Abstract
Introduction: Traumatic brain injury (TBI) remains a significant challenge in older polytrauma patients, with age being a major determinant of outcomes. While mortality predictors have been studied in general polytrauma populations, less is known about specific risk factors in older adults with TBI. Methods: This retrospective study analysed data from 304 polytrauma patients over 18 years of age treated at a Level 1 trauma centre between 2013 and 2023. Patients were divided into three age categories: 18–64 years (n = 189), 65–84 years (n = 92), and ≥85 years (n = 23). The analysis included demographics, injury patterns, clinical indicators, surgical treatments, and in-hospital mortality to identify key mortality predictors. Results: The mean age was 54.5 years (SD 22.2); 72% of patients were male. In-hospital mortality was 36.3% overall, increasing to 60.8% in patients aged ≥85. TBI severity was moderate in 25% and severe in 75% of cases. Older patients were less frequently admitted to the ICU and more often managed conservatively. ICU admission was significantly lower in patients aged 65–84 (24.5%) and ≥85 (19.4%) compared to the 18–64 group (70.0%). Multivariate analysis identified age, male sex, and severe TBI as significant predictors of 30-day mortality. Conclusions: TBI management in older polytrauma patients requires distinct approaches due to higher mortality and poorer outcomes. Age is a critical risk factor, highlighting the need for tailored triage systems and ICU strategies to improve care and prognosis in this vulnerable population.
1. Background
Elderly patients with traumatic brain injury (TBI) are at high risk of experiencing disproportionate rates of mortality, longer hospital stays, and significant long-term disabilities [1]. Additionally, this group frequently has comorbidities, such as heart disease, diabetes, and dementia, and often requires treatment with antithrombotic medications. These pre-existing conditions and associated treatments complicate the management of TBIs in this population [2]. Advanced age at the time of injury is linked to poorer functional outcomes following TBI, irrespective of the severity of the injury. Mortality rates from TBI also rise significantly with age, increasing from 71% in patients aged 65–70 to 87% in those over 80 years old [3]. Recent studies show a significant rise in polytrauma from low-height falls as patient age increases, particularly among older adults, reflecting the frailty of this specific patient group despite the low-energy nature of the trauma [4].
The timely detection of severe intracranial injuries requiring specific interventions is crucial for improving outcomes. Research shows that early surgical intervention combined with intensive rehabilitation can lead to better recovery in older patients with a TBI [5]. A key challenge in managing older polytrauma patients is their tendency to initially appear clinically stable, only to deteriorate rapidly during treatment due to reduced physiological reserves. Triage systems were primarily developed for younger trauma patients, particularly those in haemorrhagic shock, and may not adequately address the needs of older patients. Due to these challenges, older patients with TBIs have often been neglected in research, as many studies on TBIs have excluded them from their analysis [6].
The aim of this study was, therefore, to analyse data over a 10-year period to (a) describe the epidemiology of older adults (>65 years) presenting with a TBI compared with a younger cohort (<65 years) and (b) examine variables that may be predictive of short-term outcome.
2. Methods
Data for this study were sourced from the Vienna Trauma Register over a 10-year span, covering the period 1 January 2013–1 December 2023. Patients included in the study were aged ≥18 years and listed in the register with a confirmed TBI. Older patients were defined as polytraumatised individuals aged >65 years. Severe TBI was characterised by an Abbreviated Injury Scale (AIS) score of ≥3 in the head region combined with a Glasgow Coma Scale (GCS) score of ≤12. Patients with mild TBI and without any evidence of head or brain injury were excluded from the study. Polytrauma was defined as a minimal Injury Severity Score (ISS) of 16.
The Vienna Trauma Register captures detailed patient data, including age, gender, GCS score, ISS, incident location, type of transport (HEMS—helicopter emergency medical service, GEMS—ground emergency medical service), brain imaging findings, treatment, various laboratory results, discharge status, and survival outcomes. The mechanism of injury (road traffic accidents, low falls [from standing] or high falls and other types of injury) was specified. Neurosurgical procedures included extra or subdural haematoma, lobectomy, and/or decompressive craniectomy. GCS measurements are typically taken upon arrival at the emergency department unless the patient was intubated, in which case the GCS score recorded at the scene of the incident was used. The Glasgow Outcome Scale (GOS) score was retrospectively assessed using patient records and neurological evaluations at 90 days. The length of stay in the intensive care unit (ICU) and three- and six-month mortality rate were recorded. The protocol was approved by the medical ethical review committee of the Medical University of Vienna, Austria (protocol number 1961/2021).
