Bariatric Surgery Before Abdominoplasty Is Associated with Increased Perioperative Anemia, Hemoglobin Loss and Drainage Fluid Volume: Analysis of 505 Body Contouring Procedures
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Analysis
2.2. Inclusion and Exclusion Criteria
2.3. Operative Procedure
2.4. Statistics and Data Management
3. Results
3.1. Modality of Weight Loss
3.2. Patient Demographics
3.3. Hemoglobin Alternation
3.4. Drainage Fluid Volume and Drainage Inlay Time
3.5. Duration of Surgery and Hospital Stay
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, M.P.; Christopher, A.N.; Fischer, J.P. Quality-of-Life Measurement Tools after Body Contouring Surgery. Plast. Reconstr. Surg. 2021, 147, 1088e–1090e. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, J.; Bath-Hextall, F.; Maclean, J.; Stanton, W.; Soldin, M. Quality of life among adults following bariatric and body contouring surgery: A systematic review. JBI Database System Rev. Implement. Rep. 2016, 14, 240–270. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, A.; Kumar, A.; Asokan, G.; Green, L.; Ibrahim, A.; Goel, R.; Harries, R.; Kanhere, H.; Prowse, P.; Trochsler, M. Quality of Life After Bariatric and Body Contouring Surgery in the Australian Public Health System. J. Surg. Res. 2023, 285, 76–84. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, G.; Huang, J.; Shen, C.; Cai, Z.; Yin, X.; Yin, Y.; Zhang, B. A systematic review of body contouring surgery in post-bariatric patients to determine its prevalence, effects on quality of life, desire, and barriers. Obes. Rev. 2021, 22, e13201. [Google Scholar] [CrossRef]
- ElAbd, R.; AlMojel, M.; AlSabah, S.; AlRashid, A.; AlNesf, M.; Alhallabi, B.; Burezq, H. Complications Post Abdominoplasty After Surgical Versus Non-surgical Massive Weight Loss: A Comparative Study. Obes. Surg. 2022, 32, 3847–3853. [Google Scholar] [CrossRef]
- De Paep, K.; Van Campenhout, I.; Van Cauwenberge, S.; Dillemans, B. Post-bariatric Abdominoplasty: Identification of Risk Factors for Complications. Obes. Surg. 2021, 31, 3203–3209. [Google Scholar] [CrossRef]
- Sirota, M.; Weiss, A.; Billig, A.; Hassidim, A.; Zaga, J.; Adler, N. Abdominoplasty complications—What additional risks do postbariatric patients carry? J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.J.; Stewart, D.A.; Coghlan, B.; Harrison, D.H.; Jones, B.M.; Waterhouse, N. Complications of 278 consecutive abdominoplasties. J. Plast. Reconstr. Aesthet. Surg. 2006, 59, 1152–1155. [Google Scholar] [CrossRef]
