Abstract
Radical chemoradiotherapy has been used as a frontline treatment for squamous cell cancer of the anus for the last 30–40 years. Considerable acute and chronic adverse effects have been observed following radiotherapy using 2D and 3D techniques. A case of very late-onset severe chronic toxicity in a patient 26 years after radiotherapy is presented. The patient underwent radical chemoradiotherapy for squamous anal cancer stage T3N3M0 in 1998. In the anal region, cumulative doses up to 77.6 Gy (including electron boost) were administered. Durable complete regression of the disease was achieved. Fourteen years after treatment, the patient developed vast fibroatrophy of the anus and perineum, progressing within the subsequent four years to necrosis and sphincter loss. Twenty years after treatment, the asymptomatic osteonecrotic foci in the left femur appeared on MRI scans. Despite two courses of hyperbaric oxygen treatment, the fibroatrophy and subsequent necrosis of soft tissues remained progressive, but the osteonecrosis was stable. Twenty-six years after treatment, the progressive changes induced symptomatic osteomyelitis of the ischium and pubic bone. The patient now requires permanent supportive treatment. The presented case is exceptional in the very late-onset typical chronic adverse effects developing after non-conformal radiotherapy administered at high doses as part of contemporary treatment protocols. There is little evidence regarding the late onset of chronic adverse effects, since the follow-up period is usually shorter than that of the case presented. Moreover, a significant portion of patients do not survive to reach the late-onset period of adverse effects. The presented case shows that there may be long-term survivors of anal cancer in the population who were treated with outdated techniques and who still carry a risk of late-onset severe, progressive adverse effects.
1. Introduction
Chemoradiotherapy became a standard first-line treatment for anal cancer due to its ability to preserve the anal sphincter and favourable local control data obtained in phase III trials [1,2,3].
There is a sufficient body of data on acute toxicity coming from clinical trials; however, data on late toxicity are scarce due to later onset of complications frequently exceeding the length of follow-up periods in trials. Incidence data are available for gastrointestinal late effects—7.0–64.5% [4,5,6,7,8]. There are few data indicating the incidence of radiation dermatitis and fibrosis at 8.8–9.2% [8,9]. The incidence of more advanced effects—radiation-induced fibroatrophy (RIF) and necrosis—remains unknown. Hip osteoradionecrosis (ORN) has been identified as a frequent complication of older radiotherapy techniques used to treat pelvic cancer, and anal cancer has contributed up to 14.8% of cases [10].
RIF and ORN are considered significant late adverse effects with substantial functional consequences. Their pathophysiology is described in detail elsewhere [11,12,13]. The usual onset period is from 6 months to 4–5 years after radiotherapy [10,14]. Onset periods longer than 10 years after radiotherapy of anal cancer have not been referred. It is important to note that the median observation period in the referred groups is shorter than 10 years. A large population-based study (1985–2000) revealed a 5-year survival rate of 51.8% [15]. However, references coming from later periods and smaller cohorts indicate more favourable 5-year survival rates of 68.9–83.6% [16,17].
Dosimetry—total and integral doses of radiation in healthy tissues are highly predictive of late toxicity. Intensity-modulated radiotherapy (IMRT) trials have shown significant decreases in the incidence of any late toxicity compared to older 2D and 3D techniques [18,19]. Two retrospective studies document a significant decrease in late toxicity in patients who received a dose lower than 60 Gy [20,21]. Significant reductions in integral doses have been documented for particle therapy [22,23], and a reduction in late toxicity may be expected; however, a representative analysis is not yet available.
There are limited treatment options for both RIF and ORN. A modest antifibrotic effect has been revealed for anti-inflammatory agents and pentoxifylline with antioxidant vitamin E [24,25]. Other agents—TGF-b1 inhibitors, tyrosine kinase inhibitors, and PI3K/AKT/mTOR inhibitors—are being investigated in preclinical and clinical trials [26,27,28,29,30,31]. Hyperbaric oxygen has shown improvements in physical functions and general health status in nine patients with radiation-induced anal fibrosis and/or ulceration [32,33]. Prevention—via a reduction of integral doses administered to skin, soft tissues, and bones—remains the best and most effective way to cope with adverse effects.
A single case of very late-onset, slowly progressive RIF and ORN with considerable functional consequences is presented.
2. Case History
The patient, a 24-year-old male, was diagnosed with anal canal cancer stage T3N3M0 (UICC 7th edition) in 1998. There was an exophytic voluminous tumour at the ventral part of the anal verge, invading the perineum, sized 6–7 cm in longitudinal direction. The inguinal nodes were bilaterally enlarged, sized up to 20 mm, classified as metastatic. Biopsy of the primary lesion confirmed squamous cell cancer coded M8070/3. Pelvic and abdominal CT scans excluded any other metastatic lesions. MR imaging was not available at the time of diagnosis.
Radical chemoradiotherapy was indicated as part of the contemporary protocol at the Institute of radiation oncology, Prague, CZ. Two-dimensional planning was based on CT imaging. Two-dimensional treatment was administered at linear accelerators: X-rays 5 MeV, electrons 15 MeV (Orion 5, Saturne 18, CGR MeV, F-78530 Buc, CGR MeV, Paris, France). Doses in organs at risk were not separately assessed.
Treatment volume consisted of the following three shrinking CTVs.