The data sets were imported into SPSS Statistics (Thomas Reuters, IBM, Version 25), and the continuous variables were graphically tested for their distribution using histograms and normal distribution curves. The distributions and location measures of the above parameters are presented in tabular form for the three age groups (18–64 years, 65–84 years and ≥85 years). The chi-square test was used for categorical variables as part of the final statistics. The Mann–Whitney U test was used for non-normally distributed metric variables. To counteract alpha error accumulation, the Bonferroni correction was then performed. The resulting p-values are given together with the effect size r. Here, r values < 0.3 were interpreted as small effects, between 0.3 and 0.5 as medium effects, and values > 0.5 as strong effects. The variables with a p value < 0.20 were included in a multivariate model, and a backwards stepwise procedure was used. The odds ratios (ORs) and 95% confidence intervals (95% Cis) were calculated. The Hosmer–Lemeshow test was used to evaluate the goodness-of-fit of the model. Numerical variables were rounded to one decimal place, except for the effect size r (2 decimal places) and the p-values (3 decimal places). The significance level was assumed to be p = 0.05.
3. Results
3.1. Age
This study included a total of 304 polytraumatised patients. The mean age was 54.5 years (SD 22.2), and the group consisted of 219 males (72%) and 85 females (25%). The three age groups consisted of the following numbers of patients: 18–64 years (n = 189, 62.2%), 65–84 years (n = 92, 30.3%), and ≥85 years (n = 23, 7.5%) (Table 1).

Table 1.
Demography and clinical and surgical variables.
3.2. Type of Injury and Severity
Falls accounted for 161 (52.9%) of all patients. There was a significant difference in injury mechanism for men and women (p < 0.001). Men had more high-level falls (>3 m) and road accidents. There was also a significant difference in injury mechanism between age groups (p < 0.001). The incidence of low-level falls increased with age. The ISS was higher for patients aged 65-84 years (32.7, p = 0.004, r = 0.33) and over 85, respectively (32.6). Overall, it was observed that the order of the body regions most frequently affected by severe injuries (AIS ≥ 3) was identical in both groups.
3.3. Anticoagulation
A significant disparity was observed in the proportion of patients receiving anticoagulants in general at the time of their accident: 66 (57.4%) of all patients aged >65 years versus 10 (5.3%) for the younger cohort (p > 0.001). The highest mortality rate occurred among patients undergoing anticoagulant combination therapy (n = 43, 56.3%, p = 0.9) at the time of the accident, although not significantly.
3.4. Treatment and Management
Mean length of hospital stay for patients aged 65–84 years was 34 days (±49.0) and for ICU 16.2 (±15.0) days. Patient aged >85 years had the longest hospital stay, with a mean of 35 days, and shortest ICU stay (mean 14.4 days). The mean length of hospital and ICU stay for patients aged <65 years was 25.8 days (±47.4) and 14.5 (±13.0). The differences between the age groups differed regarding length of hospital stay and ICU, but not significantly (p = 0.3 and 0.6, respectively) (Table 1 and Table 2).

Table 2.
ICU data and mortality.
Of the 151 patients with subdural haematoma, 84 had a neurosurgical intervention. Patients who received neurosurgery were younger (p = 0.04), with a mean age of 75.6 (SD 7.0) years versus 80.9 (SD 8.2) years, and were more likely to be male (p = 0.02). Outcomes for those having neurosurgical intervention were significantly better (18.7% mortality) than for those without surgery (30.5% mortality, p < 0.001).
3.5. Neurological Outcome
In patients aged 65–100 years, the GOS was 2.9 ± 1.3, with a median of 3.0. Among patients < 64 years, the mean GOS was higher, at 4.1 ± 1.6 (p < 0.001, r = 0.45) (Table 3).

