Cognitive Functioning in Toxic Oil Syndrome Survivors: A Case-Control Study Four Decades After the Epidemic
Abstract
1. Introduction
2. Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Study Design and Setting
2.3. Participants
2.4. Measurements
2.4.1. Demographic and Clinical Data
2.4.2. Fatigue Measurement
2.4.3. Health-Related Quality of Life Assessment
2.4.4. Depressive Symptoms
2.4.5. Anxiety Symptoms
2.4.6. Cognitive Performance
2.5. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manuel Tabuenca, J. Toxic-allergic syndrome caused by ingestion of rapeseed oil denatured with aniline. Lancet 1981, 2, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Posada de la Paz, M.; Philen, R.M.; Borda, A.I. Toxic oil syndrome: The perspective after 20 years. Epidemiol. Rev. 2001, 23, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.M.; De La Paz, M.P.; Borda, I.A.; Ruiz-Navarro, M.D.; Philen, R.M.; Falk, H. Toxic oil syndrome: A current clinical and epidemiologic summary, including comparisons with the eosinophilia-myalgia syndrome. J. Am. Coll. Cardiol. 1991, 18, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tello, F.J.; Navas-Palacios, J.J.; Ricoy, J.R.; Conde-Zurita, J.M.; Delgado, F.C.-R.; Tellez, I.; Cabello, A.; Gil-Martín, R.; Madero-García, S. Pathology of a New Toxic Syndrome Caused by Ingestion of Adulterated Oil in Spain. Virchows Arch. [Pathol. Anat.] 1982, 397, 261–285. [Google Scholar] [CrossRef]
- Ricoy, J.R.; Cabello, A.; Rodriguez, J.; Téllez, I. Neuropathological studies on the toxic syndrome related to adulterated rapeseed oil in Spain. Brain 1983, 106 Pt 4, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Ruiz, A.; Calabozo, M.; Perez-Ruiz, F.; Mancebo, L. Toxic oil syndrome. A long-term follow-up of a cohort of 332 patients. Medicine 1993, 72, 285–295. [Google Scholar] [CrossRef]
- de la Paz, M.P.; Philen, R.M.; Gerr, F.; Letz, R.; Arroyo, M.J.F.; Vela, L.; Izquierdo, M.; Arribas, C.M.; Borda, I.A.; Ramos, A.; et al. Neurologic outcomes of toxic oil syndrome patients 18 years after the epidemic. Environ. Health Perspect. 2003, 111, 1326–1334. [Google Scholar] [CrossRef]
- del Ser, T.; Espasandín, P.; Cabetas, I.; Arredondo, J.M. Memory disorders in the toxic oil syndrome (TOS). Arch. Neurobiol. 1986, 49, 19–39. [Google Scholar]
- Kaufman, L.D.; Izquierdo Martinez, M.; Serrano, J.M.; Goez-Reino, J.J. 12-year follow-up study of epidemic Spanish toxic oil syndrome. J. Rheumatol. 1995, 22, 734–740. [Google Scholar] [PubMed]