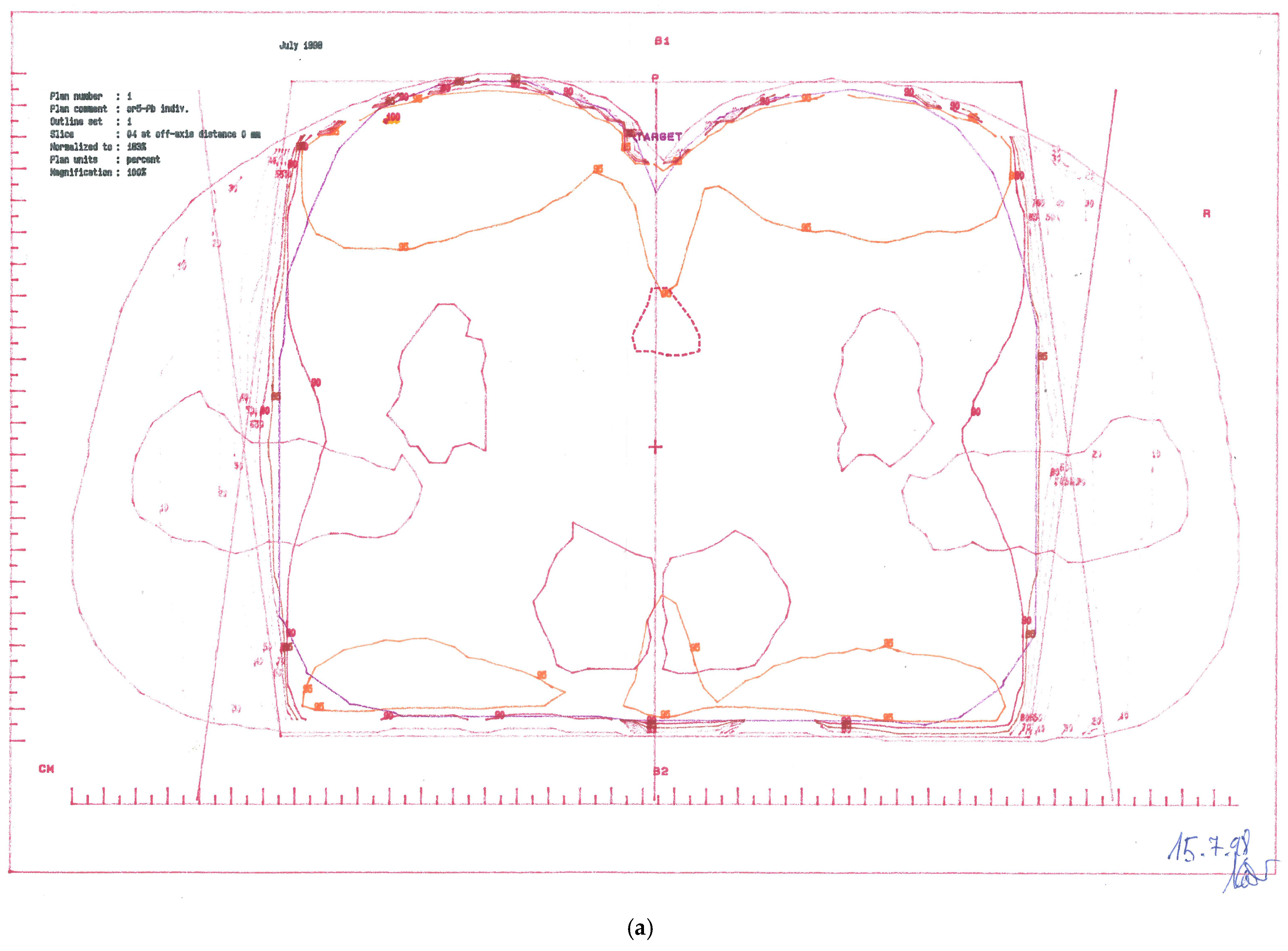
CTV-1: Primary tumour and perianal skin, whole pelvis, and groins—cumulative dose 57.6 Gy; (Figure 1a)
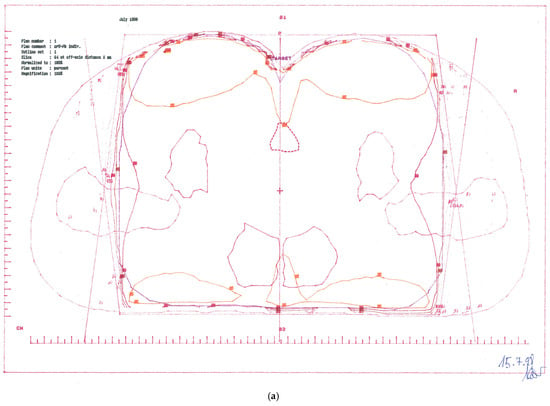
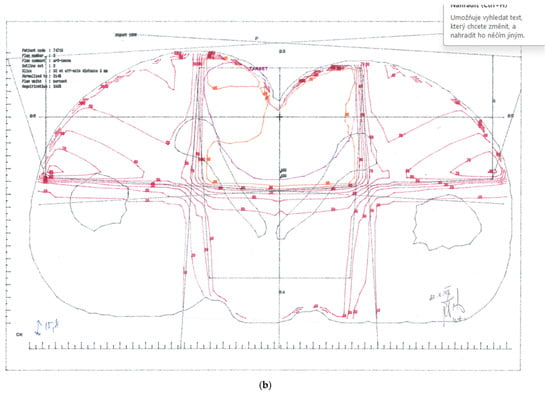
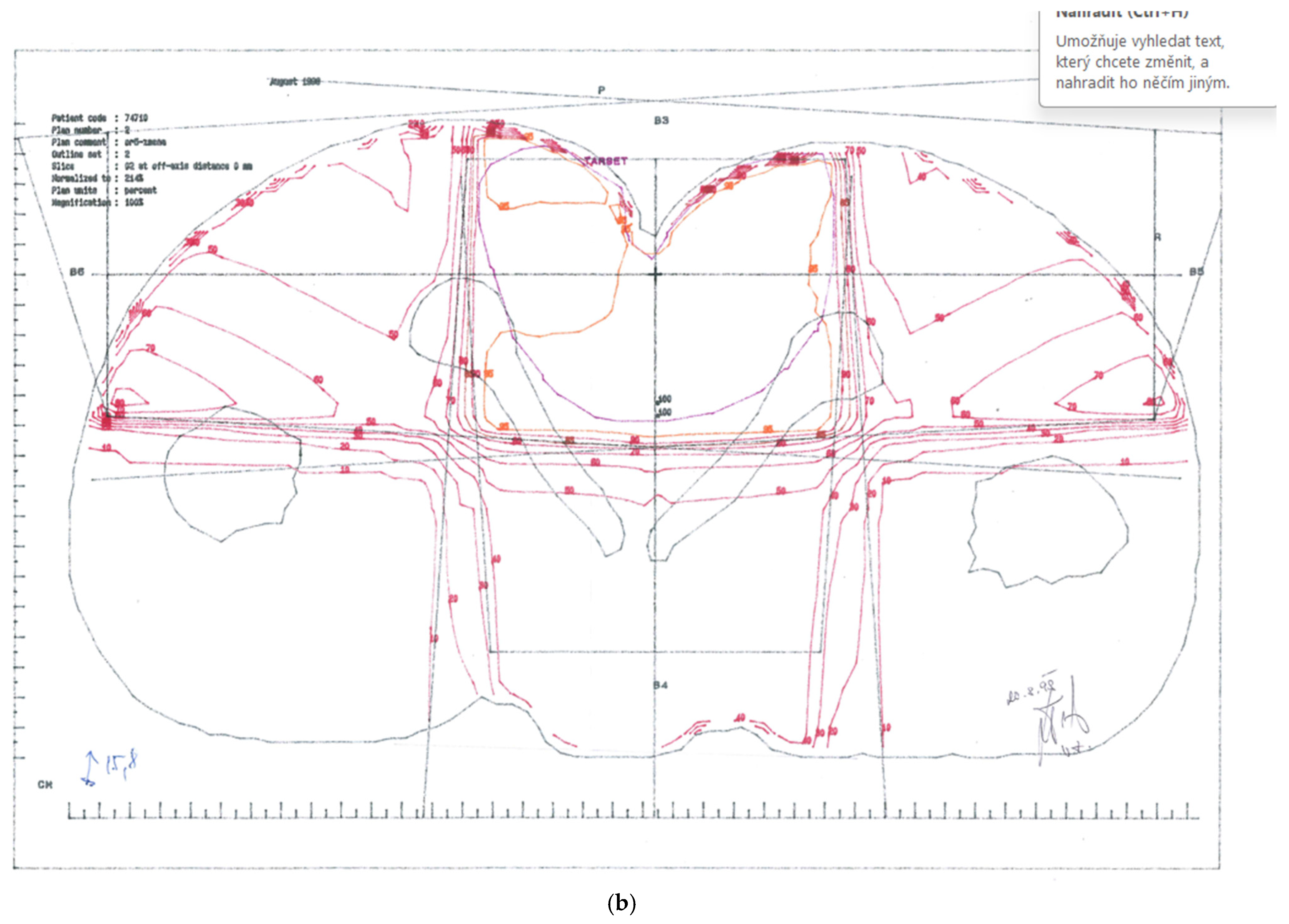
Figure 1.