- Salari, N.; Fatahi, B.; Bartina, Y.; Kazeminia, M.; Heydari, M.; Mohammadi, M.; Hemmati, M.; Shohaimi, S. The Global Prevalence of Seroma After Abdominoplasty: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2021, 45, 2821–2836. [Google Scholar] [CrossRef]
- Thacoor, A.; van den Bosch, P.; Akhavani, M.A. Surgical Management of Cosmetic Surgery Tourism-Related Complications: Current Trends and Cost Analysis Study of the Financial Impact on the UK National Health Service (NHS). Aesthet. Surg. J. 2019, 39, 786–791. [Google Scholar] [CrossRef]
- Klein, H.J.; Simic, D.; Fuchs, N.; Schweizer, R.; Mehra, T.; Giovanoli, P.; Plock, J.A. Complications After Cosmetic Surgery Tourism. Aesthet. Surg. J. 2017, 37, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Sieffert, M.R.; Fox, J.P.; Abbott, L.E.; Johnson, R.M. Obesity is associated with increased health care charges in patients undergoing outpatient plastic surgery. Plast. Reconstr. Surg. 2015, 135, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Berkane, Y.; Saget, F.; Lupon, E.; Mocquard, C.; Pluvy, I.; Watier, E.; Lellouch, A.G.; Duisit, J.; Chaput, B.; Bertheuil, N. Abdominoplasty and Lower Body Lift Surgery Improves the Quality of Life after Massive Weight Loss: A Prospective Multicenter Study. Plast. Reconstr. Surg. 2024, 153, 1101e–1110e. [Google Scholar] [CrossRef] [PubMed]
- Vater, A.M.; Fietz, J.; Schultze-Mosgau, L.E.; Lamby, P.E.; Gerauer, K.E.; Schmidt, K.; Jakubietz, R.G.; Jakubietz, M.G. Understanding the Long-Term Effects of Inverted-T-Abdominoplasty on Quality of Life: Insights from Post-Bariatric Surgery Patients. Life 2025, 15, 214. [Google Scholar] [CrossRef]
- Flores, T.; Jaklin, F.J.; Rohrbacher, A.; Schrogendorfer, K.F.; Bergmeister, K.D. Perioperative Risk Factors for Prolonged Blood Loss and Drainage Fluid Secretion after Breast Reconstruction. J. Clin. Med. 2022, 11, 808. [Google Scholar] [CrossRef]
- Collaboration, N.C.D.R.F. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Saeed, L.; Sharif, G.; Eda, S.; Raju Tullimalli, I.; Amin, A.; Riyalat, A.A.; Alrashid, F.F.; Abdelrahim, A.A. Comparative Effectiveness of Bariatric Metabolic Surgery Versus Glucagon-Like Peptide-1 Receptor Agonists on Cardiovascular Outcomes and Mortality: A Meta-Analysis. Cureus 2024, 16, e71684. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Chacon, D.; Bernardino, T.; Geraghty, F.; Carrion Rodriguez, A.; Fiani, B.; Chadhaury, A.; Pierre-Louis, M. Bariatric Surgery With Roux-En-Y Gastric Bypass or Sleeve Gastrectomy for Treatment of Obesity and Comorbidities: Current Evidence and Practice. Cureus 2022, 14, e25762. [Google Scholar] [CrossRef]
