Atypical Mycobacterium ulcerans Infection with Skip Lesions in a 68-Year-Old Male: A Rare Case and Comprehensive Literature Review
Abstract
1. Introduction
2. Materials and Methods
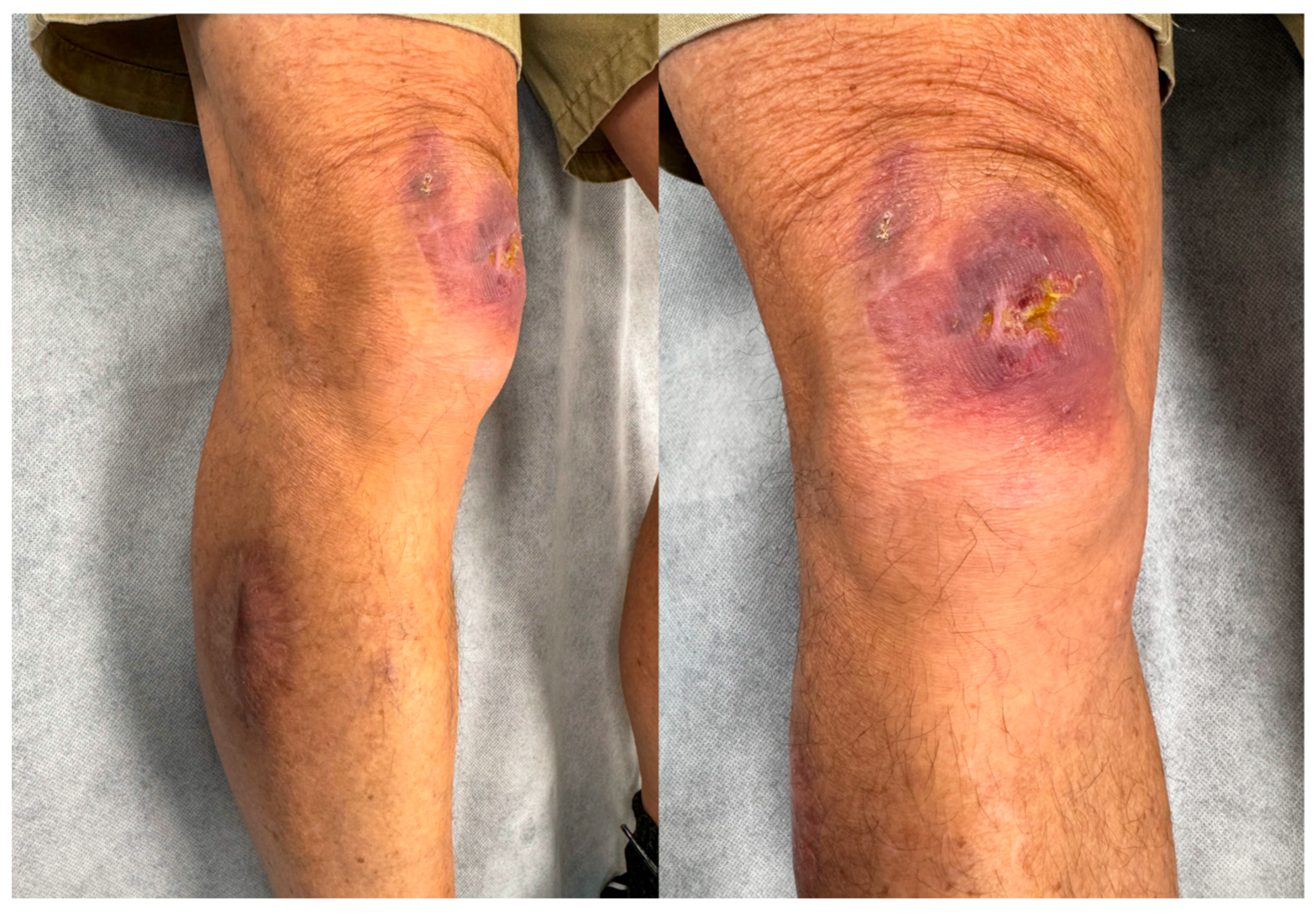
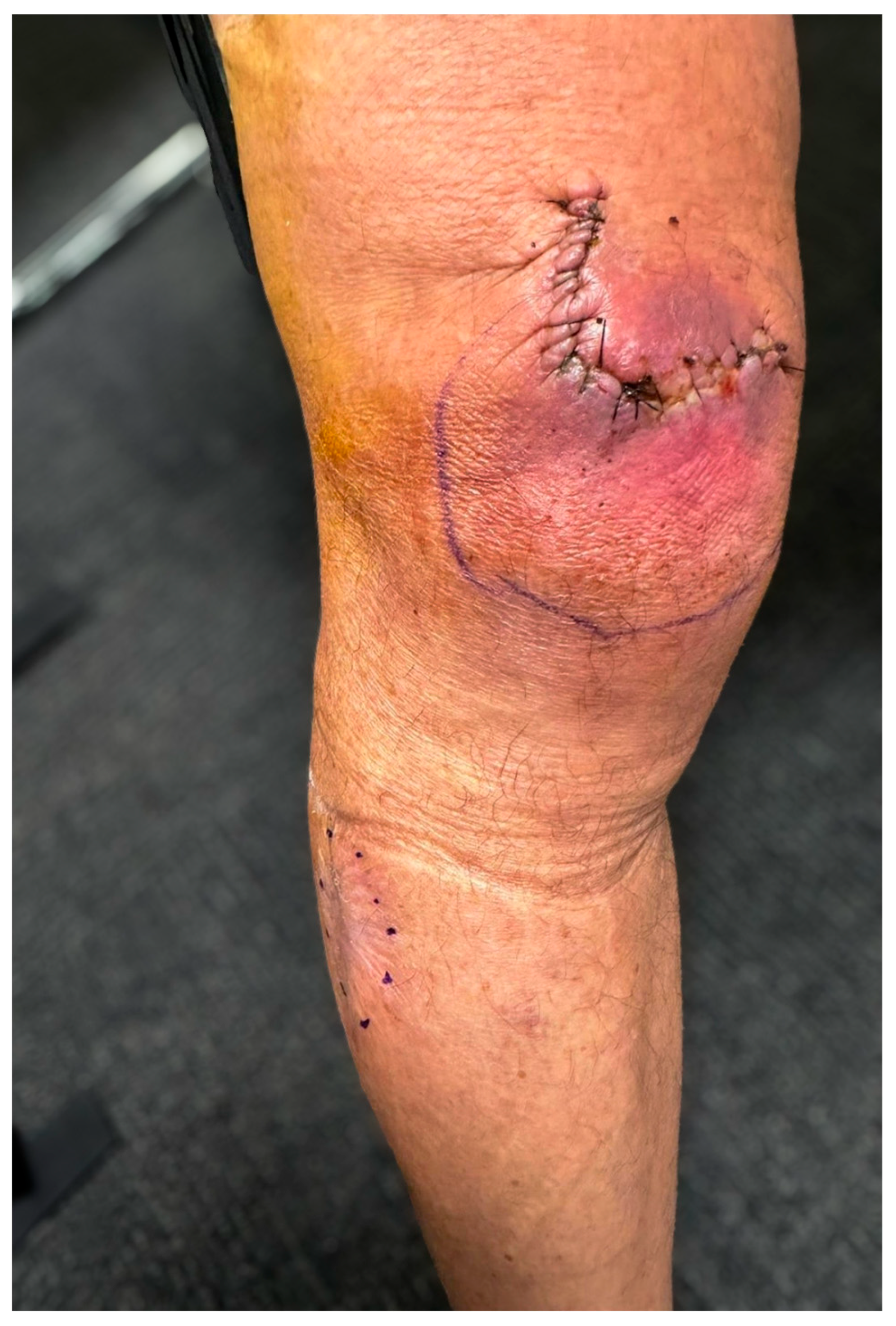
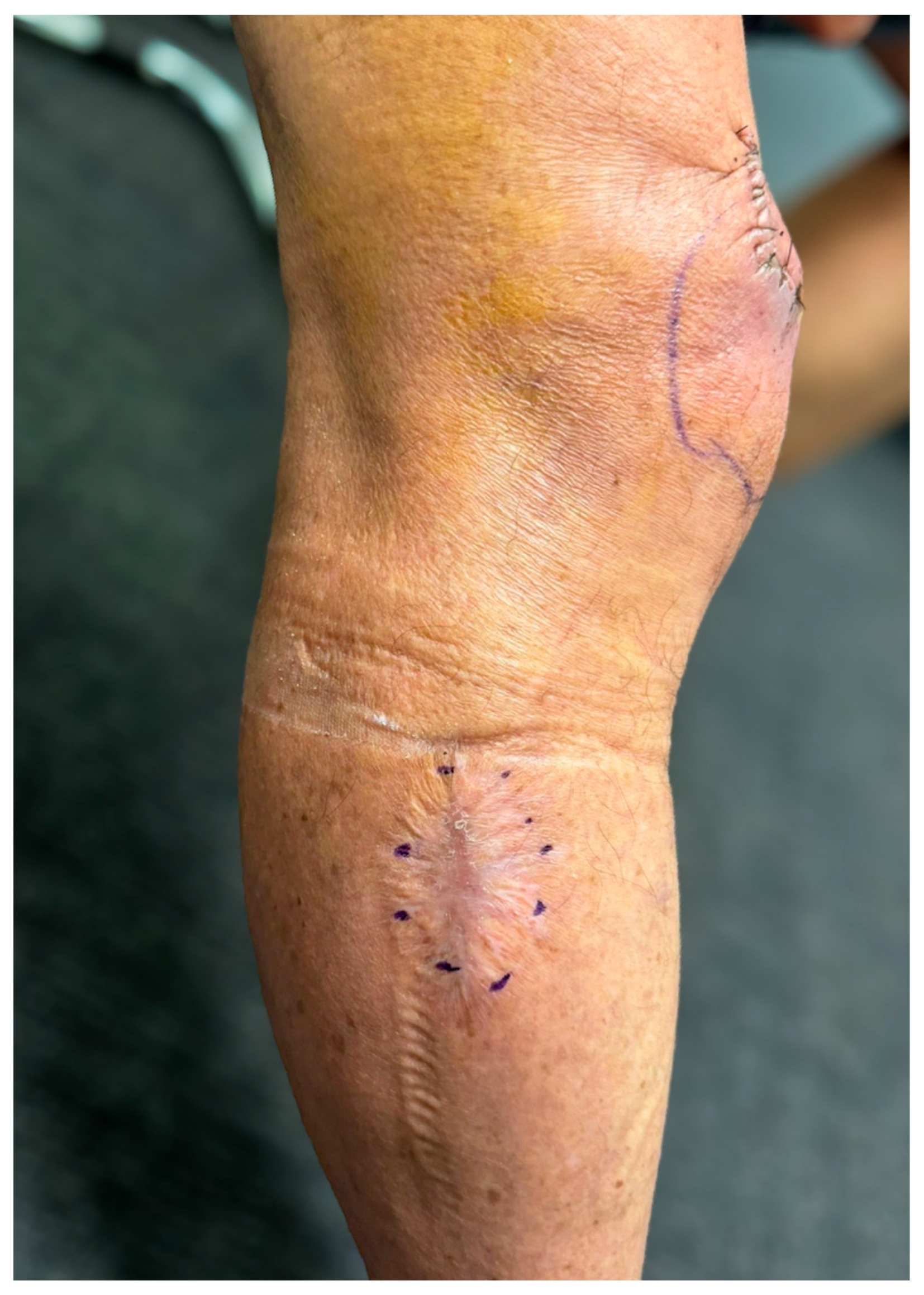
Case Presentation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLMS4BU | Buruli Lesion Management Study for Buruli Ulcer; |
| BU | Buruli ulcer; |
| eGFR | Estimated Glomerular Filtration Rate; |
| GORD | Gastroesophageal Reflux Disease; |
| HBOT | Hyperbaric Oxygen Therapy; |
| HIV | Human Immunodeficiency Virus; |
| M. ulcerans | Mycobacterium ulcerans; |
| PCR | Polymerase Chain Reaction; |
| TB | Tuberculosis; |
| WHO | World Health Organization. |
References
- McNamara, B.J.; Blasdell, K.R.; Yerramilli, A.; Smith, I.L.; Clayton, S.L.; Dunn, M.; Tay, E.L.; Gibney, K.B.; Waidyatillake, N.T.; Hussain, M.A.; et al. Comprehensive Case-Control Study of Protective and Risk Factors for Buruli Ulcer, Southeastern Australia. Emerg. Infect. Dis. 2023, 29, 2032–2043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schütte, D.; Um-Boock, A.; Mensah-Quainoo, E.; Itin, P.; Schmid, P.; Pluschke, G. Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin. PLoS Negl. Trop. Dis. 2007, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Department of Health Victoria. Victoria, Local Public Health Areas and Local Government Areas Surveillance Summary Report. Available online: https://www.health.vic.gov.au/infectious-diseases/local-government-areas-surveillance-report (accessed on 1 March 2025).