Treatment plans. (a) CTV-1 encompassing anus, perianal skin, whole pelvis, and groins (step 1). (b) CTV-2 encompassing anus, perianal skin, and dorsal pelvis (step 2).
CTV-2: Primary tumour and perianal skin, as well as dorsal pelvis—cumulative dose 67.6 Gy; (Figure 1b)
CTV-3: Primary tumour and perianal skin—cumulative dose 77.6 Gy.
A rather complicated sequential treatment regimen was planned in three steps using three different techniques, as shown in Table 1. The treatment period lasted from 15-JUL-1998 to 03-SEP-1998, with a total treatment time of 51 days (34 treatment days).

Table 1.
Volumes and dosage.
Concomitant chemotherapy was administered within the contemporary treatment protocol below.
- -
- Mitomycin C 20 mg, intravenous injection, day 1;
- -
- 5-fluoruracil 750 mg/24 h, continuous intravenous infusion, days 1–4 and 29–32.
The treatment was administered according to the prescribed plan without any interruptions. Acute toxicity was mild, not exceeding grade 2.
The effect was assessed by means of digital rectal examination (DRE) 2 months after radiotherapy. Residual induration remained on the ventral side of the anus. Fine-needle aspiration biopsy showed fibroproductive inflammatory changes, but no residual cancer cells. A CT scan 2 months after radiotherapy confirmed complete regression of the primary tumour, no regional lymphadenopathy, and no distant metastases.
One year after radiotherapy, DRE showed only small fibrotic foci about 2 cm in size on the ventral side of the anal verge. A CT scan showed no pathologic changes in the pelvis and no lymphadenopathy. There were no functional changes. The sphincter was fully functional, and defecation reflexes were fully restored.
The patient attended follow-up examinations—DRE annually and CT scan biannually for the next 11 years. There were no signs of late toxicity within 11 consecutive years after radiotherapy. Good sphincter function was maintained, and there was no radiation proctitis, no perianal fibrosis, and no skeletal abnormalities within the pelvic and femoral bones. The patient’s condition was good, and he was not limited in his daily activities.
Twelve years after radiotherapy, the patient presented with progressive perianal induration, sphincter dysfunction, and temporary limb swelling. A CT scan still showed no relapse and no lymphadenopathy.
Fourteen years after radiotherapy, the patient suffered from a subjective symptom of “painless disruption” at the perineum. From a basic physical exam, there was apparent induration of the perianal region and anal sphincter, while the rugged surface of the perianal skin resembled local relapse (an appearance very similar to skin cancer). The patient underwent multifocal biopsy of the skin; however, it did not reveal any signs of cancer, only signs of advanced skin and subdermal fibrosis. An MRI scan showed signs of perianal and periproctal fibrosis. This finding did not change for 4 years.
Eighteen years after radiotherapy, deep excavation at the site of the perineum appeared, with a rugged bottom again resembling tumour relapse. Multiple biopsies again indicated fibrosis and no cancer cells.
Nineteen years after radiotherapy, MRI had become available at the follow-up facility and the patient underwent periodic imaging. Twenty years after radiotherapy, MRI showed new skeletal changes—osteonecrosis of the right femoral head, right acetabulum, and femoral diaphysis. However, there were no subjective symptoms. Progressive sphincter dysfunction became disturbing and resulted in colostomy.
Twenty years after radiotherapy, the patient commenced hyperbaric oxygen (HBO) therapy in a hyperbaric chamber, at a pressure of 1.5 bar, breathing 100% oxygen through a mask. The patient underwent three consecutive cycles of HBO within 10 months, each cycle containing 20 sessions of 90 min, with the cycles lasting 80 days, 120 days, and 90 days. The total treatment time was 10 months.
HBO therapy had no impact on the local lesion, and the patient did not report any functional improvements.
Excavation at the site of the perineum slowly increased in size up to the 23rd year after radiation, and it completely depleted the sphincter. Then it remained stable for the next 3 years. The patient did not receive any specific therapy except for temporary antibiotic retreatment to resolve remittent wound infections in the area of fibroatrophy and necrosis.
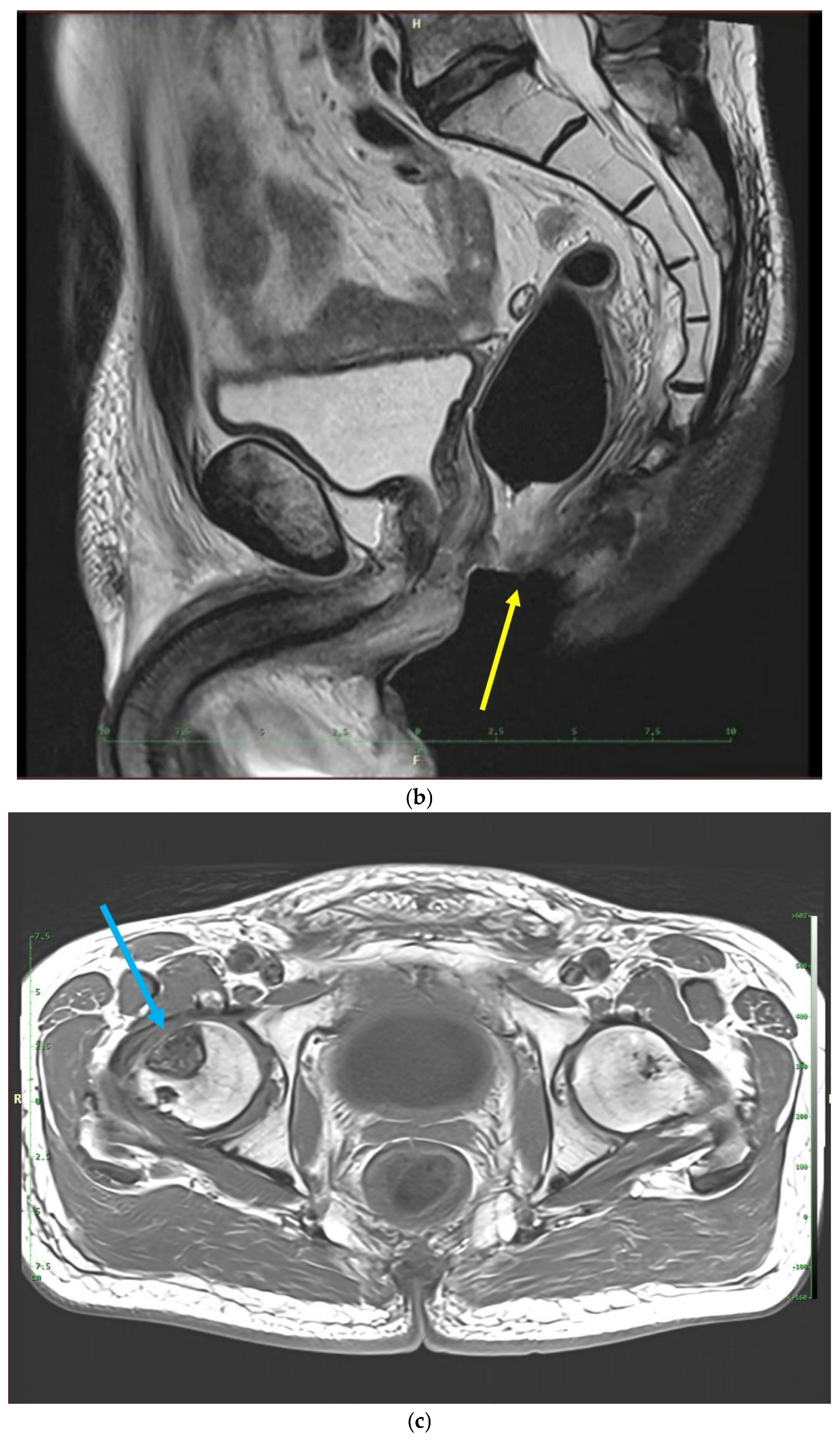
Recently, inflammatory reactions adjacent to the necrosis have slowly progressed to the lower arm of the left pubic and ischium bone and have induced mild osteomyelitis demanding antibiotic and analgesic treatment (Figure 2 and Figure 3). The osteonecrotic focus within the right femoral head and acetabulum is now stable, asymptomatic, and painless.