- Chandrakumar, H.; Khatun, N.; Gupta, T.; Graham-Hill, S.; Zhyvotovska, A.; McFarlane, S.I. The Effects of Bariatric Surgery on Cardiovascular Outcomes and Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e34723. [Google Scholar] [CrossRef]
- Jarvholm, K.; Janson, A.; Henfridsson, P.; Neovius, M.; Sjogren, L.; Olbers, T. Metabolic and bariatric surgery for adolescents with severe obesity: Benefits, risks, and specific considerations. Scand. J. Surg. 2025, 114, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Fredensborg Holm, T.; Udsen, F.W.; Giese, I.E.; Faerch, K.; Jensen, M.H.; von Scholten, B.J.; Hangaard, S. The Effectiveness of Digital Health Lifestyle Interventions on Weight Loss in People With Prediabetes: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Diabetes Sci. Technol. 2024, 19322968241292646. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Sun, Y.; Ye, W.; Liu, Y.; Korivi, M. Efficacy of time restricted eating and resistance training on body composition and mood profiles among young adults with overweight/obesity: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2025, 22, 2481127. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.X.; Beidas, O.E. Discussion: Health-Related Quality-of-Life Effects of Rectus Repair during Body Contouring Surgery in Postbariatric Patients: A Prospective Study. Plast. Reconstr. Surg. 2024, 154, 746–747. [Google Scholar] [CrossRef]
- Dalaei, F.; Dijkhorst, P.J.; Moller, S.; Klassen, A.F.; de Vries, C.E.E.; Poulsen, L.; Kaur, M.N.; Thomsen, J.B.; Hoogbergen, M.; Voineskos, S.H.; et al. Improving the Impact of BODY-Q Scores Through Minimal Important Differences in Body Contouring Surgery: An International Prospective Cohort Study. Aesthet. Surg. J. 2024, 44, 1317–1329. [Google Scholar] [CrossRef]
- Henriques, J.; Berenbaum, F.; Mobasheri, A. Obesity-induced fibrosis in osteoarthritis: Pathogenesis, consequences and novel therapeutic opportunities. Osteoarthr. Cartil. Open 2024, 6, 100511. [Google Scholar] [CrossRef]
