (0.34–2.38) | 1.97 (0.94–4.15) |
| p-value | 0.83 | 0.07 | |
| Stroke-caused readmission | 58 (7.6) | 14 (11.6) | 11 (8.1) |
| Unadjusted HR (95% CI) | 1 [Reference] | 1.36 (0.74–2.50) | 1.32 (0.68–2.54) |
| p-value | 0.31 | 0.41 | |
| Adjusted HR (95% CI) * | 1 [Reference] | 1.39 (0.74–2.62) | 1.51 (0.76–2.99) |
| p-value | 0.29 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.S.; Park, J.J.; Choi, J.-H.; Kwon, C.H.; Kim, S.H.; Jeon, K.; Chung, Y.-j.; Kim, H.Y.; Kim, H.-J. High-Sensitivity Cardiac Troponin as a Predictor of Atrial Fibrillation Detected After Stroke: Implications for Subsequent Cerebrocardiovascular Events. J. Clin. Med. 2025, 14, 7542. https://doi.org/10.3390/jcm14217542
Kim BS, Park JJ, Choi J-H, Kwon CH, Kim SH, Jeon K, Chung Y-j, Kim HY, Kim H-J. High-Sensitivity Cardiac Troponin as a Predictor of Atrial Fibrillation Detected After Stroke: Implications for Subsequent Cerebrocardiovascular Events. Journal of Clinical Medicine. 2025; 14(21):7542. https://doi.org/10.3390/jcm14217542
Chicago/Turabian StyleKim, Bum Sung, Jung Jin Park, Ji-Hoon Choi, Chang Hee Kwon, Sung Hea Kim, Kina Jeon, Yu-jin Chung, Hahn Young Kim, and Hyun-Joong Kim. 2025. "High-Sensitivity Cardiac Troponin as a Predictor of Atrial Fibrillation Detected After Stroke: Implications for Subsequent Cerebrocardiovascular Events" Journal of Clinical Medicine 14, no. 21: 7542. https://doi.org/10.3390/jcm14217542
APA StyleKim, B. S., Park, J. J., Choi, J.-H., Kwon, C. H., Kim, S. H., Jeon, K., Chung, Y.-j., Kim, H. Y., & Kim, H.-J. (2025). High-Sensitivity Cardiac Troponin as a Predictor of Atrial Fibrillation Detected After Stroke: Implications for Subsequent Cerebrocardiovascular Events. Journal of Clinical Medicine, 14(21), 7542. https://doi.org/10.3390/jcm14217542







