Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Narrative Review Construction
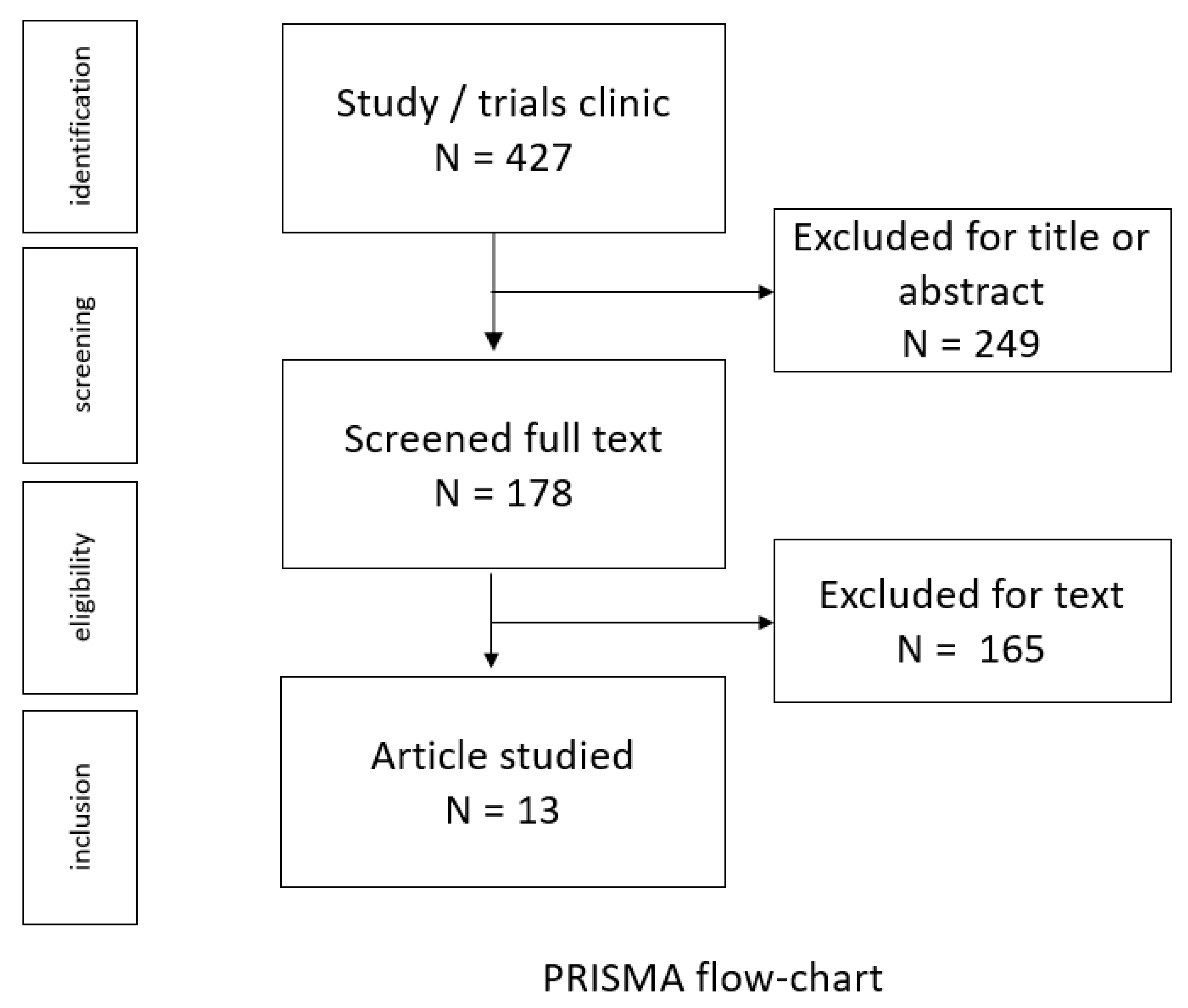
2.2. Study Selection
3. Results
| Trial Information | Period and Country | Type of Study | Inclusion/Exclusion Criteria | Design | Results |
|---|---|---|---|---|---|
| Liraglutide | |||||
| Author(s): Xavier Pi-Sunyer et al. [36] Sponsor: Novo Nordisk, Denmark | June 2011–March 2013. | Randomized, placebo-controlled trial. | Inclusion criteria: patients 18 years of age or older who had stable BMI ≥ 30, or ≥27 if the patient had treated or untreated dyslipidemia or hypertension. Exclusion criteria: type 1 or 2 diabetes, use of medications that cause clinically significant weight gain or loss, previous bariatric surgery. | 56-w. Randomized, placebo-controlled trial of 3.0 mg of liraglutide or placebo, injected s.c. once daily, as an adjunct to a reduced-calorie diet and increased physical activity. After 56 weeks, patients in the liraglutide group who did not have prediabetes at screening were randomly assigned in a 1:1 ratio to continue receiving liraglutide or to switch to placebo for 12 weeks. Patients in the placebo group continued to receive placebo. | After 56 weeks, patients in the liraglutide group had lost a mean (±SD) of 8.0 ± 6.7% (8.4 ± 7.3 kg) of their body weight, whereas patients in the placebo group had lost a mean of 2.6 ± 5.7% (2.8 ± 6.5 kg) of their body weight. Weight loss with liraglutide was maintained over 56 weeks and was similar regardless of prediabetes status. In Table 19 of the appendix of the study, we found that at week 68, 12 weeks of the follow-up period, a group who did not have prediabetes liraglutide/liraglutide: reduce weight 0.69 ± 2.58, group liraglutide/placebo 2.91 ± 3.01 group placebo/placebo: 0.28 ± 2.39. |
| Author(s): Carel W le Roux et al. [29] Sponsor: Novo Nordisk, Denmark | June 2011–March 2015. | Randomized, placebo-controlled trial. | Inclusion criteria: patients 18 years or older with stable BMI of at least 30 kg/m2, or at least 27 kg/m2 with treated or untreated dyslipidaemia, or hypertension, or both. Exclusion criteria: type 1 or type 2 diabetes, medications causing significant weight gain or loss, bariatric surgery, history of pancreatitis, major depressive