- Schulz, T.; Kirsten, T.; Langer, S.; Nuwayhid, R. Hope for the best, but prepare for the worst—Diagnostic accuracy of the American College of Surgeons National Surgical Quality Improvement Program—Risk model for patients undergoing abdominoplasty after massive weight loss—Results from a Retrospective Cohort Study. JPRAS Open 2025, 43, 347–356. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, Y.; Lyu, D.; Wang, C. The Experience and Complications of Lipoabdominoplasty for Chinese Post-bariatric Population. Aesthetic Plast. Surg. 2025, 49, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Ibrahiem, S.M.S. Investigating the Safety of Multiple Body Contouring Procedures in Massive Weight Loss Patients. Aesthetic Plast. Surg. 2022, 46, 2891–2902. [Google Scholar] [CrossRef]
- Fahmy, J.N.; Kong, L.; Benitez, T.M.; Sanders, H.M.; Wang, L.; Chung, K.C. Postbariatric Panniculectomy: Postoperative Complications by Weight Loss Surgery Type. Plast. Reconstr. Surg. 2025, 155, 354–361. [Google Scholar] [CrossRef]
- Knoedler, S.; Alfertshofer, M.; Matar, D.Y.; Sofo, G.; Hundeshagen, G.; Didzun, O.; Bigdeli, A.K.; Friedrich, S.; Schenck, T.; Kneser, U.; et al. Safety of Combined Versus Isolated Cosmetic Breast Surgery and Abdominoplasty: Insights from a Multi-institutional Database. Aesthetic Plast. Surg. 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Ottosson, J.; Clarkson, S.; Sjoberg, K. Anemia in patients ten years after bariatric surgery. Int. J. Obes. 2025, 49, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Ettleson, M.D.; Lager, C.J.; Kraftson, A.T.; Esfandiari, N.H.; Oral, E.A. Roux-en-Y gastric bypass versus sleeve gastrectomy: Risks and benefits. Minerva Chir. 2017, 72, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Du, R.; Xie, L.; Han, X.; Zhang, H.; Tu, Y.; Zhang, H.; Bao, Y.; Yu, H. Dual Hepatic Injury from Refeeding Syndrome and Starvation in a Malnourished Woman After Bariatric Surgery: A Case Report. Am. J. Case Rep. 2024, 25, e944088. [Google Scholar] [CrossRef]
- Omarov, N.; Huseynov, E. Effect of Laparoscopic Sleeve Gastrectomy on Calcium Metabolism Parameters, Vitamin D, and Parathyroid Hormone in Morbidly Obese Patients: Short-Term Results. Cureus 2024, 16, e71795. [Google Scholar] [CrossRef]
- Hung, K.C.; Weng, H.L.; Lai, Y.C.; Wu, J.Y.; Chang, Y.J.; Hung, I.Y.; Chen, I.W. Association Between Preoperative Anemia and Postoperative Acute Kidney Injury in Patients Undergoing Metabolic and Bariatric Surgery: A Multi-institute Study. Obes. Surg. 2025, 35, 1827–1837. [Google Scholar] [CrossRef]