- Bolster, M.B.; Silver, R.M. Eosinophilia-myalgia syndrome, toxic-oil syndrome, and diffuse fasciitis with eosinophilia. Curr. Opin. Rheumatol. 1994, 6, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ladona, M.G.; Izquierdo-Martinez, M.; de la Paz, M.P.P.; de la Torre, R.; Ampurdanés, C.; Segura, J.; Sanz, E.J. Pharmacogenetic profile of xenobiotic enzyme metabolism in survivors of the Spanish toxic oil syndrome. Environ. Health Perspect. 2001, 109, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Benito-León, J.; Martínez-Martín, P.; Frades, B.; Martínez-Ginés, M.; de Andrés, C.; Meca-Lallana, J.; Antigüedad, A.; Huete-Antón, B.; Rodríguez-García, E.; Ruiz-Martínez, J. Impact of fatigue in multiple sclerosis: The Fatigue Impact Scale for Daily Use (D-FIS). Mult. Scler. J. 2007, 13, 645–651. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Catalan, M.J.; Benito-Leon, J.; Moreno, A.O.; Zamarbide, I.; Cubo, E.; van Blercon, N.; Arillo, V.C.; Pondal, M.; Linazasoro, G.; et al. Impact of fatigue in Parkinson’s disease: The Fatigue Impact Scale for Daily Use (D-FIS). Qual. Life Res. 2006, 15, 597–606. [Google Scholar] [CrossRef]
- Badia, X.; Roset, M.; Herdman, M.; Kind, P. A comparison of United Kingdom and Spanish general population time trade-off values for EQ-5D health states. Med. Decis. Mak. 2001, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. PsycTESTS Dataset 2011. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Dwolatzky, T.; Whitehead, V.; Doniger, G.M.; Simon, E.S.; Schweiger, A.; Jaffe, D.; Chertkow, H. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003, 3, 4. [Google Scholar] [CrossRef]
- Doniger, G.M.; Zucker, D.M.; Schweiger, A.; Dwolatzky, T.; Chertkow, H.; Crystal, H.; Simon, E.S. Towards Practical Cognitive Assessment for Detection of Early Dementia: A 30-Minute Computerized Battery Discriminates as Well as Longer Testing. Curr. Alzheimer Res. 2005, 2, 117–124. [Google Scholar] [CrossRef]
- Schweiger, A.; Abramovitch, A.; Doniger, G.M.; Simon, E.S. A clinical construct validity study of a novel computerized battery for the diagnosis of ADHD in young adults. J. Clin. Exp. Neuropsychol. 2007, 29, 100–111. [Google Scholar] [CrossRef]
- Golan, D.; Wilken, J.; Doniger, G.M.; Fratto, T.; Kane, R.; Srinivasan, J.; Zarif, M.; Bumstead, B.; Buhse, M.; Fafard, L.; et al. Validity of a multi-domain computerized cognitive assessment battery for patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 30, 154–162. [Google Scholar] [CrossRef]