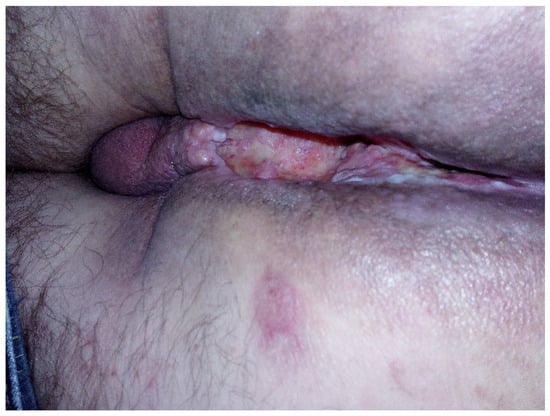
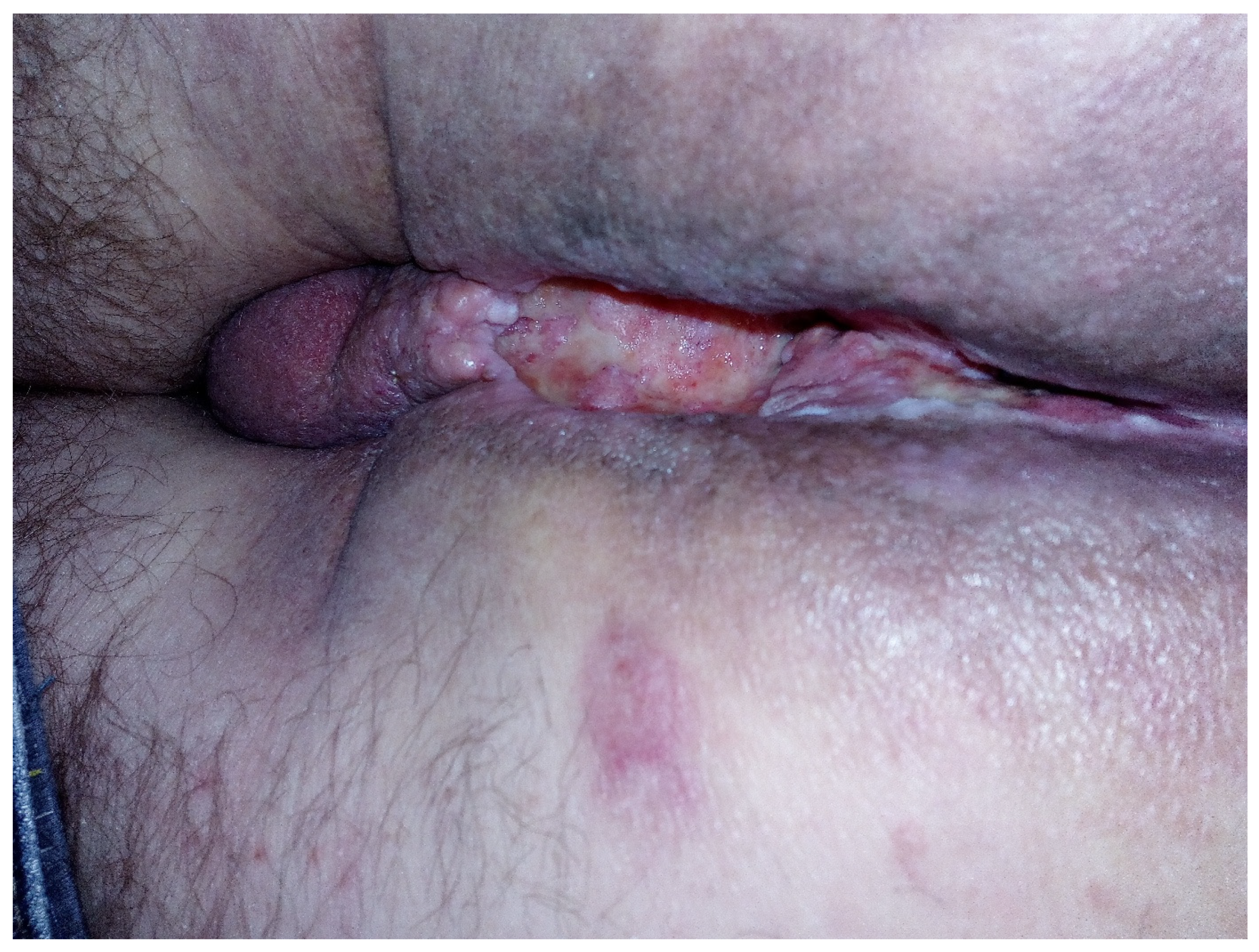
Figure 2.
Perineal excavation involving the region of the anal canal.