- Mee, P.T.; Buultjens, A.H.; Oliver, J.; Brown, K.; Crowder, J.C.; Porter, J.L.; Hobbs, E.C.; Judd, L.M.; Taiaroa, G.; Puttharak, N.; et al. Mosquitoes provide a transmission route between possums and humans for Buruli ulcer in southeastern Australia. Nat. Microbiol. 2024, 9, 377–389, Erratum in Nat. Microbiol. 2024, 9, 2463. https://doi.org/10.1038/s41564-024-01693-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallace, J.R.; Gordon, M.C.; Hartsell, L.; Mosi, L.; Benbow, M.E.; Merritt, R.W.; Small, P.L. Interaction of Mycobacterium ulcerans with mosquito species: Implications for transmission and trophic relationships. Appl. Environ. Microbiol. 2010, 76, 6215–6222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yerramilli, A.; Tay, E.L.; Stewardson, A.J.; Kelley, P.G.; Bishop, E.; Jenkin, G.A.; Starr, M.; Trevillyan, J.; Hughes, A.; Friedman, N.D.; et al. The location of Australian Buruli ulcer lesions-Implications for unravelling disease transmission. PLoS Negl. Trop. Dis. 2017, 11, e0005800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, D.P.; Friedman, N.D.; McDonald, A.; Callan, P.; Hughes, A.; Athan, E. Clinical features and risk factors of oedematous Mycobacterium ulcerans lesions in an Australian population: Beware cellulitis in an endemic area. PLoS Negl. Trop. Dis. 2014, 8, e2612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organisation. Treatment of Mycobacterium ulcerans Disease (Buruli Ulcer). Available online: https://iris.who.int/bitstream/handle/10665/77771/9789241503402_eng.pdf?sequence=1 (accessed on 1 March 2025).
- O’Brien, D.P.; Callan, P.; Friedman, N.D.; Athan, E.; Hughes, A.; McDonald, A. Mycobacterium ulcerans disease management in Australian patients: The re-emergence of surgery as an important treatment modality. ANZ J. Surg. 2019, 89, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.C.; Athan, E.; Friedman, N.D.; Hughes, A.; Walton, A.; Callan, P.; McDonald, A.; O’Brien, D.P. Epidemiology, clinical features and diagnosis of Mycobacterium ulcerans in an Australian population. Med. J. Aust. 2012, 196, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Phanzu, D.M.; Mahema, R.L.; Suykerbuyk, P.; Imposo, D.H.; Lehman, L.F.; Nduwamahoro, E.; Meyers, W.M.; Boelaert, M.; Portaels, F. Mycobacterium ulcerans infection (Buruli ulcer) on the face: A comparative analysis of 13 clinically suspected cases from the Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2011, 85, 1100–1105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruf, M.T.; Chauty, A.; Adeye, A.; Ardant, M.F.; Koussemou, H.; Johnson, R.C.; Pluschke, G. Secondary Buruli ulcer skin lesions emerging several months after completion of chemotherapy: Paradoxical reaction or evidence for immune protection? PLoS Negl. Trop. Dis. 2011, 5, e1252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, N.D.; Athan, E.; Hughes, A.J.; Khajehnoori, M.; McDonald, A.; Callan, P.; Rahdon, R.; O’Brien, D.P. Mycobacterium ulcerans disease: Experience with primary oral medical therapy in an Australian cohort. PLoS Negl. Trop. Dis. 2013, 7, e2315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, D.P.; Friedman, N.D.; Cowan, R.; Pollard, J.; McDonald, A.; Callan, P.; Hughes, A.; Athan, E. Mycobacterium ulcerans in the Elderly: More Severe Disease and Suboptimal Outcomes. PLoS Negl. Trop. Dis. 2015, 9, e0004253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, D.P.; Athan, E.; Blasdell, K.; De Barro, P. Tackling the worsening epidemic of Buruli ulcer in Australia in an information void: Time for an urgent scientific response. Med. J. Aust. 2018, 208, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Loftus, M.J.; Tay, E.L.; Globan, M.; Lavender, C.J.; Crouch, S.R.; Johnson, P.D.; Fyfe, J.A. Epidemiology of Buruli ulcer infections, Victoria, Australia, 2011–2016. Emerg. Infect. Dis. 2018, 24, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.Y.C.; Athan, E.; Friedman, N.D.; Hughes, A.; Walton, A.; O’Brien, D.P. Increased severity and spread of Mycobacterium ulcerans, southeastern Australia. Emerg Infect Dis. 2018, 24, 58–64. [Google Scholar] [CrossRef]