Table 3.
Neurological data and outcomes.
3.6. Mortality
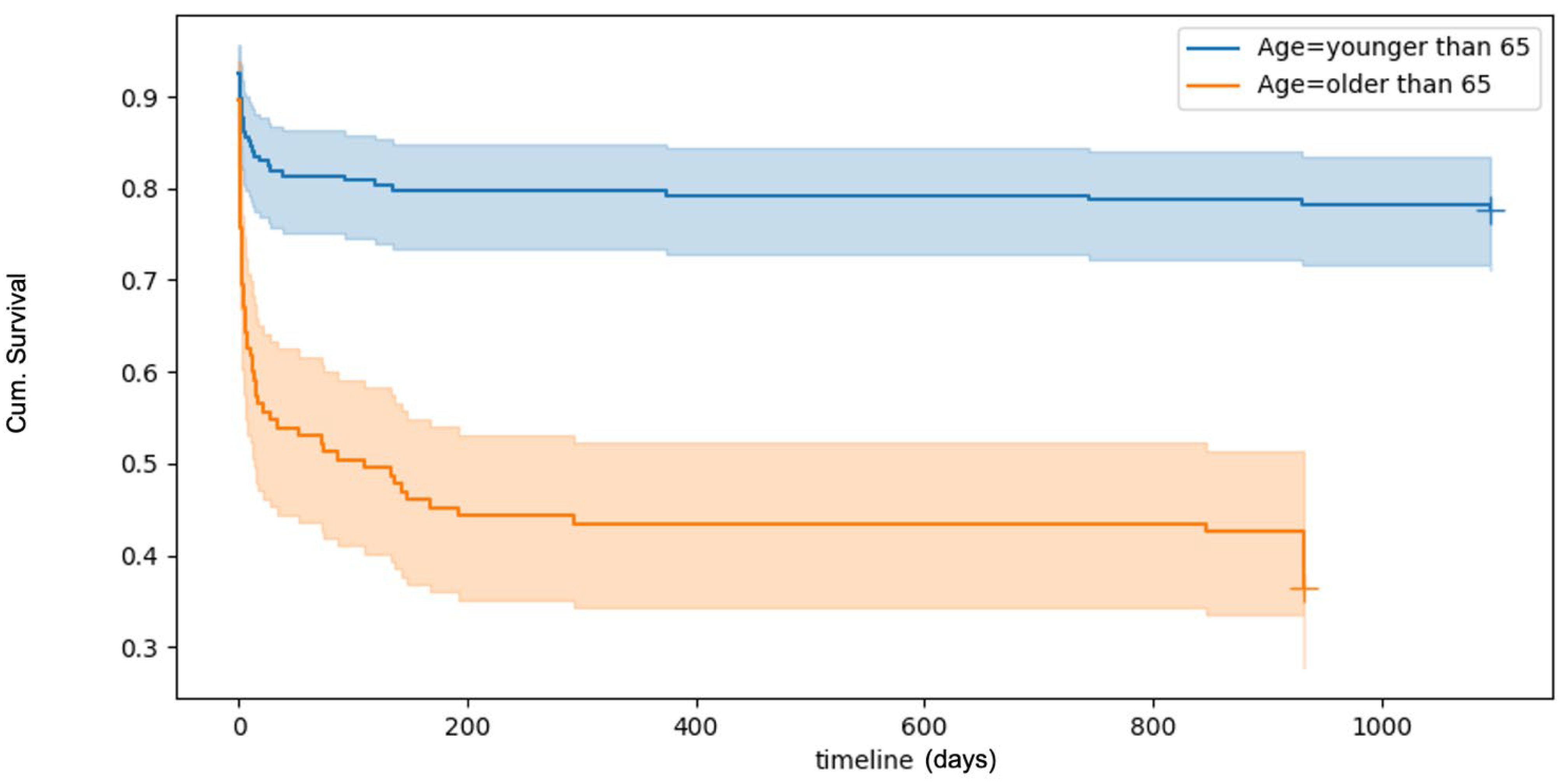
Survival analyses, visualised using Kaplan–Meier curves (Figure 1), revealed a 30-day overall mortality rate of 32.9%. Mortality rates increased with age. Logistic multivariate regression analyses identified several factors significantly associated with an increased risk of death within 30 days post-trauma. These included increasing age, male sex, and severe TBI. ICU and baseline ASA were not found to influence 30-day survival outcomes (Table 4).