- Harker, M.J.R.; Heusschen, L.; Monpellier, V.M.; Liem, R.S.L.; Van Himbeeck, M.J.J.; Nienhuijs, S.W.; Al Nawas, M.; Wiezer, R.J.; Vugts, G.; Hazebroek, E.J. Five year outcomes of primary and secondary Single-Anastomosis Duodeno-Ileal bypass with Sleeve gastrectomy (SADI-S). Obes. Surg. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Hindso, M.; Lundsgaard, A.; Marinkovic, B.; Jensen, M.H.; Hedback, N.; Svane, M.S.; Dirksen, C.; Jorgensen, N.B.; London, A.; Jeppesen, P.B.; et al. Fat absorption and metabolism after Roux-en-Y gastric bypass surgery. Metabolism 2025, 167, 156189. [Google Scholar] [CrossRef]
- Sahin, C.; Aydogdu, Y.F.; Buyukkasap, C.; Dikmen, K.; Dalgic, A. Investigation of homocysteine level after bariatric metabolic surgery, effect on vitamin B12 and folate levels. BMC Endocr. Disord. 2024, 24, 237. [Google Scholar] [CrossRef]
- Maman, Y.; Abu-Abeid, A.; Eldar, S.M.; Keidar, A. Metabolic and bariatric surgery in vegetarians and vegans. Minerva Surg. 2024, 79, 600–606. [Google Scholar] [CrossRef]
- Tang, X.; Reidlinger, D.P.; Crichton, M.; Craggs-Dino, L.; Fayet-Moore, F.; Marshall, S. Preoperative Micronutrient Repletion Strategies in Metabolic and Bariatric Surgery: A Systematic Review. J. Acad. Nutr. Diet. 2025, 125, 761–784.e6. [Google Scholar] [CrossRef] [PubMed]
- Erol, M.F.; Kayaoglu, H.A. Comparison of the Effectiveness of Single Anastomosis Sleeve Ileal Bypass and Roux-en-Y Gastric Bypass in Obese Patients with Type 2 Diabetes. Obes. Surg. 2024, 34, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Benotti, P.N.; Kaberi-Otarod, J.; Wood, G.C.; Gerhard, G.S.; Still, C.D.; Bistrian, B.R. Iron homeostasis in obesity and metabolic and bariatric surgery: A narrative review. Surg. Obes. Relat. Dis. 2024, 20, 1370–1380. [Google Scholar] [CrossRef]
- Tufail, N.; Kataria, M.; Chaudhary, A.J.; Dhillon, R.A.; Asif Naveed, M.; Mohsin, S. Comparing Holotranscobalamin and Total Vitamin B12 in Diagnosing Vitamin B12 Deficiency in Megaloblastic Anemia Patients. Cureus 2024, 16, e71278. [Google Scholar] [CrossRef]
- Moravcova, M.; Siatka, T.; Krcmova, L.K.; Matousova, K.; Mladenka, P.; Oemonom. Biological properties of vitamin B(12). Nutr. Res. Rev. 2025, 38, 338–370. [Google Scholar] [CrossRef]
