Treatment and Outcomes of COVID-19 Infection in Pregnant Women: Systematic Review of Cases Reported in Europe
Abstract
1. Introduction
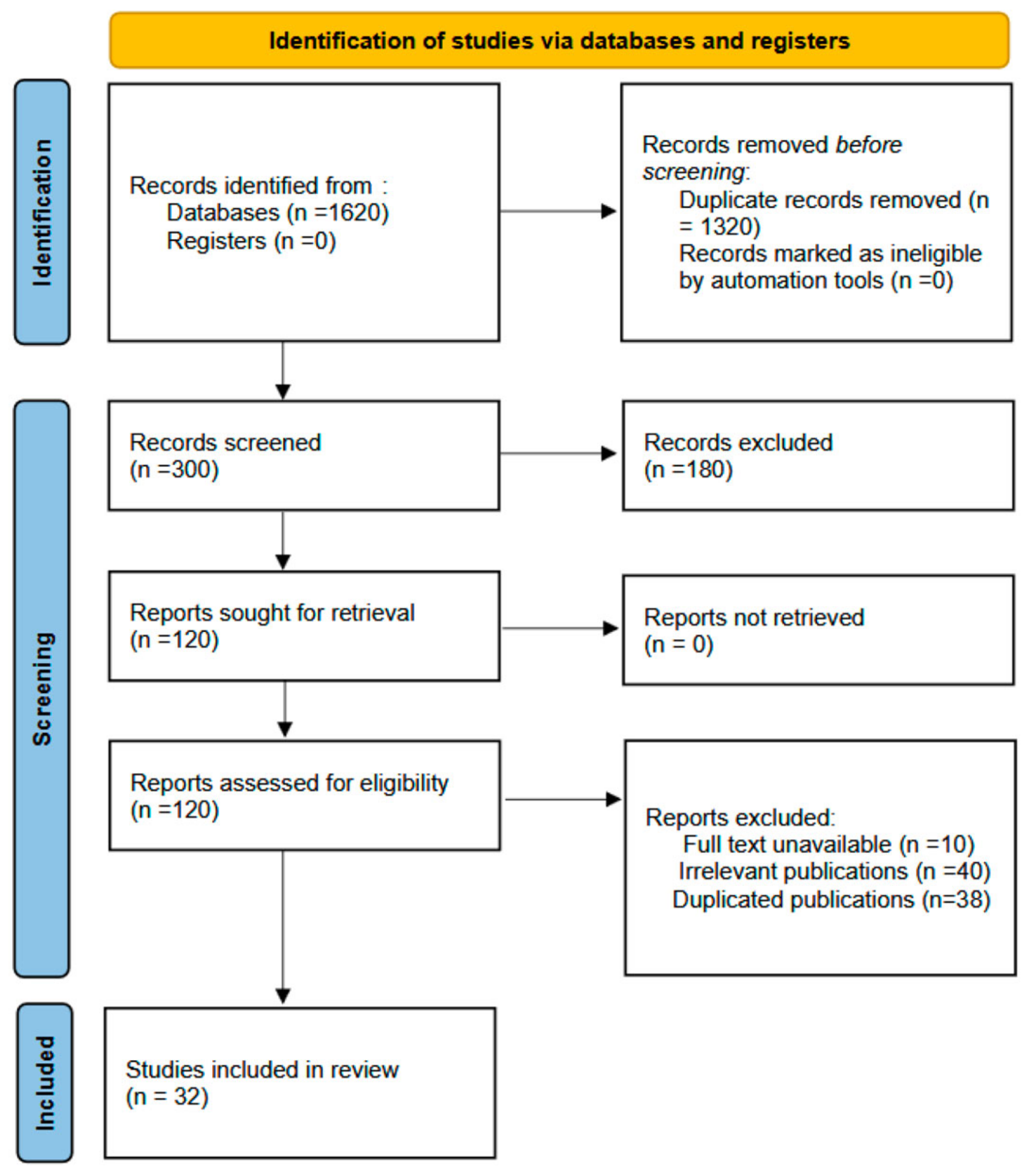
2. Materials and Methods
2.1. Data Collection and Analysis
2.2. Assessment of the Risk of Bias of Included Studies
2.3. Measures of the Study Variables
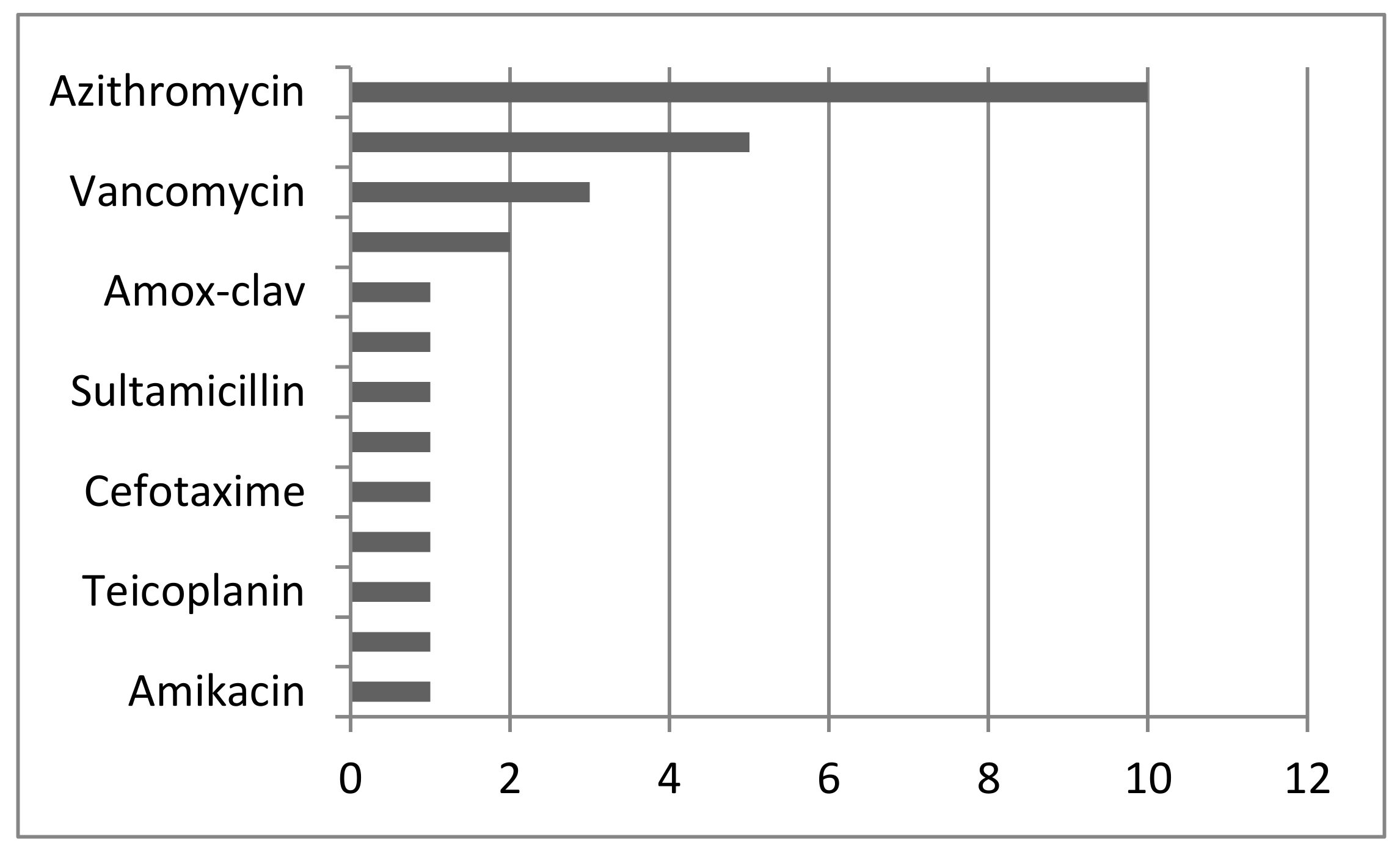
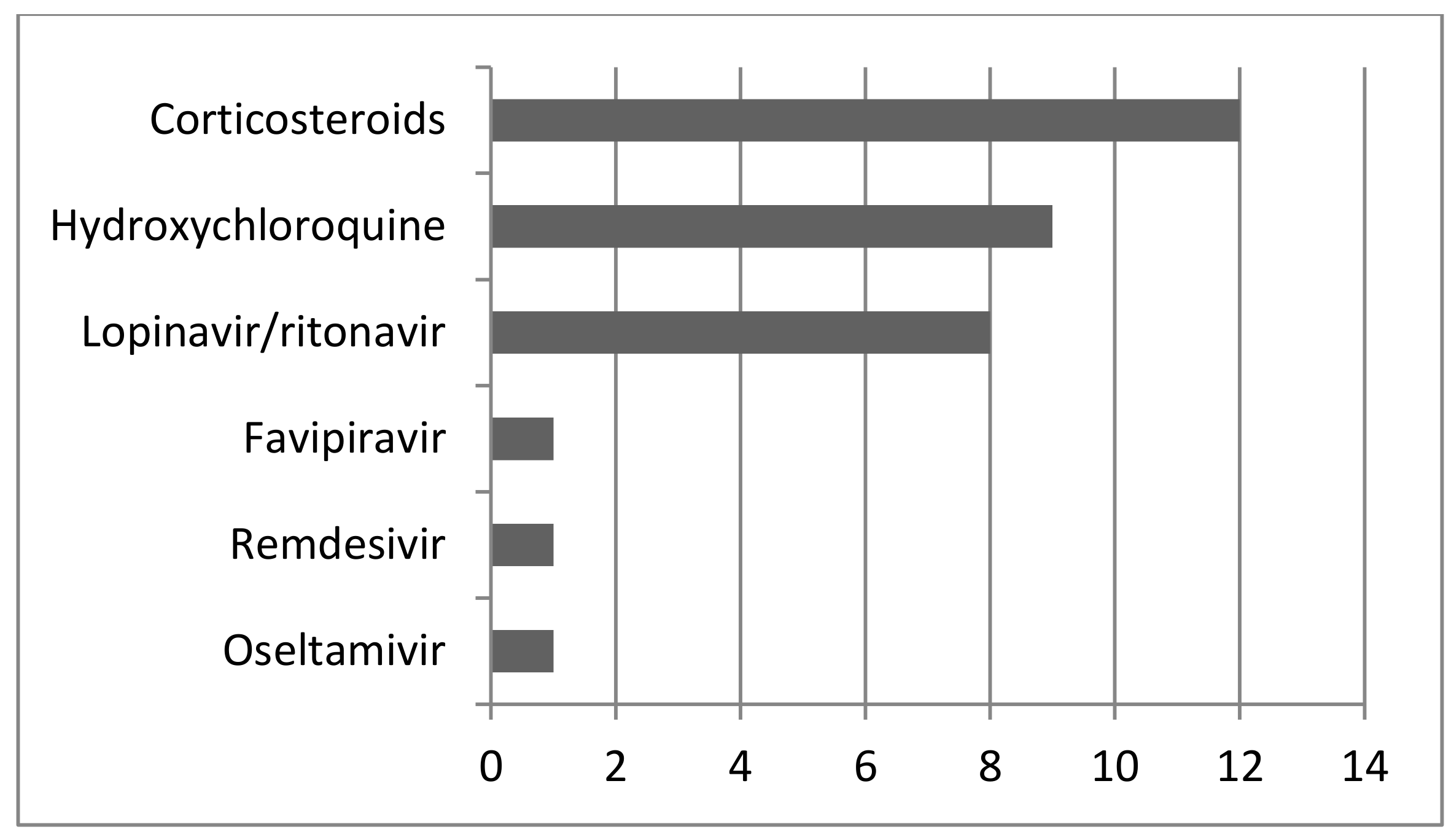
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nana, M.; Nelson-Piercy, C. COVID-19 in pregnancy. Clin. Med. 2021, 21, e446–e450. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-M.; Ahmadi, H.; Huo, L.; Lix, L.M.; Maslin, K.; Latour, J.M.; Shawe, J. COVID-19 and pregnancy: A comprehensive study of comorbidities and outcomes. BMC Public Health 2024, 24, 3157. [Google Scholar] [CrossRef] [PubMed]
- Vousden, N.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; Knight, M. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from March to September 2020: A national cohort study using the UK Obstetric Surveillance System (UKOSS). PLoS ONE 2021, 16, e0251123. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef]
- Jamieson, D.J.; Theiler, R.N.; Rasmussen, S.A. Emerging infections and pregnancy. Emerg. Infect Dis. 2006, 12, 1638–1643. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. RCOG. 2021. Available online: www.rcog.org.uk/globalassets/documents/guidelines/2021-02-19-coronavirus-covid-19-infection-in-pregnancy-v13.pdf (accessed on 10 January 2025).