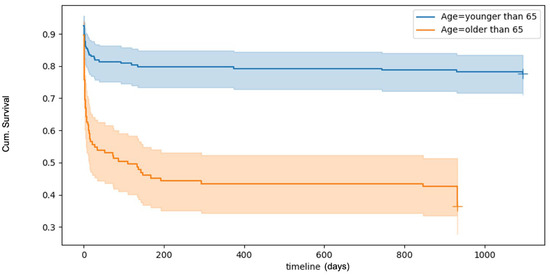
Figure 1.
Kaplan–Meyer plot of overall survival after TBI. The plot shows a poorer survival rate with increased patient age.

Table 4.
Logistic multivariate regression of parameters potentially associated with risk of death within 30 days of trauma.
4. Discussion
Our study provides insights into the epidemiology and outcomes of polytraumatized traumatic brain injuries of old patients over the past 10 years. We were able to demonstrate a correlation between age, TBI severity, sex, and higher mortality rates, and specifically in patients between 65 and 84 years of age. This finding aligns with previous studies indicating that elderly trauma patients face elevated mortality risks, particularly when presenting with severe injuries. Furthermore, older patients might benefit from ICU admission and surgical intervention. These observations underscore the critical importance of tailored management triage and treatment strategies for severely injured elderly patients to improve outcomes in this vulnerable population.
Upon arrival at the shock room, older patients’ mean GCS score was significantly higher than that of younger patients. This difference was even more pronounced when comparing median GCS values, with older patients having a mean of 14.2 versus 9.7 in the younger group. Research suggests that older adults with the same anatomical severity of TBI often exhibit a higher GCS score compared with younger patients [7]. This difference may stem partly from age-related brain atrophy, which allows larger haematomas and more significant oedema to develop before clinical symptoms become evident [8]. Consequently, this can result in under-triaging of older patients and may also account for the reduced effectiveness of triage tools in this population, therefore resulting in worse outcome [9]. Frailty screening may be used as a possibility not only for risk stratification but also to improve patient outcomes [10]. Frailty screening can serve not only as a tool for risk assessment but also as a means to enhance outcomes for patients > 65 years.
More than half (57.4%) of polytrauma patients > 65 years were on oral anticoagulants or antiplatelet agents at the time of their injury. However, their mortality rates did not significantly exceed those of older patients who were not on such medication. While some studies have suggested that anticoagulants may elevate mortality in older trauma patients by exacerbating risks, such as intracranial haemorrhages and general bleeding, others found no clear link between anticoagulation and worse outcomes. However, for traumatic brain injuries, stronger evidence connects anticoagulant or antiplatelet use to poorer prognoses [11].
Several studies have linked the use of anticoagulants or antiplatelet drugs to poorer outcomes or higher mortality in trauma patients. Karni et al. highlighted a lethal risk associated with the combination of anticoagulation therapy, age > 65, and head trauma due to intracranial haemorrhages [12]. Ivascu et al. showed the effects of an aggressive anticoagulation reversal protocol in patients previously on coumarins, which led to a 75% reduction in mortality linked to traumatic intracerebral haemorrhage in older patients [13].