or other severe psychiatric disorders. | Participants were randomly assigned, in a 2:1 ratio, to receive liraglutide 3.0 mg or placebo. Participants without prediabetes were on treatment for 56 weeks, followed by a 12-week re-randomized period; the results for this phase of the study have been reported by Xavier Pi-Sunyer et al. [36] We did not include participants without prediabetes in this trial. A total of 160 weeks plus the 12-week off-treatment follow-up period. |
Liraglutide induced greater weight loss than placebo at week 160 while on treatment (–6.1% for liraglutide
vs.
−1.9% for placebo; estimated treatment difference −4.3%, 95% CI −4.9 to −3.7, p < 0.0001). Weight loss with liraglutide treatment was sustained over 3 years. After treatment cessation at week 160, some weight was regained in the liraglutide group, although the treatment difference was still significant at week 172 (–3.2%, 95% CI −4.3 to −2.2, p < 0.0001). |
| Semaglutide | |||||
| Author(s): Domenica Rubino et al. [30] Sponsor: Novo Nordisk, Denmark | June 2018–March 2020. | Randomized, double-blind, placebo-controlled trial. | Inclusion criteria: patients 18 years or older with at least one self-reported unsuccessful dietary effort to lose weight and with BMI of 30 or higher or a BMI of 27 or higher with at least one treated or untreated weight-related comorbidity (hypertension, dyslipidemia, obstructive sleep apnea, cardiovascular disease; type 2 diabetes was excluded) were enrolled. Exclusion criteria: hemoglobin A1c of 6.5% (48 mmol/mol) or greater and a self-reported change in body weight of more than 5 kg within 90 days of screening. |
All participants initially received open-label once-weekly subcutaneous semaglutide, 0.25 mg, increased every 4 weeks to the maintenance dose of 2.4 mg once weekly by week 16, and continued to week 20. Participants receiving semaglutide, 2.4 mg, at week 20 were randomized in a 2:1 ratio using a blocking schema (block size of 6) in a double-blind manner, to continue this treatment or switch to matching placebo for 48 weeks (weeks 20–68; randomized period), with a 7-week follow-up. All participants received a lifestyle intervention from week 0 to week 68. |
During the 20-week run-in, mean body weight declined by 10.6%.