- O’Brien, D.P.; Friedman, N.D.; Walton, A.; Hughes, A.; Athan, E. Risk Factors Associated with Antibiotic Treatment Failure of Buruli Ulcer. Antimicrob. Agents Chemother. 2020, 64, e00722-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, D.P.; Friedman, N.D.; Cowan, R.; Walton, A.; Athan, E. Six vs. Eight Weeks of Antibiotics for Small Mycobacterium ulcerans Lesions in Australian Patients. Clin. Infect. Dis. 2020, 70, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Yerramilli, P.; O’Brien, D.P. Mycobacterium ulcerans in Victoria, Australia: Epidemiology and Management. In Buruli Ulcer: Mycobacterium ulcerans Disease; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Johnson, R.C.; Sáez-López, E.; Anagonou, E.S.; Kpoton, G.G.; Ayelo, A.G.; Gnimavo, R.S.; Mignanwande, F.Z.; Houezo, J.G.; Sopoh, G.E.; Addo, J.; et al. Comparison of 8 weeks standard treatment (rifampicin plus clarithromycin) vs. 4 weeks standard plus amoxicillin/clavulanate treatment [RC8 vs. RCA4] to shorten Buruli ulcer disease therapy (the BLMs4BU trial): Study protocol for a randomized controlled multi-centre trial in Benin. Trials 2022, 23, 559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandelannoote, K.; Buultjens, A.H.; Porter, J.L.; Velink, A.; Wallace, J.R.; Blasdell, K.R.; Dunn, M.; Boyd, V.; Fyfe, J.A.M.; Tay, E.L.; et al. Statistical modeling based on structured surveys of Australian native possum excreta harboring Mycobacterium ulcerans predicts Buruli ulcer occurrence in humans. eLife 2023, 14, e84983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organisation. Buruli Ulcer (Mycobacterium ulcerans Infection). Available online: https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)#:~:text=The%20disease%20has%20been%20classified,including%2C%20disseminated%20and%20mixed%20forms (accessed on 1 March 2025).
- Department of Health Victoria. Beating Buruli in Victoria. Available online: https://www.health.vic.gov.au/infectious-diseases/beating-buruli-in-victoria (accessed on 1 March 2025).
- Guarner, J. Buruli Ulcer: Review of a Neglected Skin Mycobacterial Disease. J. Clin. Microbiol. 2018, 56, 20180326. [Google Scholar] [CrossRef]
- Blasdell, K.R.; Ploeg, R.J.; Hobbs, E.C.; Muhi, S.; Riddell, S.J.; Cunneen, A.; Kelly, M.L.; Maynard, K.; Malcolm, T.R.; Islam, M.T.; et al. Experimental infection of ringtail possums (Pseudocheirus peregrinus) with Mycobacterium ulcerans, the agent of Buruli ulcer. Sci. Rep. 2024, 14, 25352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, P.D.; Hayman, J.A.; Quek, T.Y.; Fyfe, J.A.; Jenkin, G.A.; Buntine, J.A.; Athan, E.; Birrell, M.; Graham, J.; Lavender, C.J.; et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Med. J. Aust. 2007, 186, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.; Athan, E.; Friedman, N.D.; Hughes, A.J.; McDonald, A.; Callan, P.; Fyfe, J.; O’Brien, D.P. Mycobacterium ulcerans treatment—Can antibiotic duration be reduced in selected patients? PLoS Negl. Trop. Dis. 2015, 9, e0003503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, A.Y.; Seth, I.; Marcaccini, G.; Rozen, W.M.; Ross, R.J. Basal Cell Carcinoma Arising in a Previous Full-Thickness Graft Donor Site: A Case Report and Comprehensive Literature Review. J. Clin. Med. 2025, 14, 591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Study | Year | Region | Number of Cases | Skip Lesions Present | Comorbidities | Treatment | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|
| Phanzu et al. [11] | 2011 | Congo, Central Africa | 238 with 13 facial | Yes (3) | None reported | Rifampicin + streptomycin +/− surgery | Delayed treatment of facial ulcers led to complications | Early diagnosis improves outcomes, particularly in unexpected lesion locations |
| Ruf et al. [12] | 2011 | Benin, West Africa | 224 | Yes (2) | None reported | Rifampicin + Streptomycin + surgical intervention | Secondary satellite lesions developed during 8-week course | Possible immune response-mediated paradoxical reactions |