4.1. Surgical Intervention
Research shows a growing trend towards less aggressive treatment in older patients with a TBI [14]. High mortality and morbidity in this group may contribute to a self-fulfilling prophecy, influencing early surgical decisions. Research on neurosurgical outcomes in older patients remains inconclusive, with some studies reporting improved survival and function after surgery, while others suggest better outcomes with conservative management [15,16,17]. Survival after neurosurgical intervention in this study was significantly higher compared with conservative treatment. Nevertheless, in this study, younger patients with severe head injuries were more likely to undergo surgical intervention than their older counterparts (21.7% versus 5.9%). This disparity can, in part, be attributed to ongoing debate about the risks and benefits of surgical interventions in older patients with a TBI [14]. Time and age could be a possible explanation for the low numbers in the group > 65 years, as older patients often experience delays in medical intervention due to underestimating the danger and therefore causing a wait-and-see attitude in healthcare staff. ASA scores were not significantly different between the age groups; however, concomitant disease in older patients might have influenced the decisions. Although early intervention can improve survival rates, as seen in studies where prompt surgery reduced mortality from 90% to 30%, such benefits are less pronounced in the elderly due to their fragile physiological state [18]. In this study, older individuals had higher survival rates with surgery versus conservative treatment, but due to the small sample size of the studied groups, no recommendations for the timing of a neurosurgical intervention can be derived and, therefore, this needs to be further investigated.
4.2. Intensive Care Unit Management
The ICU admission of elderly patients remains a topic of debate. However, advancements in neurosurgical procedures and modern neurointensive care have improved outcomes for older patients with a TBI [19]. Merzo et al. showed that up to 55% of patients aged 70–79 and up to 30% of patients aged ≥80 years with a TBI achieved a favourable neurological outcome [20].
The analysis of ICU stays revealed comparable mean durations for older and younger polytrauma patients, with stays of 14.5 and 16.2 nights, respectively. Patients > 85 years stayed 14.4 days in the ICU. However, durations for all age groups were notably longer than those reported in other studies, suggesting that institutional practices, patient populations or injury severity may have affected the results. For example, Giannoudis et al. reported significantly shorter ICU stays, with a median of eight days for both age groups, highlighting potential differences in care protocols or regional healthcare systems [21]. A particularly important finding was the significantly lower proportion of older patients admitted to the ICU compared with their younger counterparts. While younger patients were frequently treated in intensive care following stabilisation in the shock room, many patients > 65 years were transferred directly to normal wards without ICU monitoring.
4.3. Overall Mortality
In this study, older polytrauma patients exhibit mortality rates that are significantly higher than 30 days compared with younger patients. These findings align with Giannoudis et al., who reported mortality rates of 42% in older patients compared with 20% in younger groups, with mortality rising to nearly 50% in patients > 75 years [21]. DeMaria et al. also highlighted increased mortality in older patients (≥80 years) compared with patients aged 65–79 years, while Richmond et al. noted that mortality risk escalates by approximately 5% with each additional year of age [22,23]. Age-related physiological changes also lower the threshold for traumatic stress, making older patients particularly susceptible to poor outcomes, even in cases of mild TBI [24,25].
4.4. Neurological Results
Neurological and functional outcomes, assessed using the GOS at discharge, were significantly worse in patients > 65 years. These findings are consistent with studies reporting poorer outcomes for older patients with a TBI [26]. King et al. demonstrated that patients with a GOS of three months post-trauma improved to GOS 4–5 within a year following intensive rehabilitation. These findings emphasise the potential for recovery and the importance of comprehensive long-term care strategies for older polytrauma patients [27]. However, in other areas of patient care for adults > 65 years, such as hip fractures, prompt acute management has been found to significantly reduce hospital stays and mortality rates, even for patients aged ≥60 with notable comorbidities (19). However, this study found that older patients with a TBI might experience reduced surgical interventions, have fewer admissions to the ICU and face extended hospital stays along with higher mortality rates. As recent findings suggest that early and aggressive treatment of TBIs in older adults can be as effective as it is for younger patients (4), greater emphasis and further research on this population is warranted.
4.5. Limitations
This study does have some limitations, due to its retrospective design. It focused exclusively on patients with a TBI. In addition, relevant data on underlying medications and comorbidities were not available. Knowledge of comorbidities is important, since patients with chronic comorbidities and TBI especially have different clinical needs than patients with only TBI. Saviter et al. showed a 2.07 times greater risk among older individuals for nosocomial infections, which is even more important since this is an independent predictor of poor global outcomes in severe TBI up to 5 years postinjury [28,29].