Estimated mean weight change from week 20 to week 68 was −7.9% with continued semaglutide vs. +6.9% in participants switched to placebo (difference, −14.8 [95% CI, −16.0 to −13.5] percentage points; p < 0.001). No found data for body weight at week 75, end follow-up period. |
| Author(s): John p H Wilding et al. [37] Sponsor: Novo Nordisk, Denmark | September 2019–April 2020. | Randomized, double-blind, placebo-controlled trial. | Inclusion criteria: patients 18 years or older with a BMI of ≥ 30 kg/m2 or ≥27 kg/m2 with at least one weight-related co-morbidity and a history of at least one self-reported unsuccessful dietary effort to lose weight. To be eligible for the extension, participants were required to have completed treatment with semaglutide 2.4 mg or placebo at week 68. Exclusion criteria: type 1 or 2 diabetes and obesity pharmacotherapy 90 days or less before enrolment. |
Randomized to 68 weeks of treatment with once weekly s.c. semaglutide 2.4 mg or placebo, plus lifestyle intervention. At week 68 (the end of the treatment period), participants were withdrawn from treatment (including lifestyle intervention) and followed for 7 weeks until week 75. The STEP 1 extension followed a subset of participants for an additional 45 weeks (a total of 52 weeks off-treatment) until the end-of-trial visit at week 120. |
During the main treatment phase (from baseline [week 0] to week 68), semaglutide reduced body weight more than placebo; mean weight loss was 17.3% (SD: 9.3%) with semaglutide versus 2.0% (SD: 6.1%) with placebo. After treatment withdrawal, body weight regain was observed in both the semaglutide and placebo arms. Participants regained a mean of 11.6 percentage points (SD: 7.7) of body weight in the semaglutide arm versus 1.9 percentage points (SD: 4.8) in the placebo arm. The net mean body weight loss over the full duration of the main treatment phase and off-treatment extension phase (from week 0 to week 120) was 5.6% (SD: 8.9%) in the semaglutide arm versus 0.1% (SD: 5.8%) in the placebo arm. |
| Tirzepatide | |||||
| Author(s): Louis J. Aronne et al. [38] Sponsor: Eli Lilly and Company | March 2021–18 May 2023. | Phase 3 randomized double-blind, placebo-controlled trial. | Inclusion criteria: patients 18 years or older with a BMI ≥ 30 or ≥27 and at least one weight-related complication (hypertension, dyslipidemia, obstructive sleep apnea or cardiovascular disease). Exclusion criteria: diabetes, prior surgical treatment for obesity, treatment with a medication that reduces weight loss. | After 36 w. of open-label of tirzepatide experienced a mean weight reduction of 20.9%. At week 36, those switched to placebo experienced a 14% weight regain and those continuing tirzepatide experienced an additional 5.5% weight reduction during the 52-week double-blind period. This was followed by a 36-week follow-up period. | For the treatment regimen estimand, the mean percent change in weight from week 36 to week 88 was −5.5% with tirzepatide vs. 14.0% with placebo (difference, −19.4% [95% CI, −21.2% to −17.7%]; p < 0.001. |
| Trial Information | Period and Country | Type of Study | Inclusion/Exclusion Criteria | Design | Results |
|---|---|---|---|---|---|
| Liraglutide | |||||
| Author(s): Melanie J. Davies et al. [39] Sponsor: Novo Nordisk, Denmark | June 2011–January 2013. | Randomized, double-blind, placebo-controlled, parallel-group trial. | Inclusion criteria: patients ≥ 18 years, BMI ≥ 27.0 with a stable body weight (<5-kg change in the last 3 months), diagnosed with type 2 diabetes treated with diet and exercise alone or in combination with 1 to 3 oral hypoglycemic agents (metformin, thiazolidinedione, sulfonylurea). Exclusion criteria: treatment with GLP-1 RA, DPP-4 inhibitors, insulin within the last 3 months; TSH > 6 mIU/L or <0.4 mIU/L, obesity induced by other endocrinologic disorders; current or history of treatment with medications that may cause significant weight gain, within 3 months prior to screening for this trial; previous surgical treatment for obesity. |
Liraglutide once daily. The starting dose of the trial drug was 0.6 mg. It was escalated by increments of 0.6 mg weekly to the treatment dose. This occurred over 2 weeks for the 1.8 mg treatment dose and 4 weeks for the 3.0 mg treatment dose. Participants were encouraged to follow a diet, with a 500-kcal/d deficit based on estimated total energy expenditure and exercise program. A 12-week observational off-drug follow-up period was included to assess treatment-cessation effects (total study length: 68 weeks). | Data available only in supplementary figures. eFigure 3: effects of treatment cessation → change (%) in body weight from baseline to week 68: placebo −2.7% (−2.8% at 56 weeks); Liraglutide 1.8 mg −3.6% (−5% at 56 weeks); Liraglutide 3.0 mg −4.7% (−6.7% at 56 weeks). |