- Doniger, G.M.; Jo, M.-Y.; Simon, E.S.; Crystal, H.A. Computerized cognitive assessment of mild cognitive impairment in urban African Americans. Am. J. Alzheimer’s Dis. Other Demen. 2009, 24, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Catalogna, M.; Sasson, E.; Hadanny, A.; Parag, Y.; Zilberman-Itskovich, S.; Efrati, S. Effects of hyperbaric oxygen therapy on functional and structural connectivity in post-COVID-19 condition patients: A randomized, sham-controlled trial. Neuroimage Clin. 2022, 36, 103218. [Google Scholar] [CrossRef]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 2019, 176, 1143–1157. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B.; Westlye, L.T.; Østby, Y.; Tamnes, C.K.; Jernigan, T.L.; Gamst, A.; Dale, A.M. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage 2010, 50, 1376–1383. [Google Scholar] [CrossRef]
- Roe, C.M.; Xiong, C.; Miller, J.P.; Morris, J.C. Education and Alzheimer disease without dementia: Support for the cognitive reserve hypothesis. Neurology 2007, 68, 223–228. [Google Scholar] [CrossRef]
- Seblova, D.; Berggren, R.; Lövdén, M. Education and age-related decline in cognitive performance: Systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2020, 58, 101005. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.S.; Yu, L.; Lamar, M.; Schneider, J.A.; Boyle, P.A.; Bennett, D.A. Education and cognitive reserve in old age. Neurology 2019, 92, E1041–E1050. [Google Scholar] [CrossRef]
- Bangen, K.J.; Werhane, M.L.; Weigand, A.J.; Edmonds, E.C.; Delano-Wood, L.; Thomas, K.R.; Nation, D.A.; Evangelista, N.D.; Clark, A.L.; Liu, T.T.; et al. Reduced regional cerebral blood flow relates to poorer cognition in older adults with type 2 diabetes. Front. Aging Neurosci. 2018, 10, 390812. [Google Scholar] [CrossRef]
- Petrova, M.; Prokopenko, S.; Pronina, E.; Mozheyko, E. Diabetes type 2, hypertension and cognitive dysfunction in middle age women. J. Neurol. Sci. 2010, 299, 39–41. [Google Scholar] [CrossRef]
- Daugherty, A.M. Hypertension-related risk for dementia: A summary review with future directions. Semin. Cell Dev. Biol. 2021, 116, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Polentinos-Castro, E.; Biec-Amigo, T.; Delgado-Magdalena, M.; Flores-Acosta, J.M.; Sánchez-Perruca, L.; Rabanal-Carrera, A.; Viñas-Calvo, A.; Camarelles-Guillem, F. Enfermedades crónicas y multimorbilidad en pacientes con síndrome de aceite tóxico: Estudio comparativo con población general [Chronic diseases and multimorbidiy in patients with toxic oil syndrome: A comparative study with general population.]. Rev. Esp. Salud Publica 2021, 95, e202104047. (In Spanish) [Google Scholar] [PubMed]
- Portaccio, E.; Amato, M.P. Cognitive impairment in multiple sclerosis: An update on assessment and management. NeuroSci 2022, 3, 667–676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kouvatsou, Z.; Masoura, E.; Kiosseoglou, G.; Kimiskidis, V.K. Evaluating the relationship between working memory and information processing speed in multiple sclerosis. Appl. Neuropsychol. Adult 2022, 29, 695–702. [Google Scholar] [CrossRef]