- Kaberi-Otarod, J.; Still, C.D.; Wood, G.C.; Benotti, P.N. Iron Treatment in Patients with Iron Deficiency Before and After Metabolic and Bariatric Surgery: A Narrative Review. Nutrients 2024, 16, 3350. [Google Scholar] [CrossRef] [PubMed]
- Gueant, J.L.; Gueant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar] [CrossRef]
- Faran, M.; McKechnie, T.; O’Callaghan, E.K.; Anvari, S.; Kuszaj, O.; Crowther, M.; Anvari, M.; Doumouras, A.G. Predictors of Anemia Recovery in Patients with Pre-existing Anemia Undergoing Metabolic Bariatric Surgery: A Retrospective Cohort Study. Obes. Surg. 2025, 35, 1733–1742. [Google Scholar] [CrossRef]
- Sander, J.; Torensma, B.; Siepe, J.; Schorp, T.; Schulte, T.; Schmeer, C.; Gogele, H.; Bockelmann, I.; Grabenhorst, A.; Ockert-Belz, I.; et al. Assessment of Preoperative Multivitamin Use on the Impact on Micronutrient Deficiencies in Patients with Obesity Prior to Metabolic Bariatric Surgery. Obes. Surg. 2025, 35, 1818–1826. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Yu, X.; Liang, S.; Guan, Y.; Yang, J.; Guan, B. Long-term prevalence of vitamin deficiencies after bariatric surgery: A meta-analysis. Langenbecks Arch. Surg. 2024, 409, 226. [Google Scholar] [CrossRef]
- De Luca, M.; Belluzzi, A.; Angrisani, L.; Bandini, G.; Becattini, B.; Bueter, M.; Carrano, F.M.; Chiappetta, S.; Cohen, R.V.; Copaescu, C.; et al. Meta-analysis of randomized controlled trials for the development of the International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC) guidelines on multimodal strategies for the surgical treatment of obesity. Diabetes Obes. Metab. 2025, 27, 3347–3356. [Google Scholar] [CrossRef] [PubMed]
- Shermak, M.A.; Rotellini-Coltvet, L.A.; Chang, D. Seroma development following body contouring surgery for massive weight loss: Patient risk factors and treatment strategies. Plast. Reconstr. Surg. 2008, 122, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Sun, Y.; Dang, Q.; Xu, C.; Chen, P.; Ma, T.; Ren, J. Correction of blood coagulation dysfunction and anemia by supplementation of red blood cell suspension, fresh frozen plasma, and apheresis platelet: Results of in vitro hemodilution experiments. J. Crit. Care 2015, 30, 220.e1–220.e12. [Google Scholar] [CrossRef] [PubMed]
- Kitzinger, H.B.; Cakl, T.; Wenger, R.; Hacker, S.; Aszmann, O.C.; Karle, B. Prospective study on complications following a lower body lift after massive weight loss. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 231–238. [Google Scholar] [CrossRef]