| Semaglutide | |||||
| Author(s): Lone B Enebo et al. [40] Sponsor: Novo Nordisk, Denmark | July 2018–Dec 2019. | Randomized, placebo-controlled, multiple-ascending dose, phase 1b trial. | Inclusion criteria: patients 18–55 years of age with a BMI of 27–0-39–9 kg/m2 and who were otherwise healthy. Exclusion criteria: Individuals aged 40 years or older with an estimated 10-year atherosclerotic cardiovascular disease risk of 5% or higher at screening were excluded from the trial. | The trial included six sequential overlapping cohorts, and in each cohort, eligible participants were randomly assigned (3:1) to once-weekly subcutaneous cagrilintide (0.16, 0.30, 0.60, 1.2, 2.4, or 4.5 mg) or matched placebo, in combination with once-weekly subcutaneous semaglutide 2.4 mg, without lifestyle interventions. In each cohort, the doses of cagrilintide and semaglutide were co-escalated in 4-week intervals to the desired dose over 16 weeks, participants were treated at the target dose for 4 weeks and then followed up for 5 weeks. | In the Appendix, Figure S3, page 13, the 5 weeks of follow-up with weight regain are shown for each dosage of cagrilintide with semaglutide 2.4 mg. |
| Liraglutide and semagludide | |||||
| Author(s): Patrick M O’Neil et al. [26] Sponsor: Novo Nordisk, Denmark | Oct 2015–Feb 2016. | Randomized, double-blind, placebo and active-controlled, multicentre, parallel-group, dose-ranging, phase 2 trial. | Inclusion criteria: patients 18 years or older without diabetes, and with a BMI of ≥30 kg/m2 that was not of endocrine etiology. Eligible individuals must have undergone at least one previous unsuccessful non-surgical weight loss. Exclusion criteria: diabetes mellitus; TSH > 6 mIU/L or <0.4 mIU/L; treatment with glucose-lowering agent(s) within 90 days before screening; previous surgical treatment for obesity. | The study consisted of a 1-week screening period, 52 weeks of treatment, and a post-treatment follow-up of 7 weeks. | A prespecified analysis of observed weight change from baseline at week 59 showed slightly smaller mean reductions in the active treatment groups than at week 52 due to off-treatment weight regain. Mean changes at week 59 for semaglutide escalated on the 4-weekly schedule were −4.9% (SD 6.2; 0.05 mg) to −13.5% (7.9; 0.4 mg), for the 2-weekly escalation were −12.0% (7.9; 0.3 mg) and −15.5% (9.3; 0.4 mg), for liraglutide 3.0 mg was −7.7% (6.9), and for pooled placebo was −1.8% (5.5). A post-hoc analysis of participants still on treatment at week 52 (regardless of the treatment group) who also had week 59 data showed a positive correlation between the amount of weight regained off treatment and the amount lost from baseline to week 52. |
| Beinaglutide | |||||
| Author(s): Kang Chen et al. [41] Sponsor: Shanghai Benemae Pharmaceutical Corporation | September 2019–October 2020. | Multicentre, randomized, double-blind, placebo-controlled study | Inclusion criteria: patients aged 18–70 years, BMI of 28 kg/m2 or higher or a BMI of 24–27.9 kg/m2 with weight-related co-morbidities Exclusion criteria: diabetes; use of weight-loss drugs; weight-reduction surgery; psychiatric disorder. | 16-week beinaglutide or placebo (4-week dose escalation and 12-week dose maintenance) regimen subcutaneously, and underwent a 12-week post-treatment observation. | The mean body weight change at week 16 was −6.0% and −2.4% in the beinaglutide and placebo groups, respectively (treatment difference: −3.6%; 95% CI: −4.6% and −2.6%; p < 0.0001). Beinaglutide resulted in a weight regain rate of 0.78% during the post-treatment observation (12 weeks). |
| Trial Information | Period and Country | Type of Study | Inclusion/Exclusion Criteria | Design | Results |
|---|---|---|---|---|---|
| Liraglutide | |||||