- Chabriat, H.; Lesnik Oberstein, S. Cognition, mood and behavior in CADASIL. Cereb. Circ. Cogn. Behav. 2022, 3, 100043. [Google Scholar] [CrossRef]
- Jolly, A.A.; Anyanwu, S.; Koohi, F.; Morris, R.G.; Markus, H.S. Prevalence of Fatigue and Associations with Depression and Cognitive Impairment in Patients With CADASIL. Neurology 2025, 104, e213335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Katz, D.I.; Cohen, S.I.; Alexander, M.P. Mild traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.F.I.; Cockle, E. Investigating the effect of fatigue and psychological distress on information processing speed in the postacute period after mild traumatic brain injury in premorbidly healthy adults. Arch. Clin. Neuropsychol. 2021, 36, 918–929. [Google Scholar] [CrossRef]
- Bai, L.; Bai, G.; Wang, S.; Yang, X.; Gan, S.; Jia, X.; Yin, B.; Yan, Z. Strategic white matter injury associated with long-term information processing speed deficits in mild traumatic brain injury. Hum. Brain Mapp. 2020, 41, 4431–4441. [Google Scholar] [CrossRef]
- Nuño, L.; Gómez-Benito, J.; Carmona, V.R.; Pino, O. A systematic review of executive function and information processing speed in major depression disorder. Brain Sci. 2021, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Biasi, M.M.; Manni, A.; Pepe, I.; Abbatantuono, C.; Gasparre, D.; Iaffaldano, P.; Simone, M.; De Caro, M.F.; Trojano, M.; Taurisano, P.; et al. Impact of depression on the perception of fatigue and information processing speed in a cohort of multiple sclerosis patients. BMC Psychol. 2023, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Diniz, B.S.; Butters, M.A.; Albert, S.M.; Dew, M.A.; Reynolds, C.F., 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: Systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 2013, 202, 329–335. [Google Scholar] [CrossRef]
- Yang, L.; Deng, Y.-T.; Leng, Y.; Ou, Y.-N.; Li, Y.-Z.; Chen, S.-D.; He, X.-Y.; Wu, B.-S.; Huang, S.-Y.; Zhang, Y.-R.; et al. Depression, Depression Treatments, and Risk of Incident Dementia: A Prospective Cohort Study of 354,313 Participants. Biol. Psychiatry 2023, 93, 802–809. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; Monforte, A.D.; et al. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef]