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic Dis. 2021, 18, E66. [Google Scholar] [CrossRef]
- Blumberg, D.A.; Underwood, M.A.; Hedriana, H.L.; Lakshminrusimha, S. Vertical transmission of SARS–CoV-2: What is the optimal definition? Am. J. Perinatol. 2020, 37, 769–772. [Google Scholar] [CrossRef]
- Shah, P.S.; Diambomba, Y.; Acharya, G.; Morris, S.K.; Bitnun, A. Classification system and case defini-tion for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet. Gynecol. Scand. 2020, 99, 565–568. [Google Scholar] [CrossRef]
- World Health Organization. Definition and Categorization of the Timing of Mother-to-Child Transmission of SARS-CoV-2 Scientific Brief. 2021. Available online: https://apps.who.int/iris/handle/10665/339422 (accessed on 11 November 2024).
- Oshay, R.R.; Chen, M.Y.; Fields, B.K.; Demirjian, N.L.; Lee, R.S.; Mosallaei, D.; Gholamrezanezhad, A. COVID-19 in pregnancy: A systematic review of chest CT findings and associated clinical features in 427 patients. Clin. Imaging 2021, 75, 75–82. [Google Scholar] [CrossRef]
- Mattar, C.N.; Kalimuddin, S.; Sadarangani, S.P.; Tagore, S.; Thain, S.; Thoon, K.C.; Hong, E.Y.; Kanneganti, A.; Ku, C.W.; Chan, G.M.; et al. Pregnancy Outcomes in COVID-19: A Prospective Cohort Study in Singapore. Ann. Acad. Med. 2020, 49, 857–869. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, J.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Agbontaen, K.O.; Somasundram, K.; Baker, M. Critical COVID-19 in a 24-week pregnant woman with 32 days of invasive mechanical ventilation before delivery of fetus: A case of successful collaborative multidisciplinary care. BMJ Case Rep. 2021, 14, e243516. [Google Scholar] [CrossRef]
- Ambrož, R.; Stašek, M.; Molnár, J.; Špička, P.; Klos, D.; Hambálek, J.; Skanderová, D. Spontaneous liver rupture following SARS-CoV-2 infection in late pregnancy: A case report. World J. Clin. Cases 2022, 10, 5042–5050. [Google Scholar] [CrossRef] [PubMed]
- Anness, A.; Siddiqui, F. COVID-19 complicated by hepatic dysfunction in a 28-week pregnant woman. BMJ Case Rep. 2020, 13, e237007. [Google Scholar] [CrossRef]
- Bağlı, İ.; Öcal, E.; Yavuz, M.; Uzundere, O.; Bozkurt, F. Maternal deaths due to COVID-19 disease: The cases in a single center pandemic hospital in the south east of Turkey. J. Obstet. Gynaecol. Res. 2021, 47, 4067–4076. [Google Scholar] [CrossRef]
- Berić, S.; Nesek Adam, V.; Šklebar, I.; Klancir, T.; Žižak, M.; Mličević, M. Whole Range of Respiratory Support in a Pregnant Woman with a Severe Form of COVID-19 Infection: A Case Report. Acta Clin. Croat. 2023, 62, 154–159. [Google Scholar] [CrossRef]
- Clemenza, S.; Zullino, S.; Vacca, C.; Simeone, S.; Serena, C.; Rambaldi, M.P.; Ottanelli, S.; Vannuccini, S.; Bonizzoli, M.; Peris, A.; et al. Perinatal outcomes of pregnant women with severe COVID-19 requiring extracorporeal membrane oxygenation (ECMO): A case series and literature review. Arch. Gynecol. Obstet. 2022, 305, 1135–1142. [Google Scholar] [CrossRef]