| Author(s): Simona Ferjan et al. [45] Sponsor: Ministry of Health, Republic of Slovenia, Tertiary Care Scientific grant number 20120047 of the University Medical Centre Ljubljana. | Published in 2017. | Prospective randomized open-label study. | Inclusion criteria: type A phenotype of PCOS, including the concomitant presence of hyperandrogenemia on either the biochemical or the clinical level, menses abnormalities, and PCO morphology. Aged 18 years or older to menopause and obese (BMI ≥ 30). Exclusion criteria: significant cardiovascular, kidney or hepatic disease, personal or family history of medullary thyroid carcinoma, known history of gallbladder disease or pancreatitis. | Pretreated with liraglutide 3.0 mg for 12 weeks. After stopping liraglutide, they were switched to metformin (MET) 1000 mg twice daily (BID) alone (n = 12) or combined treatment (COMBO) with metformin 1000 mg twice daily and sitagliptin 100 mg daily (QD) (n = 12). Lifestyle intervention was reinforced after liraglutide cessation in both groups. Reducing diet of 500–800 kcal/. A total of 30 min of moderate-intensity physical activity daily was promoted. The second part of the treatment (after stopping liraglutide) lasted 12 weeks. | Before randomization, the average weight loss induced with 12-week treatment with liraglutide 3 mg was 5.1–3.6 kg. Weight regain in 12 weeks after liraglutide cessation did not correlate with weight loss achieved with liraglutide before randomization. Subjects treated with MET alone regain on average 4.7 ± 2.7 kg (p = 0.002) compared with 0.9 ± 2.5 kg in COMBO group (p = 0.147). BMI increased by 1.7 ± 0.9 kg/m2 in MET arm (p = 0.002) compared with statistically insignificant increase of 0.3 ± 0.8 kg/m2 in COMBO. |
| Author(s): Joan Khoo et al. [46] Sponsor: National Medical Research Council of Singapore | September 2014–July 2016. | Prospective randomized pilot study. | Inclusion criteria: Asians patients non-diabetic, abdominally obese (BMI > 28 kg/m2, waist circumference [WC] ≥ 90 cm in men or ≥80 cm in women), diagnosed with NAFLD and steatohepatitis, in the absence of other causes of hepatic steatosis and chronic liver disease. Exclusion criteria: history of excessive alcohol intake; weight-loss medications. | Structured combined diet-exercise programme (DE) group vs. Liraglutide (LI) group. 26 weeks of active weight loss phase (weeks 0–26) followed by 26 weeks of weight maintenance phase (weeks 27–52). | DE and LI groups had significant (p < 0.01) and similar reductions in weight (−3.5 ± 3.3 vs. −3.0 ± 2.2 kg), LFF (−8.1 ± 13.2 vs. −7.0 ± 7.1%), serum alanine aminotransferase (−39 ± 35 vs. −26 ± 33 U/L) and caspase-cleaved cytokeratin-18 (cCK-18) (−206 ± 252 vs. −130 ± 158 U/L) at 26 weeks. At 52 weeks, the LI group significantly (p < 0.05) regained weight (1.8 ± 2.1 kg), LFF (4.0 ± 5.3%) and cCK-18 (72 ± 126 U/L), whereas these were unchanged in the DE group. |
| Author(s): Camilla K. Svensson et al. [47] Sponsor: Supported by an unrestricted grant (Dr. Fink-Jensen) and Novo Nordisk A/S, Capital Region Psychiatry Research Group, The foundation of King Christian X, and grants from the Lundbeck Foundation (Dr. Jespersen and Dr. Svensson). | May 2013–February 2016. | Randomized, double-blinded, placebo-controlled trial. | Inclusion criteria: overweight/obese patients with prediabetes, diagnosed with a schizophrenia-spectrum disorder and treated with clozapine or olanzapine. Exclusion criteria: type 1 and type 2 diabetes, other serious somatic illnesses. | Randomly assigned to receive either liraglutide or placebo At baseline and every four weeks until the end of treatment (week 16), and at the one-year follow-up (week 68). Changes in medication, blood tests, psychiatric and somatic diagnoses, and diet and exercise habits were recorded. Treatment between 16 and 68 weeks was by clinician’s choice. | One year after the end of treatment (68 weeks from baseline) the liraglutide group (within-group analyses) had a significant increase in body weight, BMI, waist circumference, LDL and HDL. Compared to the placebo group (between-group analyses), the liraglutide group maintained a significant body weight loss of 3.8 kg (p = 0.04) and a reduction in BMI of 1.6 kg/m2 (p = 0.02) from baseline to one-year follow-up. Many drops or exclusion of patients for various reasons. |
| Semaglutide | |||||
| Author(s): John Blundell et al. [48] Sponsor: Novo Nordisk, Danmark | Published in 2017. | Single-center, randomized, double-blind, placebo-controlled, two-period crossover trial. | Inclusion criteria: patients 18 years or older, BMI of 30 to 45 kg/m2 and stable body weight. Exclusion criteria: diabetes; previous surgical treatment for obesity. | Two 12-week crossover treatment periods, separated by a wash-out period of 5 to 7 weeks. Randomized 1:1 to one of two treatment sequences: semaglutide– placebo or placebo–semaglutide. | After 12 weeks of treatment with semaglutide, a change from baseline in mean body weight of −5.0 kg was observed, vs. +1.0 kg with placebo. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HRQoL | Health-related quality of life |
| QALYs | Quality-adjusted life-years |
| BMI | Body mass index |