- Krupp, L.B.; Elkins, L.E. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology 2000, 55, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Dobryakova, E.; DeLuca, J.; Genova, H.M.; Wylie, G.R. Neural correlates of cognitive fatigue: Cortico-striatal circuitry and effort-reward imbalance. J. Int. Neuropsychol. Soc. 2013, 19, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Téllez, I.; Cabello, A.; Franch, O.; Ricoy, J.R. Chromatolytic changes in the central nervous system of patients with the toxic oil syndrome. Acta Neuropathol. 1987, 74, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ban, S.; Wang, M.; Hua, F.; Wang, L.; Cheng, X.; Tang, Y.; Zhou, H.; Zhai, Y.; Du, X.; et al. Reduced resting-state brain functional network connectivity and poor regional homogeneity in patients with CADASIL. J. Headache Pain. 2019, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.Y.W.; Shea, Y.F.; Chiu, P.K.C.; Kwan, J.S.K.; Mak, H.K.F. Diagnostic Efficacy of Voxel-Mirrored Homotopic Connectivity in Vascular Dementia as Compared to Alzheimer’s Related Neurodegenerative Diseases-A Resting State fMRI Study. Life 2021, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kampaite, A.; Gustafsson, R.; York, E.N.; Foley, P.; MacDougall, N.J.J.; Bastin, M.E.; Chandran, S.; Waldman, A.D.; Meijboom, R. Brain connectivity changes underlying depression and fatigue in relapsing-remitting multiple sclerosis: A systematic review. PLoS ONE 2024, 19, e0299634. [Google Scholar] [CrossRef]
- Drysdale, A.T.; Grosenick, L.; Downar, J.; Dunlop, K.; Mansouri, F.; Meng, Y.; Fetcho, R.N.; Zebley, B.; Oathes, D.J.; Etkin, A.; et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017, 23, 28–38. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Zhong, X.; Wang, Q.; Zhou, H.; Liu, M.; Zhang, M.; Hou, L.; Wu, Z.; Zhang, S.; et al. Disrupted dynamic functional connectivity of hippocampal subregions mediated the slowed information processing speed in late-life depression. Psychol. Med. 2023, 53, 6500–6510. [Google Scholar] [CrossRef]
- Greicius, M.D.; Flores, B.H.; Menon, V.; Glover, G.H.; Solvason, H.B.; Kenna, H.; Reiss, A.L.; Schatzberg, A.F. Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 2007, 62, 429–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Altered amygdala connectivity in individuals with chronic traumatic brain injury and comorbid depressive symptoms. Front. Neurol. 2015, 6, 231. [Google Scholar] [CrossRef]

| Variable | Overall (n = 100) | Control (n = 50) | Patient (n = 50) | p Value |
|---|---|---|---|---|
| Sex, n (%) | 0.284 a | |||
| Male | 32 (32.0) | 19 (38.0) | 13 (26.0) | |
| Female | 68 (68.0) | 31 (62.0) | 37 (74.0) | |
| Age, mean (standard deviation) | 59.3 (8.0) | 58.7 (8.2) | 59.9 (7.8) | 0.449 b |
| Education, n (%) | 0.546 a | |||
| Illiterate or primary studies | 44 (44.0) | 20 (40.0) | 24 (48.0) | |