| Friedman et al. [13] | 2013 | Bellarine Peninsula, Victoria, Australia | 43 | No | Diabetes, malignancy, immunosuppression | Rifampicin + Ciprofloxacin/Clarithromycin + Surgery | Potential efficacy of shortened antibiotic course (4 weeks) | Genomic differences in M. ulcerans strains may affect treatment response |
| O’Brien et al. [14] | 2015 | Bellarine Peninsula (Barwon Health) | 327 | Yes (3) | Elderly population | Rifampicin + Ciprofloxacin + Clarithromycin | Older patients more likely to have multiple lesions | Potential role of immune response in causing multiple lesions |
| O’Brien et al. [15] | 2017 | Bellarine Peninsula, Australia | 70 | Yes (3 cases) | Immunosuppression | Rifampicin + Clarithromycin | Full recovery, but delayed healing in patients with skip lesions | Delayed treatment may lead to skip lesions |
| Loftus et al. [16] | 2019 | Victoria, Australia | 55 | Yes (4 cases) | Hypercholesterolemia | Rifampicin + Clarithromycin | Successful healing in most cases, additional surgical debridement needed | Skip lesions are rare but present; early intervention is key |
| Tai et al. [17] | 2019 | Ghana, West Africa | 320 | No | Immunosuppression | Rifampicin + Streptomycin | Significant healing in all cases, no recorded skip lesions | Study focused on single lesions, low skip lesion occurrence |
| O’Brien et al. [18] | 2020 | Victoria, Australia | 85 | Yes (5 cases) | Diabetes, hypertension, immunosuppression | Rifampicin + Clarithromycin + Surgery | Good recovery, delayed healing in 2 cases with skip lesions | Skip lesions associated with delayed treatment |
| O’Brien et al. [19] | 2020 | Australia (coastal areas) | 100 | No | Hypertension, diabetes | Rifampicin + Moxifloxacin + Surgery | Successful recovery, no cases of skip lesions recorded | Environmental factors may play a role in increasing incidence |
| Yerramilli et al. [20] | 2021 | Victoria, Australia | 120 | Yes (3 cases) | Hypertension, hypercholesterolemia | Rifampicin + Clarithromycin | Full recovery in most cases, surgical debridement required | Mornington Peninsula cases attributed to environmental factors |
| Johnson et al. [21] | 2022 | Benin, West Africa (BLMS4BU trial) | 186 | No | HIV/TB | Rifampicin + Clarithromycin for 8 weeks OR Amoxi/clav for 4 weeks | Ongoing trial, potential for shortened 4-week triple therapy course | Study suggests bactericidal activity prevents spread |
| Vandelannoote et al. [22] | 2023 | Mornington Peninsula, Australia | 60 | Yes (4 cases) | Hypertension, GORD | Rifampicin + Clarithromycin | Full resolution with combined medical and surgical management | Possum feces identified as a potential environmental source |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuomo, R.; Seth, I.; Marcaccini, G.; Lu, P.Y.J.; Rozen, W.M.; O’Brien, D.P. Atypical Mycobacterium ulcerans Infection with Skip Lesions in a 68-Year-Old Male: A Rare Case and Comprehensive Literature Review. J. Clin. Med. 2025, 14, 3853. https://doi.org/10.3390/jcm14113853
Cuomo R, Seth I, Marcaccini G, Lu PYJ, Rozen WM, O’Brien DP. Atypical Mycobacterium ulcerans Infection with Skip Lesions in a 68-Year-Old Male: A Rare Case and Comprehensive Literature Review. Journal of Clinical Medicine. 2025; 14(11):3853. https://doi.org/10.3390/jcm14113853
Chicago/Turabian StyleCuomo, Roberto, Ishith Seth, Gianluca Marcaccini, Phil Y. J. Lu, Warren M. Rozen, and Daniel P. O’Brien. 2025. "Atypical Mycobacterium ulcerans Infection with Skip Lesions in a 68-Year-Old Male: A Rare Case and Comprehensive Literature Review" Journal of Clinical Medicine 14, no. 11: 3853. https://doi.org/10.3390/jcm14113853
APA StyleCuomo, R., Seth, I., Marcaccini, G., Lu, P. Y. J., Rozen, W. M., & O’Brien, D. P. (2025). Atypical Mycobacterium ulcerans Infection with Skip Lesions in a 68-Year-Old Male: A Rare Case and Comprehensive Literature Review. Journal of Clinical Medicine, 14(11), 3853. https://doi.org/10.3390/jcm14113853