| EOC | Ente Ospedaliero Cantonale |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| s/c | subcutaneously |
| GLP-1 RA | Glucacon-like peptide 1 receptor agonists |
| COPD | Chronic obstructive pulmonary disease |
| RYGB | Roux-en-Y gastric bypass |
| SG EWL | Sleeve gastrectomy Excess body weight loss |
| GIP | Gastric inhibitory polypeptide |
| PCOS | Polycystic ovary syndrome |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhaskaran, K.; Dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagi, M.A.; Ahmed, H.; Rezq, M.A.A.; Sangroongruangsri, S.; Chaikledkaew, U.; Almalki, Z.; Thavorncharoensap, M. Economic costs of obesity: A systematic review. Int. J. Obes. 2024, 48, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjöholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.A.; Taube, M.; Carlsson, B.; Peltonen, M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N. Engl. J. Med. 2020, 383, 1535–1543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mital, S.; Nguyen, H.V. Cost-Effectiveness of Antiobesity Drugs for Adolescents with Severe Obesity. JAMA Netw. Open 2023, 6, e2336400. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pontiroli, A.E.; Ceriani, V.; Tagliabue, E. Compared with Controls, Bariatric Surgery Prevents Long-Term Mortality in Persons with Obesity Only Above Median Age of Cohorts: A Systematic Review and Meta-Analysis. Obes. Surg. 2020, 30, 2487–2496. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424, Erratum in Obes. Facts 2016, 9, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atallah, R.; Filion, K.B.; Wakil, S.M.; Genest, J.; Joseph, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. Long-term effects of 4 popular diets on weight loss and cardiovascular risk factors: A systematic review of randomized controlled trials. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Noparatayaporn, P.; Thavorncharoensap, M.; Chaikledkaew, U.; Bagepally, B.S.; Thakkinstian, A. Incremental Net Monetary Benefit of Bariatric Surgery: Systematic Review and Meta-Analysis of Cost-Effectiveness Evidences. Obes. Surg. 2021, 31, 3279–3290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diao, X.; Gao, L.; Yang, Y.; Chen, X.; Gong, J.; Qian, Y.; Yang, W.; Chinese Obesity and Metabolic Surgery Collaborative. Knowledge and Attitudes Towards Obesity and Bariatric Surgery in University Students: A National Survey. Obes. Surg. 2022, 32, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22 (Suppl. 3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, D.H.; Van Gaal, L.F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Rissanen, A.; Andersen, T.; Boldrin, M.; Golay, A.; Koppeschaar, H.P.; Krempf, M.; European Multicentre Orlistat Study Group. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet 1998, 352, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Weissman, N.J.; Anderson, C.M.; Sanchez, M.; Chuang, E.; Stubbe, S.; Bays, H.; Shanahan, W.R.; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 2010, 363, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gilden, A.H.; Catenacci, V.A.; Taormina, J.M. Obesity. Ann. Intern. Med. 2024, 177, ITC65–ITC80. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D.; Endocrine Society. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362, Erratum in J. Clin. Endocrinol. Metab. 2015, 100, 2135–2136. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Batterham, R.L.; Bhatta, M.; Buscemi, S.; Christensen, L.N.; Frias, J.P.; Jódar, E.; Kandler, K.; Rigas, G.; Wadden, T.A.; et al. Two-year effects of semaglutide in adults with overweight or obesity: The STEP 5 trial. Nat. Med. 2022, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Neil, P.M.; Garvey, W.T.; Gonzalez-Campoy, J.M.; Mora, P.; Ortiz, R.V.; Guerrero, G.; Claudius, B.; Pi-Sunyer, X.; Satiety and Clinical Adiposity—Liraglutide Evidence in individuals with and without diabetes (SCALE) study groups. Effects of liraglutide 3.0 mg on weight and risk factors in hispanic versus non-hipanic populations: Subgroup analysis from scale randomized trials. Endocr. Pract. 2016, 22, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Dong, X.; Li, Y.; Li, Y.; Lim, S.; Liu, M.; Ning, Z.; Rasmussen, S.; Skjøth, T.V.; Yuan, G.; et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes Obes. Metab. 2021, 23, 404–414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaku, K.; Yamada, Y.; Watada, H.; Abiko, A.; Nishida, T.; Zacho, J.; Kiyosue, A. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: A randomized trial. Diabetes Obes. Metab. 