| Secondary or higher | 56 (56.0) | 30 (60.0) | 26 (52.0) | |
| Central nervous system-acting medications, n (%) | 39 (39.0) | 10 (20.0) | 29 (58.0) | <0.001 a |
| Arterial hypertension, n (%) | 42 (42.0) | 9 (18.0) | 33 (66.0) | <0.001 a |
| Diabetes mellitus, n (%) | 15 (15.0) | 3 (6.0) | 12 (24.0) | 0.025 a |
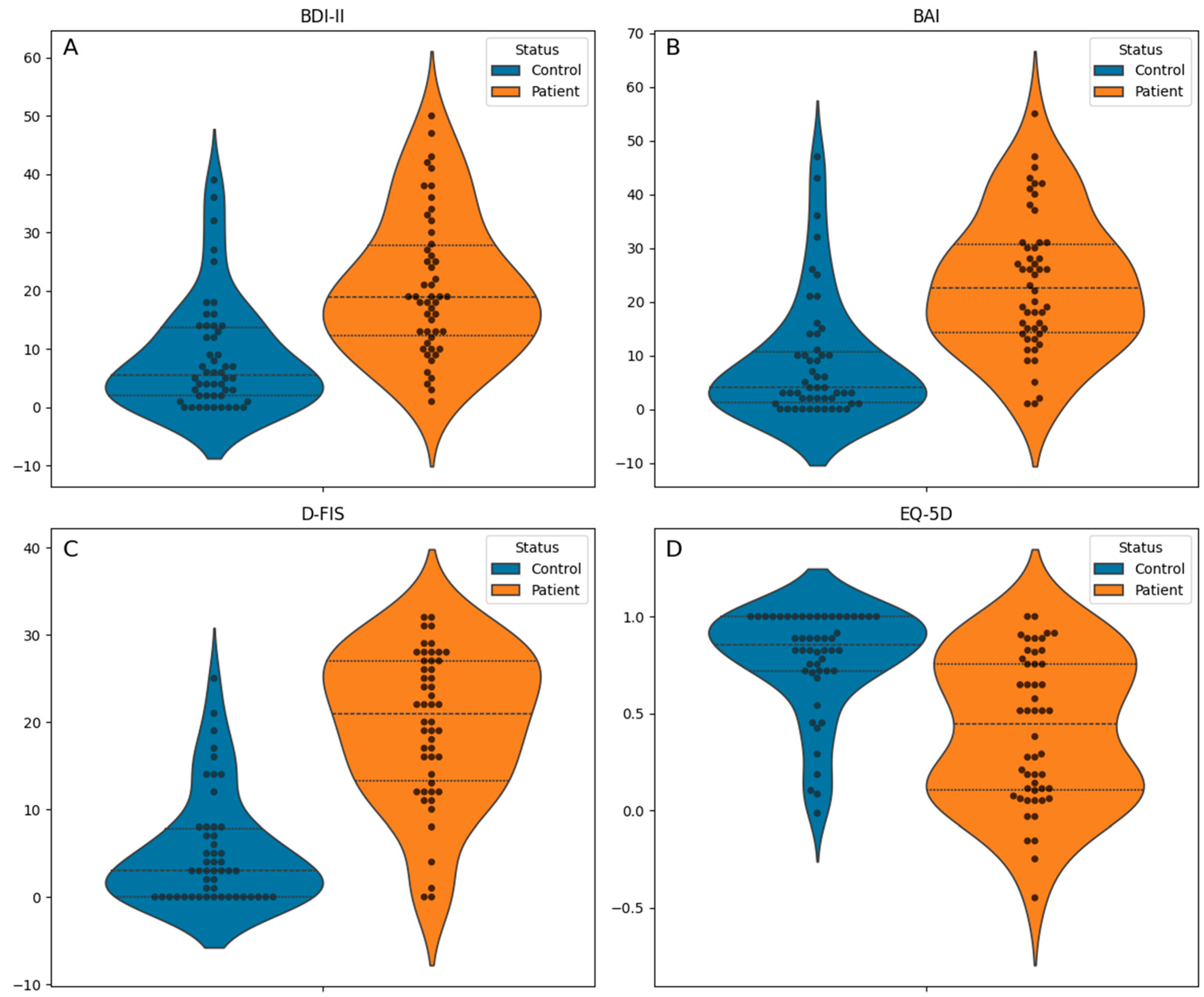
| EQ-5D index, median [Q1, Q3] | 0.7 [0.3, 0.9] | 0.9 [0.7, 1.0] | 0.4 [0.1, 0.8] | <0.001 c |
| Fatigue Impact Scale for Daily Use, mean (standard deviation) | 12.4 (10.4) | 5.2 (6.3) | 19.7 (8.5) | <0.001 b |
| Beck Anxiety Inventory, median [Q1, Q3] | 14.0 [3.0, 26.0] | 4.0 [1.2, 10.8] | 22.5 [14.2, 30.8] | <0.001 c |
| Beck Depression Inventory, median [Q1, Q3] | 13.0 [4.8, 21.0] | 5.5 [2.0, 13.8] | 19.0 [12.2, 27.8] | <0.001 c |
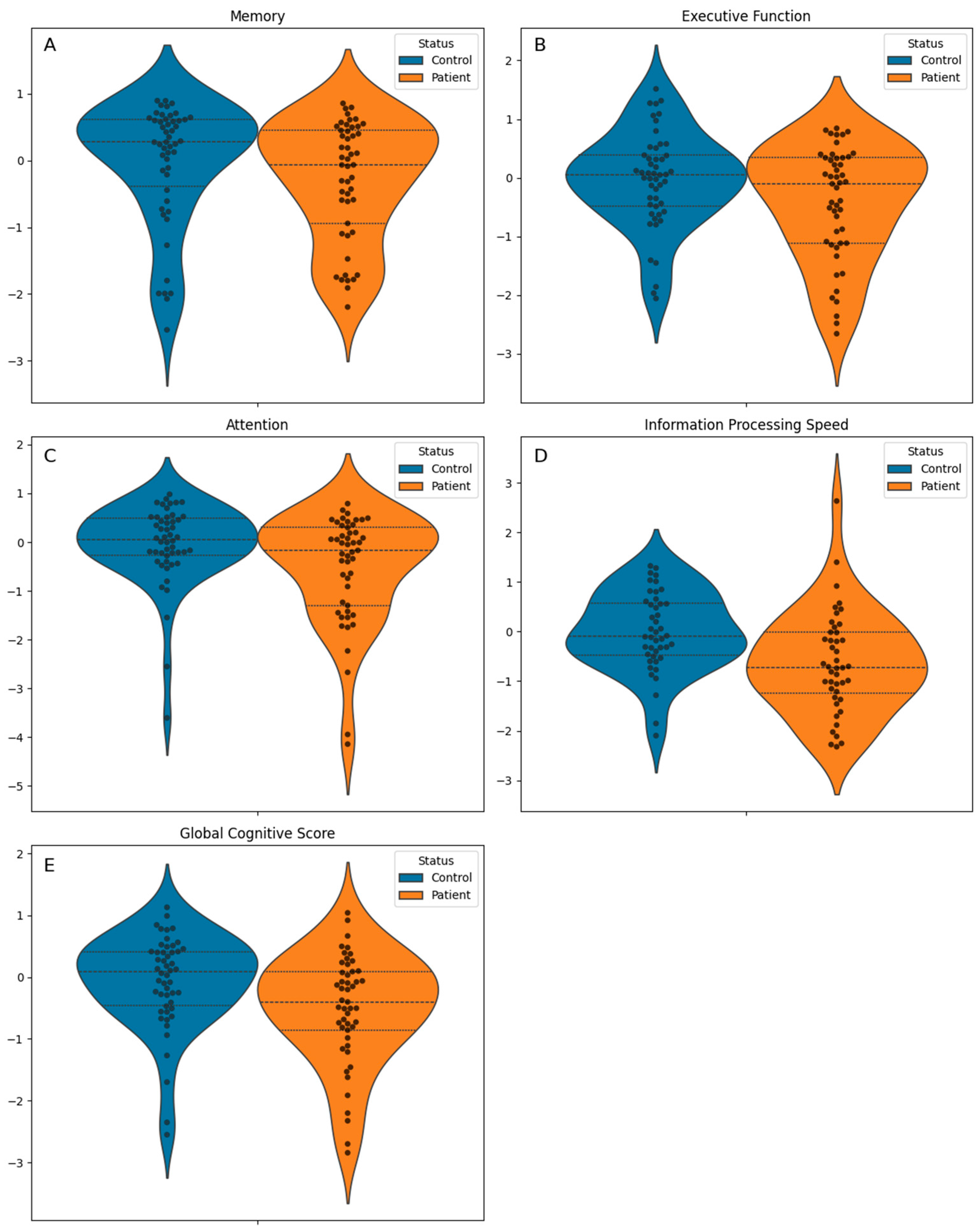
| Global Cognitive Score, mean (standard deviation) | −0.3 (0.9) | −0.1 (0.8) | −0.5 (0.9) | 0.010 b |
| Cognitive domains | ||||
| Memory, median [Q1, Q3] | 0.2 [−0.6, 0.5] | 0.3 [−0.4, 0.6] | −0.1 [−0.9, 0.4] | 0.070 c |
| Executive function, mean (standard deviation) | −0.2 (0.9) | −0.0 (0.8) | −0.4 (1.0) | 0.036 b |
| Attention, median [Q1, Q3] | −0.0 [−0.5, 0.4] | 0.1 [−0.3, 0.5] | −0.2 [−1.3, 0.3] | 0.024 c |
| Information processing speed, mean (standard deviation) | −0.3 (1.0) | −0.0 (0.8) | −0.6 (1.0) | 0.002 b |
| Predictor Variable | Global Cognitive Score Coefficient (p Value) | Memory Coefficient (p Value) | Executive Function Coefficient (p Value) | Attention Coefficient (p Value) | Information Processing Speed Coefficient (p Value) |
|---|---|---|---|---|---|
| Diabetes mellitus | –0.183 (0.294) | 0.044 (0.824) | –0.318 (0.107) | –0.472 (0.050) | 0.170 (0.523) |
| Educational level | 0.437 (0.001) | 0.657 (<0.001) | 0.229 (0.119) | 0.213 (0.231) | 0.644 (0.001) |
| Age squared | –0.002 (0.019) | –0.003 (0.004) | –0.001 (0.271) | –0.003 (0.021) | –0.000 (0.910) |
| Age | 0.176 (0.071) | 0.284 (0.012) | 0.057 (0.603) | 0.254 (0.058) | –0.020 (0.904) |
| Arterial hypertension | 0.220 (0.115) | 0.262 (0.101) | 0.218 (0.164) | 0.199 (0.295) | 0.215 (0.273) |
| Sex (female) | –0.356 (0.006) | 0.025 (0.864) | –0.517 (<0.001) | –0.453 (0.010) | –0.439 (0.018) |
| Toxic oil syndrome diagnosis | –0.382 (0.006) | –0.307 (0.050) | –0.274 (0.074) | –0.369 (0.048) | –0.606 (0.002) |
| Predictor Variable | Depression/Anxiety Composite Coefficient | p Value | Fatigue Impact Scale for Daily Use Coefficient | p Value | Use of Central Nervous System-Acting Medications Coefficient | p Value |
|---|---|---|---|---|---|---|
| Diabetes mellitus | 0.590 | 0.187 | 4.561 | 0.042 | 0.102 | 0.457 |