- Edwards, M.A.; Powers, K.; Vosburg, R.W.; Zhou, R.; Stroud, A.; Obeid, N.R.; Pilcher, J.; Levy, S.; McArthur, K.; Basishvili, G.; et al. American Society for Metabolic and Bariatric Surgery: Postoperative care pathway guidelines for Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2025, 21, 523–536. [Google Scholar] [CrossRef]

| Patient Characteristics | Abdominoplasty—Lifestyle Change | Abdominoplasty—Bariatric Surgery | |
|---|---|---|---|
| Number | 80 | 124 | |
| Gender | F | 64 (80%) | 113 (91.13%) |
| M | 16 (20%) | 11 (8.87%) | |
| Age (years) | Mean | 42.82 | 42.87 |
| STD | ±11.33 | ±10.98 | |
| BMI (kg/m2) | Mean | 26.17 | 26.33 |
| STD | ±4.58 | ±3.72 | |
| Hb pre-op (g/dL) | Mean | 14.02 | 13.24 |
| STD | ±1.22 | ±1.38 | |
| Hb post-op (g/dL) | Mean | 11.71 | 10.68 |
| STD | ±1.59 | ±1.48 | |
| Hb difference pre-post | Mean | −2.30 | −2.56 |
| STD | ±1.43 | ±1.62 | |
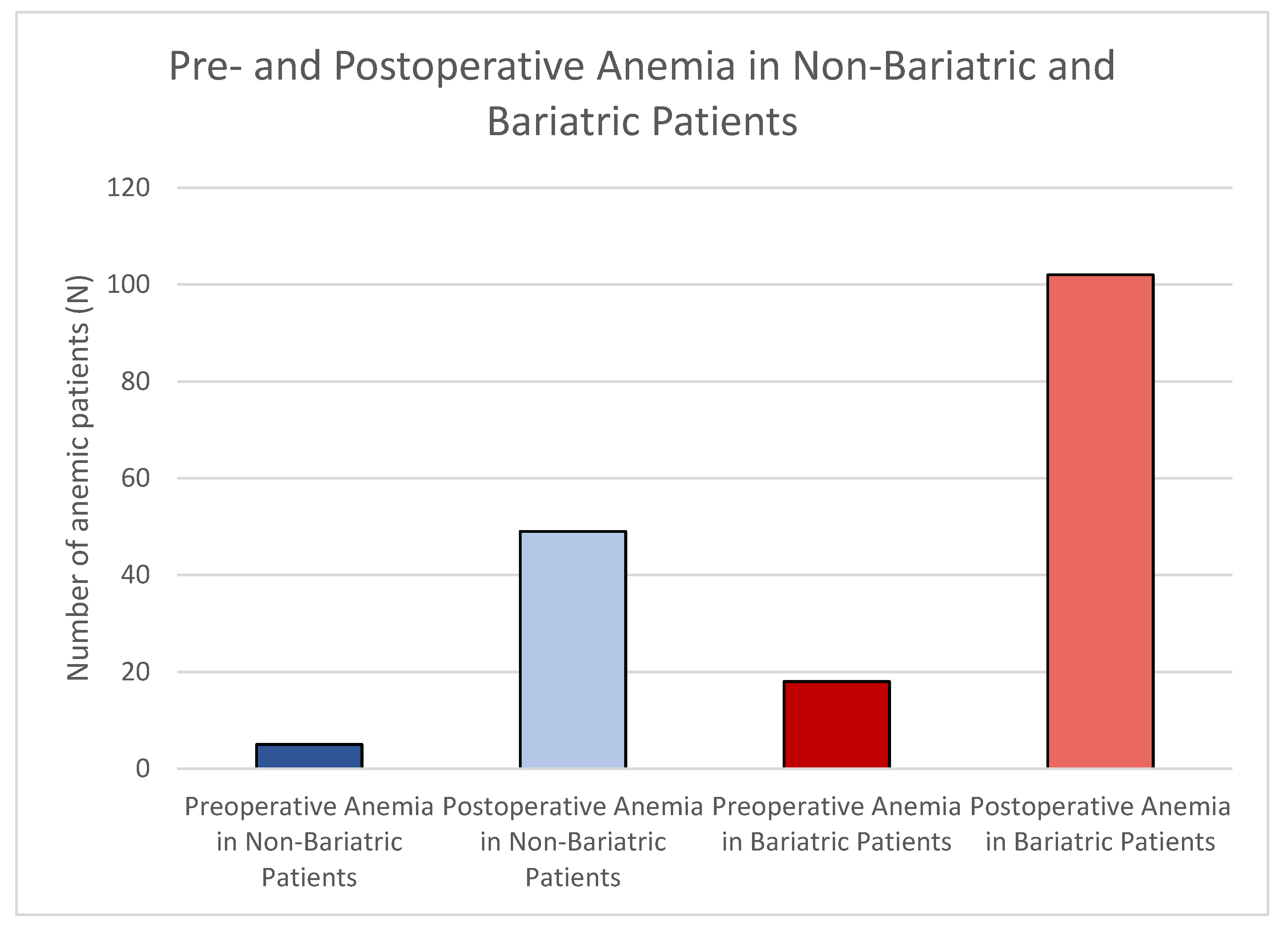
| Anemia pre-op | Yes | 5 (6.25%) | 18 (14.52%) |
| No | 75 (93.75%) | 106 (85.48%) | |
| Anemia post-op | Yes | 49 (61.25%) | 102 (82.26%) |
| No | 31 (38.75%) | 22 (17.74%) | |
| Resection weight (g) | Mean | 1364.90 | 1442.28 |
| STD | ±1108.29 | ±1359.51 | |
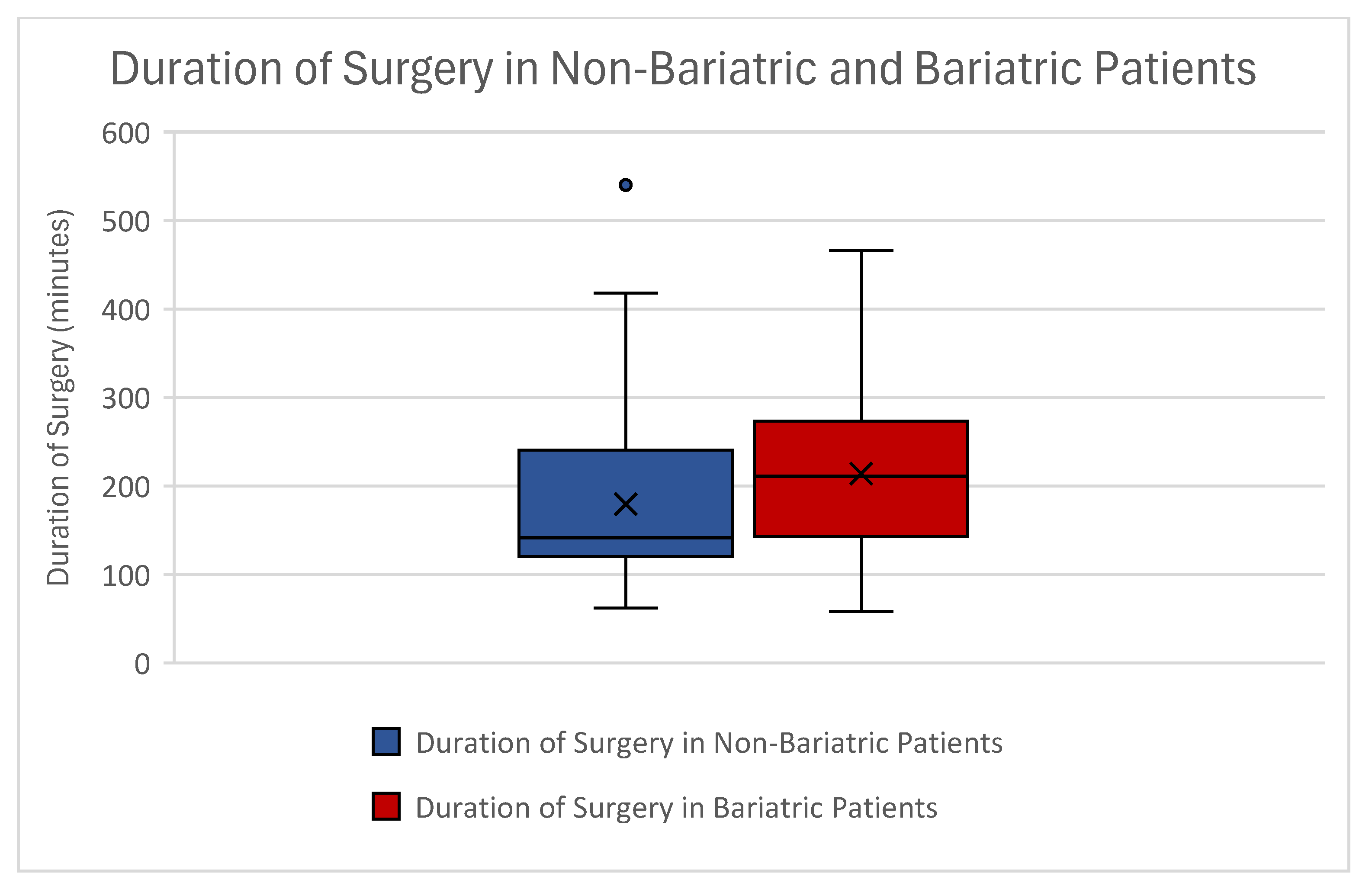
| Duration of surgery (min) | Mean | 179.43 | 213.89 |
| STD | ±89.86 | ±87.26 | |
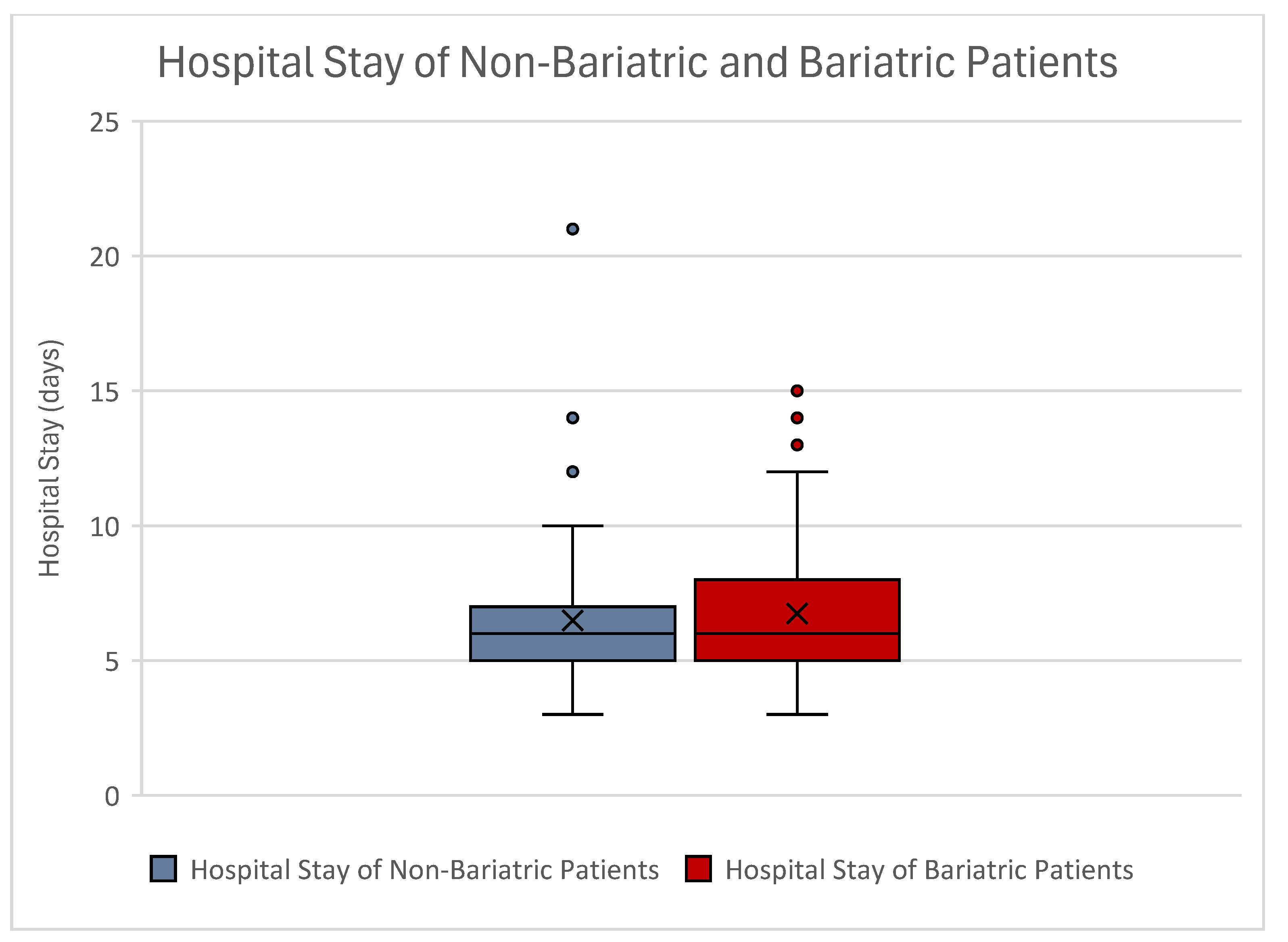
| Hospital stay (days) | Mean | 6.49 | 6.74 |
| STD | ±2.68 | ±2.40 | |
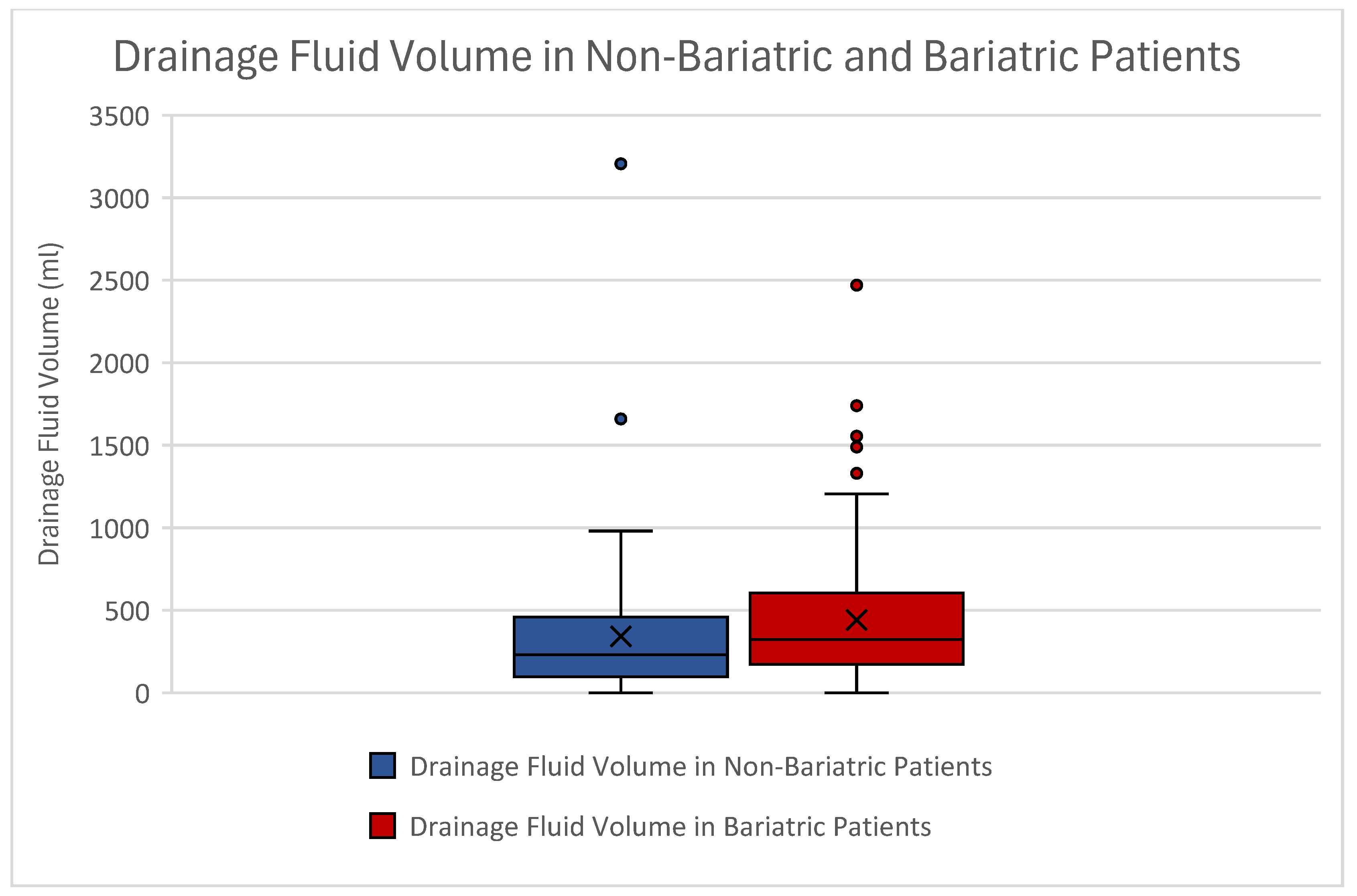
| Drainage volume (mL) | Mean | 342.21 | 442.38 |
| STD | ±429.77 | ±405.42 | |
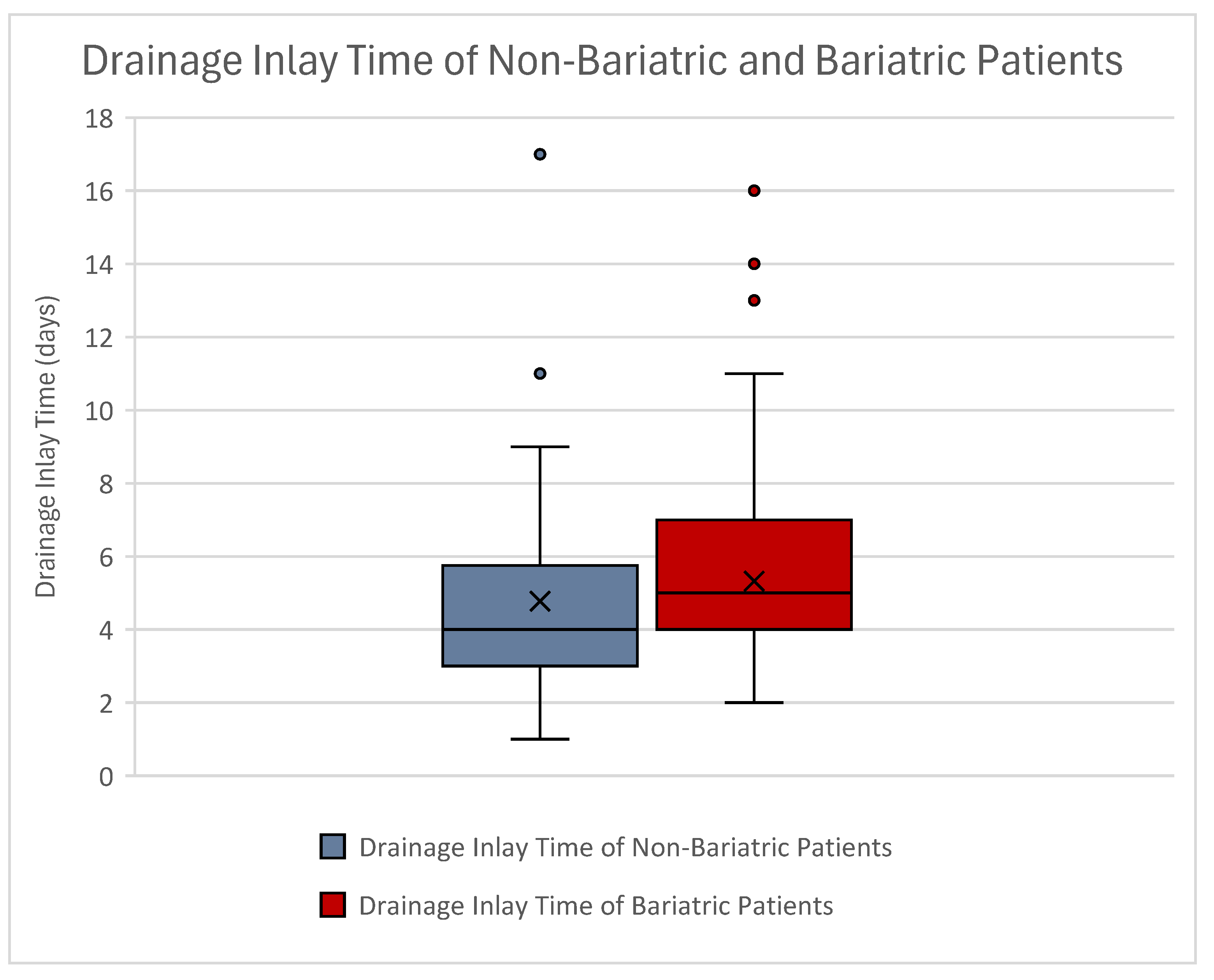
| Drainage inlay time (days) | Mean | 4.78 | 5.32 |
| STD | ±2.42 | ±2.38 |
| T-Test for Equality of Means | |||||||
|---|---|---|---|---|---|---|---|
| F | Sig. | T | df | One-Sided p | Two-Sided p | Mean Difference | |
| Hemoglobin preoperative | 0.486 | 0.486 | 4.127 | 202 | <0.001 | <0.001 | 0.7818 |
| Hemoglobin postoperative | 0.678 | 0.411 | 4.678 | 202 | <0.001 | <0.001 | 1.0253 |
| Hemoglobin difference | 1.227 | 0.269 | −1.164 | 202 | 0.123 | 0.246 | −0.258 |
| T-Test for Equality of Means | |||||||
|---|---|---|---|---|---|---|---|
| F | Sig. | T | df | One-Sided p | Two-Sided p | Mean Difference | |
| Anemia preoperative | 14.865 | <0.001 | −1.976 | 201.102 | 0.025 | 0.05 | −0.082 |
| Anemia postoperative | 38.646 | <0.001 | −3.245 | 139.724 | <0.001 | 0.001 | −0.2100 |