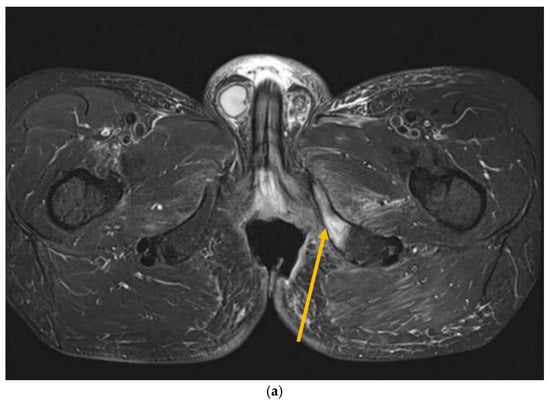
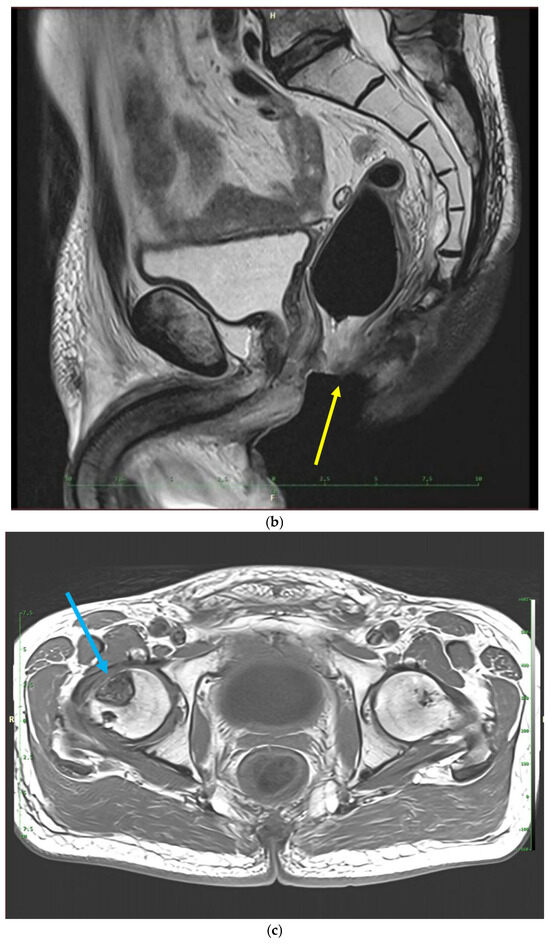
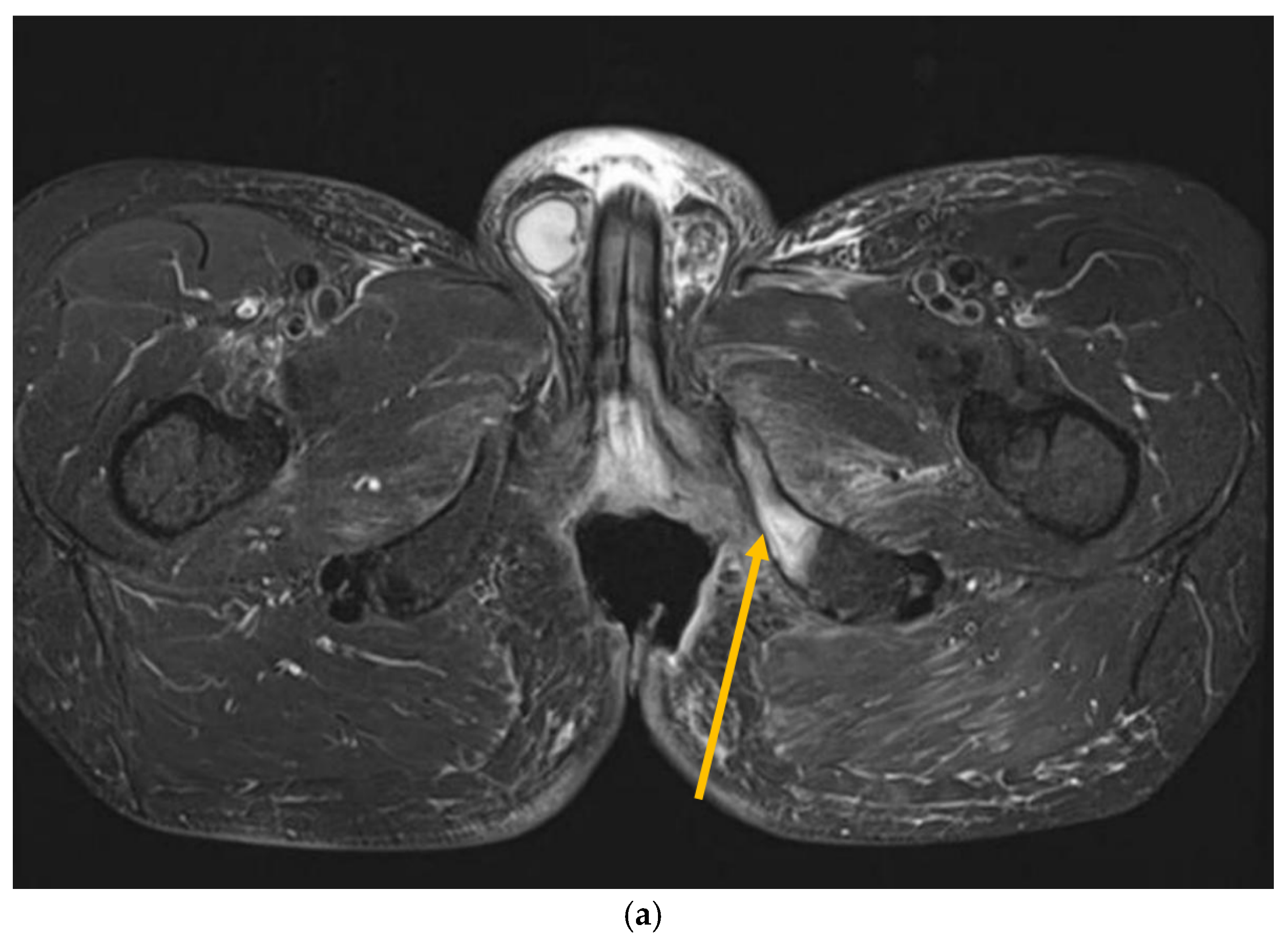
Figure 3.
MRI scans 26 years after radiotherapy: T1-weighted axial MRI scan shows vast perineal and anal defects progressing to the left lower arm of the pubis with signs of induced osteomyelitis (orange arrow) (a); T2-weighted sagittal scan shows vast perineal and anal defects including absence of anal sphincter (yellow arrow) (b); and T1-tse weighted axial scans show the osteoradionecrosis of the right hip (blue arrow) (c).
The patient is now 26 years post-radiotherapy. He is naturally limited by his irreversible colostomy and weak mucinous rectal secretions, which are unpredictably released as a result of the absent sphincter. The recently developed osteomyelitis is a substantial complication limiting the patient in his job—he is a driver of agriculture machines. The patient experiences negligible limitations resulting from the osteoradionecrosis of his right hip.
3. Discussion
Chronic adverse effects of radiotherapy including radiation-induced fibroatrophy (RIF) and osteoradionecrosis (ORN) may be expected and have been consistently described [10,14,34]. RIF after radiotherapy for anal cancer may proceed to necrosis and fistulation, followed by various functional consequences. Sphincter dysfunction and faecal incontinence have been predominantly described [8,35,36,37]. Various fistulations have been reported in 0.7–22% of cases and faecal incontinence in 0.5–10.9% [38,39]. Abdominoperineal resection, or at least derivation colostomy, follows frequently. The colostomy rate resulting from chronic adverse effects is estimated at 1.5–12% [7,20,40,41,42] (Table 2). The colostomy rate resulting from local relapse (either as part of salvage surgery—abdominoperineal resection—or as a separate derivation colostomy only) is about 3 times higher than that resulting from toxicity, i.e., 9–22% vs. 0–6.3% [7,20,40,41,42,43,44] (Table 2).