| Educational level | 0.020 | 0.951 | 2.620 | 0.108 | –0.067 | 0.502 |
| Age | –0.003 | 0.900 | 0.020 | 0.843 | 0.002 | 0.742 |
| Arterial hypertension | –0.003 | 0.994 | 1.345 | 0.445 | 0.024 | 0.824 |
| Sex (female) | 0.040 | 0.902 | 1.304 | 0.418 | 0.117 | 0.243 |
| Toxic oil syndrome diagnosis | 1.695 | <0.001 | 13.062 | <0.001 | 0.328 | 0.003 |
| Global Cognitive Score | Memory | Attention | Executive Function | Information Processing Speed | |
|---|---|---|---|---|---|
| Average Direct Effect | 0.055 | −0.02 | 0.15 | 0.114 | −0.164 |
| Average Causal Mediation Effects (composite variable of depression/anxiety) | −0.062 | −0.241 | 0.003 | −0.076 | 0.005 |
| Average Causal Mediation Effects (Fatigue Impact Scale for Daily Use) | −0.351 | −0.051 | −0.508 | −0.302 | −0.402 |
| Average Causal Mediation Effects (Central Nervous System-Acting Medications) | −0.019 | −0.007 | −0.018 | −0.014 | −0.045 |
| Total Effect | −0.377 | −0.318 | −0.372 | −0.278 | −0.606 |
| Mediated proportion | 1.146 | 0.938 | 1.404 | 1.409 | 0.729 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapeña-Motilva, J.; Ruiz-Ortiz, M.; Doniger, G.M.; Nogales, M.A.; Giménez de Bejar, V.; Álvarez-Sesmero, S.; Morales, M.; Bartolomé, F.; Alquézar, C.; Lahiri, D.; et al. Cognitive Functioning in Toxic Oil Syndrome Survivors: A Case-Control Study Four Decades After the Epidemic. J. Clin. Med. 2025, 14, 3746. https://doi.org/10.3390/jcm14113746
Lapeña-Motilva J, Ruiz-Ortiz M, Doniger GM, Nogales MA, Giménez de Bejar V, Álvarez-Sesmero S, Morales M, Bartolomé F, Alquézar C, Lahiri D, et al. Cognitive Functioning in Toxic Oil Syndrome Survivors: A Case-Control Study Four Decades After the Epidemic. Journal of Clinical Medicine. 2025; 14(11):3746. https://doi.org/10.3390/jcm14113746
Chicago/Turabian StyleLapeña-Motilva, José, Mariano Ruiz-Ortiz, Glen M. Doniger, María Antonia Nogales, Verónica Giménez de Bejar, Sonia Álvarez-Sesmero, Montserrat Morales, Fernando Bartolomé, Carolina Alquézar, Durjoy Lahiri, and et al. 2025. "Cognitive Functioning in Toxic Oil Syndrome Survivors: A Case-Control Study Four Decades After the Epidemic" Journal of Clinical Medicine 14, no. 11: 3746. https://doi.org/10.3390/jcm14113746
APA StyleLapeña-Motilva, J., Ruiz-Ortiz, M., Doniger, G. M., Nogales, M. A., Giménez de Bejar, V., Álvarez-Sesmero, S., Morales, M., Bartolomé, F., Alquézar, C., Lahiri, D., García-Cena, C., & Benito-León, J. (2025). Cognitive Functioning in Toxic Oil Syndrome Survivors: A Case-Control Study Four Decades After the Epidemic. Journal of Clinical Medicine, 14(11), 3746. https://doi.org/10.3390/jcm14113746