2018, 20, 1202–1212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolotkin, R.L.; Gabriel Smolarz, B.; Meincke, H.H.; Fujioka, K. Improvements in health-related quality of life over 3 years with liraglutide 3.0 mg compared with placebo in participants with overweight or obesity. Clin. Obes. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundgren, J.R.; Janus, C.; Jensen, S.B.K.; Juhl, C.R.; Olsen, L.M.; Christensen, R.M.; Svane, M.S.; Bandholm, T.; Bojsen-Møller, K.N.; Blond, M.B.; et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N. Engl. J. Med. 2021, 384, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Wharton, S.; Blevins, T.; Connery, L.; Rosenstock, J.; Raha, S.; Liu, R.; Ma, X.; Mather, K.J.; Haupt, A.; Robins, D.; et al. Daily Oral GLP-1 Receptor Agonist Orforglipron for Adults with Obesity. N. Engl. J. Med. 2023, 389, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Birkenfeld, A.L.; McGowan, B.; Mosenzon, O.; Pedersen, S.D.; Wharton, S.; Carson, C.G.; Jepsen, C.H.; Kabisch, M.; Wilding, J.P.H. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: A randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 2018, 392, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Kaplan, L.M.; Frías, J.P.; Wu, Q.; Du, Y.; Gurbuz, S.; Coskun, T.; Haupt, A.; Milicevic, Z.; Hartman, M.L.; et al. Triple-Hormone-Receptor Agonist Retatrutide for Obesity—A Phase 2 Trial. N. Engl. J. Med. 2023, 389, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; le Roux, C.W.; Stefanski, A.; Aronne, L.J.; Halpern, B.; Wharton, S.; Wilding, J.P.H.; Perreault, L.; Zhang, S.; Battula, R.; et al. Tirzepatide for Obesity Treatment and Diabetes Prevention. N. Engl. J. Med. 2024, 392, 958–971. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.V.; Wilding, J.P.H.; Skjøth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409, Erratum in Lancet 2017, 389, 1398. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. STEP 4 Investigators. Effect of Continued Weekly Subcutaneous Semaglutide vs. Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Office Fédéral de la Santé Publique OFSP, Liste Des Spécialités (LS). Available online: https://www.xn--spezialittenliste-yqb.ch/ShowPreparations.aspx?searchType=ATCCODE&searchValue=A10BJ06 (accessed on 10 January 2025).
- Baig, K.; Dusetzina, S.B.; Kim, D.D.; Leech, A.A. Medicare Part D Coverage of Antiobesity Medications—Challenges and Uncertainty Ahead. N. Engl. J. Med. 2023, 388, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.; le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment with Tirzepatide for Maintenance of Weight Reduction in Adults with Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687–699, Erratum in JAMA 2016, 315, 90. [Google Scholar] [CrossRef] [PubMed]
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C.W. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2·4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, L.; Shan, Z.; Wang, G.; Qu, S.; Qin, G.; Yu, X.; Xin, W.; Hsieh, T.H.; Mu, Y. Beinaglutide for weight management in Chinese individuals with overweight or obesity: A phase 3 randomized controlled clinical study. Diabetes Obes. Metab. 2024, 26, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Aberle, J.; Bardtrum, L.; Christiansen, E.; Knop, F.K.; Gabery, S.; Pedersen, S.D.; Buse, J.B. Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): A multicentre, randomised, phase 3b trial. Lancet 2023, 402, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Aroda, V.R.; do Vale, R.D.; Holst-Hansen, T.; Laursen, P.N.; Rosenstock, J.; Rubino, D.M.; Garvey, W.T.; OASIS 1 Investigators. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Ferjan, S.; Janez, A.; Jensterle, M. Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Prevented Weight Regain in Obese Women with Polycystic Ovary Syndrome Previously Treated with Liraglutide: A Pilot Randomized Study. Metab. Syndr. Relat. Disord. 2017, 15, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Khoo, J.; Hsiang, J.C.; Taneja, R.; Koo, S.H.; Soon, G.H.; Kam, C.J.; Law, N.M.; Ang, T.L. Randomized trial comparing effects of weight loss by liraglutide with lifestyle modification in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.K.; Larsen, J.R.; Vedtofte, L.; Jakobsen, M.S.L.; Jespersen, H.R.; Jakobsen, M.I.; Koyuncu, K.; Schjerning, O.; Nielsen, J.; Ekstrøm, C.T.; et al. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiatr. Scand. 2019, 139, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mailhac, A.; Pedersen, L.; Pottegård, A.; Søndergaard, J.; Mogensen, T.; Sørensen, H.T.; Thomsen, R.W. Semaglutide (Ozempic®) Use in Denmark 2018 Through 2023—User Trends and off-Label Prescribing for Weight Loss. Clin. Epidemiol. 