Due to the small sample size of 23 patients aged over 85 years, the results for this group are questionable, even though other authors have reported similar findings. The study’s retrospective design and small sample size further limit the generalisability of its findings. GOS outcome data were collected at three months, but the possibility of further improvement beyond this period cannot be ruled out. In fact, it has been suggested that functional status is best assessed at six months or later. Furthermore, frailty was not assessed in the study patients; frailty is now acknowledged as a key factor influencing outcomes, particularly in older ICU patients [30].
5. Conclusions
The range of severe and costly outcomes associated with TBI in older adults further underscores the critical importance of addressing this issue. While age remains an important consideration in therapy and intensive care, it should not be the sole determining factor. Greater emphasis should be placed on a patient’s functional capacity, anticipated quality of life, and individual preferences. These aspects should play a central role in the decision-making process, particularly in light of evolving patient demographics
Author Contributions
A.N., D.P. and S.A. contributed to conception and design of this study. A.N., H.K.W., L.S. and D.P. contributed to the analysis of data. D.W., K.D. and J.L. contributed to the interpretation of data. Article drafts were written by A.N. and critically revised by all authors. All authors have read and agreed to the published version of the manuscript.
Funding
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Institutional Review Board Statement
The study was approved by the medical ethical review committee of the Medical University of Vienna (protocol number 1961/2021, approval date 9 November 2021). The procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest regarding the presented work.
References
- Thompson, H.J.; McCormick, W.C.; Kagan, S.H. Traumatic brain injury in older adults: Epidemiology, outcomes, and future implications. J. Am. Geriatr. Soc. 2006, 54, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.P.; LaDuke, Z.J.; Cain-Nielsen, A.H.; Hemmila, M.R.; Wahl, W.L. Effect of Preinjury Oral Anticoagulants on Outcomes Following Traumatic Brain Injury from Falls in Older Adults. Pharmacotherapy 2020, 40, 604–613. Available online: https://pubmed.ncbi.nlm.nih.gov/32515829/ (accessed on 19 January 2025). [CrossRef] [PubMed]
- Hawley, C.; Sakr, M.; Scapinello, S.; Salvo, J.; Wrenn, P. Traumatic brain injuries in older adults—6 years of data for one UK trauma centre: Retrospective analysis of prospectively collected data. Emerg. Med. J. 2017, 34, 509–516. Available online: https://emj.bmj.com/content/34/8/509 (accessed on 19 January 2025). [CrossRef] [PubMed]
- Weihs, V.; Frenzel, S.; Dedeyan, M.; Hruska, F.; Staats, K.; Hajdu, S.; Negrin, L.L.; Aldrian, S. 25-Year experience with adult polytraumatized patients in a European level 1 trauma center: Polytrauma between 1995 and 2019. What has changed? A retrospective cohort study. Arch. Orthop. Trauma Surg. 2023, 143, 2409–2415. Available online: https://link.springer.com/article/10.1007/s00402-022-04433-1 (accessed on 30 January 2025). [CrossRef]
- Castaño-Leon, A.M.; Gomez, P.A.; Jimenez-Roldan, L.; Paredes, I.; Munarriz, P.M.; Delgado-Fernandez, J.; Perez, I.P.; Gomez, L.M.M.; Sinovas, O.E.; Posadas, G.G.; et al. The impact of early surgery on mortality and functional recovery in older adults with traumatic intracranial lesions: A propensity score-based analysis. Acta Neurochir. 2024, 166, 443. Available online: https://pubmed.ncbi.nlm.nih.gov/39503799/ (accessed on 1 February 2025). [CrossRef]