| Spearman Rho Correlation Analysis | |||
|---|---|---|---|
| Spearman Rho | Drainage Volume | Corr. Coefficient | 1.000 |
| Sig. (2-tailed) | . | ||
| N | 204 | ||
| Bariatric surgery | Corr. Coefficient | 0.182 | |
| Sig. (2-tailed) | 0.009 | ||
| N | 204 | ||
| Hemoglobin postoperative | Corr. Coefficient | −0.145 | |
| Sig. (2-tailed) | 0.039 | ||
| N | 204 | ||
| Anemia postoperative | Corr. Coefficient | 0.158 | |
| Sig. (2-tailed) | 0.024 | ||
| N | 204 | ||
| Resection weight | Corr. Coefficient | 0.247 | |
| Sig. (2-tailed) | <0.001 | ||
| N | 204 | ||
| T-Test for Equality of Means | |||||||
|---|---|---|---|---|---|---|---|
| F | Sig. | T | df | One-Sided p | Two-Sided p | Mean Difference | |
| Duration of Surgery | 0.017 | 0.897 | −2.722 | 202 | 0.004 | 0.007 | −34.4621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, T.; Schön, J.; Glisic, C.; Pfoser, K.; Kerschbaumer, C.; Mayrl, M.S.; Schrögendorfer, K.F.; Bergmeister, K.D. Bariatric Surgery Before Abdominoplasty Is Associated with Increased Perioperative Anemia, Hemoglobin Loss and Drainage Fluid Volume: Analysis of 505 Body Contouring Procedures. J. Clin. Med. 2025, 14, 3783. https://doi.org/10.3390/jcm14113783
Flores T, Schön J, Glisic C, Pfoser K, Kerschbaumer C, Mayrl MS, Schrögendorfer KF, Bergmeister KD. Bariatric Surgery Before Abdominoplasty Is Associated with Increased Perioperative Anemia, Hemoglobin Loss and Drainage Fluid Volume: Analysis of 505 Body Contouring Procedures. Journal of Clinical Medicine. 2025; 14(11):3783. https://doi.org/10.3390/jcm14113783
Chicago/Turabian StyleFlores, Tonatiuh, Jana Schön, Christina Glisic, Kristina Pfoser, Celina Kerschbaumer, Martin S. Mayrl, Klaus F. Schrögendorfer, and Konstantin D. Bergmeister. 2025. "Bariatric Surgery Before Abdominoplasty Is Associated with Increased Perioperative Anemia, Hemoglobin Loss and Drainage Fluid Volume: Analysis of 505 Body Contouring Procedures" Journal of Clinical Medicine 14, no. 11: 3783. https://doi.org/10.3390/jcm14113783
APA StyleFlores, T., Schön, J., Glisic, C., Pfoser, K., Kerschbaumer, C., Mayrl, M. S., Schrögendorfer, K. F., & Bergmeister, K. D. (2025). Bariatric Surgery Before Abdominoplasty Is Associated with Increased Perioperative Anemia, Hemoglobin Loss and Drainage Fluid Volume: Analysis of 505 Body Contouring Procedures. Journal of Clinical Medicine, 14(11), 3783. https://doi.org/10.3390/jcm14113783