2024, 16, 307–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burki, T. European Commission classifies obesity as a chronic disease. Lancet Diabetes Endocrinol. 2021, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Maclean, P.S.; Bergouignan, A.; Cornier, M.A.; Jackman, M.R. Biology’s response to dieting: The impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R581–R600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H.; Bays, H.; Czernichow, S.; Uddén Hemmingsson, J.; Hjelmesæth, J.; Hoffmann Morville, T.; Koroleva, A.; Skov Neergaard, J.; Vélez Sánchez, P.; Wharton, S.; et al. Once-Weekly Semaglutide in Persons with Obesity and Knee Osteoarthritis. N. Engl. J. Med. 2024, 391, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, T.; Antonowicz, S.S.; Markar, S.R. Cancer Risk Following Bariatric Surgery-Systematic Review and Meta-analysis of National Population-Based Cohort Studies. Obes. Surg. 2019, 29, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Benjamin, M.M.; Srinivasan, S.; Morin, E.E.; Shishatskaya, E.I.; Schwendeman, S.P.; Schwendeman, A. Battle of GLP-1 delivery technologies. Adv. Drug Deliv. Rev. 2018, 130, 113–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sjöström, L.; Lindroos, A.K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.; Wellman, R.; Emiliano, A.; Smith, S.R.; Odegaard, A.O.; Murali, S.; Williams, N.; Coleman, K.J.; Courcoulas, A.; Coley, R.Y.; et al. Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann. Intern. Med. 2018, 169, 741–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Athanasiadis, D.I.; Martin, A.; Kapsampelis, P.; Monfared, S.; Stefanidis, D. Factors associated with weight regain post-bariatric surgery: A systematic review. Surg. Endosc. 2021, 35, 4069–4084. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.B.; Renström, F.; Aczél, S.; Folie, P.; Biraima-Steinemann, M.; Beuschlein, F.; Bilz, S. Efficacy of the Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide for the Treatment of Weight Regain After Bariatric surgery: A Retrospective Observational Study. Obes. Surg. 2023, 33, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gasoyan, H.; Pfoh, E.R.; Schulte, R.; Le, P.; Butsch, W.S.; Rothberg, M.B. One-Year Weight Reduction with Semaglutide or Liraglutide in Clinical Practice. JAMA Netw. Open 2024, 7, e2433326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): A double-blind, randomised, phase 3 trial. Lancet 2021, 398, 143–155, Erratum in Lancet 2021, 398, 212. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.W.; Mailhac, A.; Løhde, J.B.; Pottegård, A. Real-world evidence on the utilization, clinical and comparative effectiveness, and adverse effects of newer GLP-1RA-based weight-loss therapies. Diabetes Obes. Metab. 2025, 27 (Suppl. 2), 66–88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, C.; Yang, S.; Zhou, Z. GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: Data from cardiovascular outcome trials. Endocrine 2020, 68, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.; Chhatwal, J.; Xiao, J.; Jirapinyo, P.; Thompson, C.C. Semaglutide vs. Endoscopic Sleeve Gastroplasty for Weight Loss. JAMA Netw. Open 2024, 7, e246221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, A.S.; Auerbach, P.; Barrientos-Perez, M.; Gies, I.; Hale, P.M.; Marcus, C.; Mastrandrea, L.D.; Prabhu, N.; Arslanian, S.; NN8022-4180 Trial Investigators. A Randomized, Controlled Trial of Liraglutide for Adolescents with Obesity. N. Engl. J. Med. 2020, 382, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarenghi, M.; Capelli, S.; Galligani, G.; Giana, A.; Preatoni, G.; Turri Quarenghi, R. Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies. J. Clin. Med. 2025, 14, 3791. https://doi.org/10.3390/jcm14113791
Quarenghi M, Capelli S, Galligani G, Giana A, Preatoni G, Turri Quarenghi R. Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies. Journal of Clinical Medicine. 2025; 14(11):3791. https://doi.org/10.3390/jcm14113791
Chicago/Turabian StyleQuarenghi, Massimo, Silvia Capelli, Giulia Galligani, Arianna Giana, Giorgia Preatoni, and Rosamaria Turri Quarenghi. 2025. "Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies" Journal of Clinical Medicine 14, no. 11: 3791. https://doi.org/10.3390/jcm14113791
APA StyleQuarenghi, M., Capelli, S., Galligani, G., Giana, A., Preatoni, G., & Turri Quarenghi, R. (2025). Weight Regain After Liraglutide, Semaglutide or Tirzepatide Interruption: A Narrative Review of Randomized Studies. Journal of Clinical Medicine, 14(11), 3791. https://doi.org/10.3390/jcm14113791