- Peters, M.E. Traumatic brain injury in older adults: Shining light on a growing public health crisis. Int. Rev. Psychiatry 2020, 32, 1–2. Available online: https://www.tandfonline.com/doi/abs/10.1080/09540261.2019.1683959 (accessed on 19 January 2025). [CrossRef]
- Bick, H.; Wasfie, T.; Labond, V.; Hella, J.R.; Pearson, E.; Barber, K.R. Traumatic brain injury in the elderly with high Glasgow coma scale and low injury severity scores: Factors influencing outcomes. Am. J. Emerg. Med. 2022, 51, 354–357. [Google Scholar] [CrossRef]
- Abdi, H.; Sanchez-Molina, D.; Garcia-Vilana, S.; Rahimi-Movaghar, V. Quantifying the effect of cerebral atrophy on head injury risk in elderly individuals: Insights from computational biomechanics and experimental analysis of bridging veins. Injury 2023, 54, 111125. [Google Scholar] [CrossRef]
- Ruge, T.; Carlsson, A.C.; Hellstrom, M.; Wihlborg, P.; Undén, J. Is medical urgency of elderly patients with traumatic brain injury underestimated by emergency department triage? Upsala J. Med. Sci. 2020, 125, 58. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7054978/ (accessed on 26 January 2025). [CrossRef]
- Egodage, T.; Ho, V.P.; Bongiovanni, T.; Knight-Davis, J.; Adams, S.D.; Digiacomo, J.; Swezey, E.; Posluszny, J.; Ahmed, N.; Prabhakaran, K.; et al. Geriatric trauma triage: Optimizing systems for older adults—A publication of the American Association for the Surgery of Trauma Geriatric Trauma Committee. Trauma Surg. Acute Care Open 2024, 9, e001395. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11253746/ (accessed on 26 January 2025). [CrossRef]
- Vehviläinen, J.; Virta, J.J.; Skrifvars, M.B.; Reinikainen, M.; Bendel, S.; Ala-Kokko, T.; Hoppu, S.; Laitio, R.; Siironen, J.; Raj, R. Effect of antiplatelet and anticoagulant medication use on injury severity and mortality in patients with traumatic brain injury treated in the intensive care unit. Acta Neurochir. 2023, 165, 4003. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10739466/ (accessed on 19 January 2025). [CrossRef] [PubMed]
- Karni, A.; Holtzman, R.; Bass, T.; Zorman, G.; Carter, L.; Rodriguez, L.; Bennett-Shipman, V.J.; Lottenberg, L. Traumatic head injury in the anticoagulated elderly patient: A lethal combination. Am. Surg. 2001, 67, 1098–1100. [Google Scholar] [CrossRef]
- Ivascu, F.A.; Howells, G.A.; Junn, F.S.; Bair, H.A.M.; Bendick, P.J.; Janczyk, R.J. Rapid warfarin reversal in anticoagulated patients with traumatic intracranial hemorrhage reduces hemorrhage progression and mortality. J. Trauma—Inj. Infect. Crit. Care 2005, 59, 1131–1139. Available online: https://journals.lww.com/jtrauma/fulltext/2005/11000/rapid_warfarin_reversal_in_anticoagulated_patients.18.aspx (accessed on 7 March 2025). [CrossRef]
- Laic, R.A.G.; Sloten, J.V.; Depreitere, B. Neurosurgical treatment in elderly patients with Traumatic brain injury: A 20-year follow-up study. Brain Spine 2023, 3, 101723. [Google Scholar] [CrossRef]
- Antoni, A.; Heinz, T.; Leitgeb, J. Polytrauma and concomitant traumatic brain injury. Trauma Surg. 2017, 120, 722–727. [Google Scholar]
- Wan, X.; Liu, S.; Wang, S.; Zhang, S.; Yang, H.; Ou, Y. Elderly Patients with Severe Traumatic Brain Injury Could Benefit from Surgical Treatment. World Neurosurg. 2016, 89, 147–152. [Google Scholar] [CrossRef]