Table 2.
Late effects and colostomy results described in selected studies on anal cancer.
The onset of fibrosis and its sequelae is usually described much earlier than in this case, some 4–12 months after radiotherapy [43,45]. However, the median follow-up times in the referred anal cancer patient groups (22–100 months) may not be long enough to provide reproducible information on late- or very late-onset chronic toxicity [7,20,40,41,42] (Table 2).
In general, there are only a few references describing very late-onset chronic adverse effects. Different localizations, fractionation schedules, treatment volumes, and organs are concerned [46]. Johansson et al. presented long observation periods after chest wall radiotherapy for breast cancer, up to 30 years [47]. RF was shown to develop in the first decade, not later. Other effects like brachial plexus neuropathy and paralysis were observed later, even after 30 years. Moreover, there is the question of applicability. Any observation study presented 20 years or more after the treatment period is naturally based on rather obsolete techniques of radiotherapy and obsolete fractionation regimens. It is questionable, then, to what degree the results are applicable to current treatments beyond simple awareness of particular adverse effects.
The median period to ORN onset is less than 4 years after radiotherapy [10]. However, very long periods have also been observed for jaw ORN—up to 38 and 45 years after radiotherapy [48,49]. ORN has more frequently been described in relation to older techniques of radiotherapy predominantly involving the jaw. The hip presents as the second most frequent region at risk of ORN. Again, it is related to extensive irradiation with outdated techniques used for pelvic radiation, e.g., the “box” technique. Anal cancer radiotherapy comprised 14.8% of hip ORN cases in a survey covering the period of 1980–2020 [10].
The presented case shows a very late onset of two chronic adverse effects: skin and sphincter fibroatrophy/necrosis and osteoradionecrosis. These effects are rather exceptional in terms of delayed onset. Fibrosis slowly proceeded to fibroatrophy and finally necrosis, with consequent anatomic and functional impairment. Single successive events that are relevant to specific stages of RIF have been described in detail by Delanian et al. [45]. The final effect has presented as continuously and slowly increasing vast tissue defects—complete sphincter disintegration and loss, both anatomic and functional in this case. In contrast to the observed fibroatrophy, the exceptionally delayed onset of unilateral hip ORN has remained stable and asymptomatic and has not developed any functional disturbances despite no consistent treatment except hyperbaric oxygen.
Both adverse effects are apparently related to the radiotherapy technique. A full dose was homogeneously administered to the entire pelvis via the “box” technique, while the prescribed dose was maximal in terms of contemporary recommendations and references [50,51]. Moreover, the electron boost covered the subcutaneous tissue and skin adjacent to the primary tumour in the range relevant to current tissue loss and disintegration. A lower dose administered to the pelvic bone (outside the volume of the 10 Gy boost dose) may have contributed to the different behaviour of soft tissue (continuously progressive fibroatrophy and necrosis) and bone (no progression of necrosis for the last 8 years).
The severe chronic adverse effects, at least the perineal and sphincter fibrosis, may have been expected if the total dose was considered for the combined hyper and normofractionation regimen. But the late onset of severe adverse effects was not predictable. However, there may be some bias considering what is the “usual” onset time of these adverse effects. Patient groups with a consistent follow-up regime and reproducible data present rather short median follow-up times (Table 1). Late-onset cases are referred to as “exceptional” since they are presumably usually excluded from references and observations. Moreover, for anal cancer, the 5-year survival rates are referred between 65% and 85% [42,52,53], and, although limited, the data for 10-year survival reveal a rate up to 75% [54]. A large population-based study (1985–2000) indicated the 5-year survival rate as only 51.8% [15]. Survival data may produce some bias in estimating the true risk of chronic adverse effects with a very late onset.
The treatment options for developed fibrosis and fibroatrophy are limited. Favourable results have been achieved with anti-inflammatory agents or pentoxifylline combined with the antioxidant tocopherol [24,25]. Overall, the effects are classified as “modest”. However, the treatment was not prescribed in the referred case. Other agents—TGF-b1 inhibitors, tyrosine kinase inhibitors, and PI3K/AKT/mTOR inhibitors—are being investigated in preclinical and clinical trials; treatment is not available for a routine practice. HBO is another available option [32,33,55]. Its efficacy has been assessed predominantly in terms of improvements in functional deficits, not observed in the referred case. HBO remained without significant effects. However, in the stage of developed fibroatrophy and necrosis, any significant improvement can hardly be expected with any method, and treatment can only be employed with the aim to preclude further progression. Sphincter loss and functional impairment can be compensated only by colostomy with or without abdominoperineal resection. Any functional restoration can also be hardly expected. There is a more effective option for osteoradionecrosis compensation using total hip joint endoprosthesis. However, the asymptomatic course and lack of progression still allow us to postpone surgery in this case.
Finally, the presented case stresses the importance of prevention—more favourable dosimetry. It confirms the significance of reasonable dosage and the use of more advanced technologies, such as high-voltage radiation (IMRT, VMAT) or particle therapy, to achieve higher conformity and lower doses outside the target volume. Looking to the future, fewer chronic adverse effects may be expected, including late-onset effects. However, there are still plenty of long-term survivors undergoing follow-up, who were treated some decades ago by obsolete techniques and with higher doses, the distribution of which may not be reproducible. These patients carry a “heritage” of unfavourable dosimetry and remain at risk of late-onset severe adverse effects.
4. Conclusions
The late adverse effects of radiotherapy may keep developing even decades after treatment within the framework of a very complicated pathophysiology, finally resulting in substantial functional and anatomic impairment. The treatment options are very limited. The risk of these adverse effects should be considered in long-term survivors after radiotherapy administered with outdated techniques and unfavourable dosimetry. Mutilating complications are demonstrated in the presented case of anal canal cancer; nevertheless, survivors of various other malignancies may also be similarly affected.
Author Contributions
Conceptualization P.V.; methodology P.V., resources P.V. and J.K., writing—original draft preparation P.V. and J.K., writing review and editing B.O. and A.H., visualization B.O. and A.H., supervision P.V. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Proton Therapy Center Czech (protocol nr. PTC_VR_54/2025 of 17 April 2025).