- Brazinova, A.; Rehorcikova, V.; Taylor, M.S.; Buckova, V.; Majdan, M.; Psota, M.; Peeters, W.; Feigin, V.; Theadom, A.; Holkovic, L.; et al. Epidemiology of Traumatic Brain Injury in Europe: A Living Systematic Review. J. Neurotrauma 2021, 38, 1411–1440. Available online: https://pubmed.ncbi.nlm.nih.gov/26537996/ (accessed on 1 February 2025). [CrossRef]
- Seelig, J.M.; Becker, D.P.; Miller, J.D.; Greenberg, R.P.; Ward, J.D.; Choi, S.C. Traumatic acute subdural hematoma: Major mortality reduction in comatose patients treated within four hours. N. Engl. J. Med. 1981, 18, 304. [Google Scholar] [CrossRef]
- Guidet, B.; de Lange, D.W.; Flaatten, H. Should this elderly patient be admitted to the ICU? Intensive Care Med. 2018, 44, 1926–1928. Available online: https://link.springer.com/article/10.1007/s00134-018-5054-7 (accessed on 7 March 2025). [CrossRef]
- Merzo, A.; Lenell, S.; Nyholm, L.; Enblad, P.; Lewén, A. Promising clinical outcome of elderly with TBI after modern neurointensive care. Acta Neurochir. 2016, 158, 125–133. Available online: https://pubmed.ncbi.nlm.nih.gov/26577639/ (accessed on 7 March 2025). [CrossRef]
- Giannoudis, P.V.; Harwood, P.J.; Court-Brown, C.; Pape, H.C. Severe and multiple trauma in older patients; incidence and mortality. Injury 2009, 40, 362–367. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, E.J.; Kenney, P.R.; Merriam, M.A.; Casanova, L.A.; Gann, D.S. Survival after trauma in geriatric patients. Ann. Surg. 1987, 206, 738–743. Available online: https://pubmed.ncbi.nlm.nih.gov/3689009/ (accessed on 14 March 2025). [CrossRef] [PubMed]
- Richmond, T.S.; Kauder, D.; Strumpf, N.; Meredith, T. Characteristics and outcomes of serious traumatic injury in older adults. J. Am. Geriatr. Soc. 2002, 50, 215–222. Available online: https://pubmed.ncbi.nlm.nih.gov/12028201/ (accessed on 14 March 2025). [CrossRef] [PubMed]
- Karibe, H.; Hayashi, T.; Hirano, T.; Kameyama, M.; Nakagawa, A.; Tominaga, T. Surgical Management of Traumatic Acute Subdural Hematoma in Adults: A Review. Neurol. Med.-Chir. 2014, 54, 887–894. [Google Scholar] [CrossRef]
- Hackenberg, K.; Unterberg, A. Schädel-Hirn-Trauma. Nervenarzt 2016, 87, 203–216. Available online: https://link.springer.com/article/10.1007/s00115-015-0051-3 (accessed on 14 March 2025). [CrossRef]
- Launey, Y.; Coquet, A.; Lasocki, S.; Dahyot-Fizelier, C.; Huet, O.; Le Pabic, E.; Roquilly, A.; Seguin, P. Factors associated with an unfavourable outcome in elderly intensive care traumatic brain injury patients—A retrospective multicentre study. BMC Geriatr. 2022, 22, 1004. Available online: https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-022-03651-x (accessed on 19 January 2025). [CrossRef]
- King, J.T.; Carlier, P.M.; Marion, D.W. Early Glasgow Outcome Scale Scores Predict Long-Term Functional Outcome in Patients with Severe Traumatic Brain Injury. J. Neurotrauma 2005, 22, 947–954. [Google Scholar] [CrossRef]
- Kesinger, M.R.; Kumar, R.G.; Wagner, A.K.; Puyana, J.C.; Peitzman, A.P.; Billiar, T.R.; Sperry, J.L. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J. Trauma. Acute Care Surg. 2015, 78, 396–402. [Google Scholar] [CrossRef]
- Saviteer, S.M.; Samsa, G.P.; Rutala, W.A. Nosocomial infections in the elderly. Am. J. Med. 1988, 84, 661–666. [Google Scholar] [CrossRef]
- Galimberti, S.; Graziano, F.; Maas, A.I.R.; Isernia, G.; Lecky, F.; Jain, S.; Sun, X.; Gardner, R.C.; Taylor, S.R.; Markowitz, A.J.; et al. Effect of frailty on 6-month outcome after traumatic brain injury: A multicentre cohort study with external validation. Lancet Neurol. 2022, 21, 153–162. Available online: https://pubmed.ncbi.nlm.nih.gov/35065038/ (accessed on 23 March 2025). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).