Informed Consent Statement
Informed consent was obtained from the subject involved in the case report. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript.
| CT | Computer tomography |
| CTV | Clinical target volume |
| DRE | Digital rectal examination |
| HBO | Hyperbaric oxygen |
| IMRT | Intensity-modulated radiation therapy |
| ORN | Osteoradionecrosis |
| MRI | Magnetic resonance imaging |
| RIF | Radiation-induced fibroatrophy |
| VMAT | Volumetric modulated arch therapy |
References
- Northover, J.; Glynne-Jones, R.; Sebag-Montefiore, D.; James, R.; Meadows, H.; Wan, S.; Jitlal, M.; Ledermann, J. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br. J. Cancer 2010, 102, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 2008, 299, 1914. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Pajak, T.; Flam, M.; Hoffman, J.; Markoe, A.; Wolkov, H.; Paris, K. Dose escalation in chemoradiation for anal cancer: Preliminary results of RTOG 92-08. Cancer J. Sci. Am. 1996, 2, 205–211. [Google Scholar]
- Lestrade, L.; De Bari, B.; Montbarbon, X.; Pommier, P.; Carrie, C. Radiochemotherapy and brachytherapy could be the standard treatment for anal canal cancer in elderly patients? A retrospective single-centre analysis. Med. Oncol. 2013, 30, 402. [Google Scholar] [CrossRef]
- Ferrigno, R.; Nakamura, R.A.; Novaes, P.E.R.D.S.; Pellizzon, A.C.A.; Maia, M.A.C.; Fogarolli, R.C.; Salvajoli, J.V.; Filho, W.J.D.; Lopes, A. Radiochemotherapy in the conservative treatment of anal canal carcinoma: Retrospective analysis of results and radiation dose effectiveness. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1136–1142. [Google Scholar] [CrossRef]
- Mai, S.K.; Welzel, G.; Hermann, B.; Bohrer, M.; Wenz, F. Long-term outcome after combined radiochemotherapy for anal cancer—Retrospective analysis of efficacy, prognostic factors, and toxicity. Onkologie 2008, 31, 251–257. [Google Scholar] [CrossRef]
- De Bari, B.; Lestrade, L.; Pommier, P.; Maddalo, M.; Buglione, M.; Magrini, S.M.; Carrie, C. Could concomitant radio-chemotherapy improve the outcomes of early-stage node negative anal canal cancer patients? A retrospective analysis of 122 patients. Cancer Investig. 2015, 33, 114–120. [Google Scholar] [CrossRef]
- Pan, Y.B.; Maeda, Y.; Wilson, A.; Glynne-Jones, R.; Vaizey, C.J. Late gastrointestinal toxicity after radiotherapy for anal cancer: A systematic literature review. Acta Oncol. 2018, 57, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Mikkelsen, A.H.; Kronborg, C.; Oggesen, B.T.; Faaborg, P.M.; Serup-Hansen, E.; Spindler, K.-L.G.; Christensen, P. Management of late adverse effects after chemoradiation for anal cancer. Acta Oncol. 2021, 60, 1688–1701. [Google Scholar] [CrossRef]
- Xu, S.H.; Tang, J.S.; Shen, X.Y.; Niu, Z.X.; Xiao, J.L. Osteoradionecrosis of the Hip, a Troublesome Complication of Radiation Therapy: Case Series and Systematic Review. Front. Med. 2022, 9, 858929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Jereczek-Fossa, B.A.; Orecchia, R. Radiotherapy-induced mandibular bone complications. Cancer Treat. Rev. 2002, 28, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, D.B.; Imboden, J.B. Update in rheumatology: Evidence published in 2014. Ann. Intern. Med. 2015, 162, W122-6. [Google Scholar] [CrossRef] [PubMed]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bilimoria, K.Y.; Bentrem, D.J.; Rock, C.E.; Stewart, A.K.; Ko, C.Y.; Halverson, A. Outcomes and prognostic factors for squamous-cell carcinoma of the anal canal: Analysis of patients from the National Cancer Data Base. Dis. Colon Rectum 2009, 52, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Ghareeb, A.E.; Jha, A.; Van der Voet, H.; Garg, D.; Jha, M. Anal cancer survival: A socioeconomic analysis. Ann. R. Coll. Surg. Engl. 2021, 103, 191–196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martin, D.; Rödel, C.; Fokas, E. Chemoradiotherapy for anal cancer: Are we as good as we think? Strahlenther. Onkologie 2019, 195, 369–373. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.A.; Winter, K.; Myerson, R.J.; Goodyear, M.D.; Willins, J.; Esthappan, J.; Haddock, M.G.; Rotman, M.; Parikh, P.J.; Safran, H.; et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 27–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, M.; Ho, H.; Skelton, J.; Guerrieri, M.; Guiney, M.; Chao, M.; Blakey, D.; Macleod, C.; Amor, H.; Subramanian, B.; et al. Intensity-modulated Radiotherapy for Anal Cancer: Dose-Volume Relationship of Acute Gastrointestinal Toxicity and Disease Outcomes. Clin. Oncol. 2018, 30, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Ortholan, C.; Ramaioli, A.; Peiffert, D.; Lusinchi, A.; Romestaing, P.; Chauveinc, L.; Touboul, E.; Peignaux, K.; Bruna, A.; de La Roche, G.; et al. Anal canal carcinoma: Early-stage tumors < or =10 mm (T1 or Tis): Therapeutic options and original pattern of local failure after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Engineer, R.; Mallik, S.; Mahantshetty, U.; Shrivastava, S. Impact of radiation dose on locoregional control and survival on squamous cell carcinoma of anal canal. Radiother. Oncol. 2010, 95, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Wo, J.Y.; Plastaras, J.P.; Metz, J.M.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Adams, J.; Baglini, C.; Ryan, D.P.; Murphy, J.E.; et al. Pencil Beam Scanning Proton Beam Chemoradiation Therapy with 5-Fluorouracil and Mitomycin-C for Definitive Treatment of Carcinoma of the Anal Canal: A Multi-institutional Pilot Feasibility Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ojerholm, E.; Kirk, M.L.; Thompson, R.F.; Zhai, H.; Metz, J.M.; Both, S.; Ben-Josef, E.; Plastaras, J.P. Pencil-beam scanning proton therapy for anal cancer: A dosimetric comparison with intensity-modulated radiotherapy. Acta Oncol. 2015, 54, 1209–1217. [Google Scholar] [CrossRef]
- Dittmann, K.H.; Mayer, C.; Ohneseit, P.A.; Raju, U.; Andratschke, N.H.; Milas, L.; Rodemann, H.P. Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: A COX-2 independent mechanism. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 203–212. [Google Scholar] [CrossRef]
- Delanian, S. Striking regression of radiation-induced fibrosis by a combination of pentoxifylline and tocopherol. Br. J. Radiol. 1998, 71, 892–894. [Google Scholar] [CrossRef]
- Flechsig, P.; Dadrich, M.; Bickelhaupt, S.; Jenne, J.; Hauser, K.; Timke, C.; Peschke, P.; Hahn, E.W.; Gröne, H.-J.; Yingling, J.; et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin. Cancer Res. 2012, 18, 3616–3627. [Google Scholar] [CrossRef]
- Bueno, L.; de Alwis, D.P.; Pitou, C.; Yingling, J.; Lahn, M.; Glatt, S.; Trocóniz, I.F. Semi-mechanistic modelling of the tumour growth inhibitory ef fects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur. J. Cancer 2008, 44, 142–150. [Google Scholar] [CrossRef]
- Bonner, J.C. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004, 15, 255–273. [Google Scholar] [CrossRef]
- Nishioka, Y.; Azuma, M.; Kishi, M.; Aono, Y. Targeting platelet-derived growth factor as a therapeutic approach in pulmonary fibrosis. J. Med. Investig. 2013, 60, 175–183. [Google Scholar] [CrossRef]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef]
- Eke, I.; Makinde, A.Y.; Aryankalayil, M.J.; Sandfort, V.; Palayoor, S.T.; Rath, B.H.; Liotta, L.; Pierobon, M.; Petricoin, E.F.; Brown, M.F.; et al. Exploiting radiation-induced signaling to increase the susceptibility of resistant Cancer cells to targeted drugs: AKT and mTOR inhibitors as an ex ample. Mol. Cancer Ther. 2018, 17, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Vera-Rosas, A.; Aguiar, D.; Domínguez, A.; Cabrera-Vicente, A.; Martín-Barrientos, P.; Cabrera, R.; Salas-Salas, B.G.; Ferrera-Alayón, L.; Ribeiro, I.; Chicas-Sett, R.; et al. Prospective Pilot study of Quality of Life in patients with severe late-radiation-toxicity treated by Low hyperbaric-oxygen-therapy. Clin. Transl. Radiat. Oncol. 2023, 40, 100620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bennett, M.H.; Feldmeier, J.; Hampson, N.B.; Smee, R.; Milross, C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst. Rev. 2016, 4, Cd005005. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wei, J.; Meng, L.; Wang, H.; Qu, C.; Chen, X.; Xin, Y.; Jiang, X. Advances in pathogenic mechanisms and management of radiation-induced fibrosis. Biomed. Pharmacother. 2020, 121, 109560. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.A.; Raus, I.; Jung, K.; Schüler, P.; Herrmann, M.K.; Hennies, S.; Vorwerk, H.; Hille, A.; Hess, C.F.; Christiansen, H. High-grade acute organ toxicity as a positive prognostic factor in primary radiochemotherapy for anal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1467–1478. [Google Scholar] [CrossRef]
- Oblak, I.; Petric, P.; Anderluh, F.; Velenik, V.; Fras, P. Long term outcome after combined modality treatment for anal cancer. Radiol. Oncol. 2012, 46, 145–152. [Google Scholar] [CrossRef][Green Version]
- Eng, C.; Chang, G.J.; You, Y.N.; Das, P.; Xing, Y.; Delclos, M.; Wolff, R.A.; Rodriguez-Bigas, M.A.; Skibber, J.; Ohinata, A.; et al. Long-term results of weekly/daily cisplatin-based chemoradiation for locally advanced squamous cell carcinoma of the anal canal. Cancer 2013, 119, 3769–3775. [Google Scholar] [CrossRef]
- Wexler, A.; Berson, A.M.; Goldstone, S.E.; Waltzman, R.; Penzer, J.; Maisonet, O.G.; McDermott, B.; Rescigno, J. Invasive anal squamous-cell carcinoma in the HIV-positive patient: Outcome in the era of highly active antiretroviral therapy. Dis. Colon Rectum. 2008, 51, 73–81. [Google Scholar] [CrossRef]
- Fraunholz, I.; Weiss, C.; Eberlein, K.; Haberl, A.; Rödel, C. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1425–1432. [Google Scholar] [CrossRef]
- Peiffert, D.; Tournier-Rangeard, L.; Gérard, J.P.; Lemanski, C.; François, E.; Giovannini, M.; Cvitkovic, F.; Mirabel, X.; Bouché, O.; Luporsi, E.; et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: Final analysis of the randomized UNICANCER ACCORD 03 trial. J. Clin. Oncol. 2012, 30, 1941–1948, Erratum in J. Clin. Oncol. 2012, 30, 3903. [Google Scholar] [CrossRef] [PubMed]
- Young, S.C.; Solomon, M.J.; Hruby, G.; Frizelle, F.A. Review of 120 anal cancer patients. Color. Dis. 2009, 11, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Axelsson, A.; Haglind, E.; Bock, D.; Angenete, E. Long-term survival after treatment for primary anal cancer- results from the Swedish national ANCA cohort study. Acta Oncol. 2022, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.P.; Ayzac, L.; Hun, D.; Romestaing, P.; Coquard, R.; Ardiet, J.M.; Mornex, F. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother. Oncol. 1998, 46, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Madhu, J.; Marshall, F.; Palma, N. Ten-year results of chemoradiation for anal cancer: Focus on late morbidity. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 65–69. [Google Scholar]
- Delanian, S.; Lefaix, J.L. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131. [Google Scholar] [CrossRef] [PubMed]
- van Geel, A.N.; Lans, T.E.; Haen, R.; Tjong Joe Wai, R.; Menke-Pluijmers, M.B. Partial mastectomy and m. latissimus dorsi reconstruction for radiation-induced fibrosis after breast-conservin cancer therapy World. J. Surg. 2011, 355, 568–572. [Google Scholar]
- Johansson, S.; Svensson, H.; Denekamp, J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulos, A.; Zarra, T.; Ehrenfeld, M.; Otto, S. Osteoradionecrosis of the jaws: Definition, epidemiology, staging and clinical and radiological findings. A concise review. Int. Dent. J. 2018, 68, 22–30. (In English) [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musbah, T.M.; Mupparapu, M. Diagnosis and management of late onset osteoradionecrosis of the mandible. J. Orofac. Sci. 2013, 5, 71–73. [Google Scholar] [CrossRef]
- Eschwege, F.; Lasser, P.; Chavy, A.; Wibault, P.; Kac, J.; Rougier, P.; Bognel, C. Squamous cell carcinoma of the anal canal: Treatment by external beam irradiation. Radiother. Oncol. 1985, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Papillon, J.; Montbarbon, J.F. Epidermoid carcinoma of the anal canal. A series of 276 cases. Dis. Colon Rectum. 1987, 30, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Slørdahl, K.S.; Klotz, D.; Olsen, J.Å.; Skovlund, E.; Undseth, C.; Abildgaard, H.L.; Brændengen, M.; Nesbakken, A.; Larsen, S.G.; Hanekamp, B.A.; et al. Treatment outcomes and prognostic factors after chemoradiotherapy for anal cancer. Acta Oncol. 2021, 60, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Leon, O.; Guren, M.; Hagberg, O.; Glimelius, B.; Dahl, O.; Havsteen, H.; Naucler, G.; Svensson, C.; Tveit, K.M.; Jakobsen, A.; et al. Anal carcinoma—Survival and recurrence in a large cohort of patients treated according to Nordic guidelines. Radiother. Oncol. 2014, 113, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.R.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Oncologic outcomes of squamous cell carcinoma of the anal canal after chemoradiation therapy. Korean J. Clin. Oncol. 2016, 12, 41–47, pISSN: 1738-8082, eISSN: 2288-4084. [Google Scholar] [CrossRef]
- Delanian, S.; Lefaix, J.L. Current management for late normal tissue injury: Radiation-induced fibrosis and necrosis. Semin. Radiat. Oncol. 2007, 17, 99–107. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).