- Craina, M.; Iacob, D.; Dima, M.; Bernad, S.; Silaghi, C.; Moza, A.; Pantea, M.; Gluhovschi, A.; Bernad, E. Clinical, Laboratory, and Imaging Findings of Pregnant Women with Possible Vertical Transmission of SARS-CoV-2-Case Series. Int. J. Environ. Res. Public Health 2022, 19, 10916. [Google Scholar] [CrossRef]
- De Nardo, M.C.; Bellomo, A.R.; Perfetti, F.; Battaglia, F.A.; Lichtner, M.; Lubrano, R. Impact of joint management of a COVID-19 mother and her newborn on the virus transmission: A case report. Virol. J. 2021, 18, 130. [Google Scholar] [CrossRef]
- Esposito, P.; Cappadona, F.; Sangregorio, F.; Costa, E.; Mallia, L.; Zanetti, V.; Nescis, L.; Bianzina, S.; Ferrari, F.; Patroniti, N.A.; et al. Combined extracorporeal CO2 removal and renal replacement therapy in a pregnant patient with COVID-19: A case report. G. Ital. Nefrol. 2023, 40, 2023. [Google Scholar]
- Federici, L.; Picone, O.; Dreyfuss, D.; Sibiude, J. Successful continuation of pregnancy in a patient with COVID-19-related ARDS. BMJ Case Rep. 2020, 13, e237511. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.; Falcão, V.; Pinto, M.J.; Ramalho, C. Peripheral facial paralysis as presenting symptom of COVID-19 in a pregnant woman. BMJ Case Rep. 2020, 13, e237146. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Piscitelli, M.; Adodo, D.K.; Thomas, C.; Dessap, A.M.; Bagate, F.; Folliguet, T. Successful Use of Extracorporeal Membrane Oxygenation Postpartum as Rescue Therapy in a Woman With COVID-19. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2140–2143. [Google Scholar] [CrossRef]
- Giavoli, C.; Iurlaro, E.; Morelli, V.; Rodari, G.; Ronchi, A.; Pietrasanta, C.; Pugni, L.; Tubiolo, D.; Properzi, P.; Pesenti, A.; et al. Case Report: Late-Onset Congenital Adrenal Hyperplasia and Acute Covid-19 Infection in a Pregnant Woman: Multidisciplinary Management. Front. Endocrinol. 2021, 11, 602535. [Google Scholar] [CrossRef]
- Lyra, J.; Valente, R.; Rosário, M.; Guimarães, M. Cesarean Section in a Pregnant Woman with COVID-19: First Case in Portugal. Acta Med. Port. 2020, 33, 429–431. [Google Scholar] [CrossRef]
- Maier, J.T.; Zickler, D.; Metz, M.; Jebens, A.; Jarchau, U.; Fischer, J.; Kimmel, V.; Prueter, E.; Hellmeyer, L. Peripartum Covid-19 Pneumonia with Severe ARDS—A Case Report. Z. Geburtshilfe Neonatol. 2021, 225, 183–187. [Google Scholar] [CrossRef]
- Garcia-Manau, P.; Garcia-Ruiz, I.; Rodo, C.; Sulleiro, E.; Maiz, N.; Catalan, M.; Fernández-Hidalgo, N.; Balcells, J.; Antón, A.; Carreras, E.; et al. Fetal Transient Skin Edema in Two Pregnant Women with Coronavirus Disease 2019 (COVID-19). Obstet. Gynecol. 2020, 136, 1016–1020. [Google Scholar] [CrossRef]
- Mihajlovic, S.; Savic, P.; Potparevic, N.; Lackovic, M. Outcomes of mechanical ventilation in COVID-19 pregnant patients. Hippokratia 2022, 26, 32–37. [Google Scholar]
- Diago-Muñoz, D.; Martínez-Varea, A.; Pérez-Sancho, E.; Diago-Almela, V. Severe COVID-19 Infection during Pregnancy Requiring ECMO: Case Report and Review of the Literature. J. Pers. Med. 2023, 13, 263. [Google Scholar] [CrossRef]
- Palalioglu, R.M.; Mahammadaliyeva, A.; Erbiyik, H.I.; Muhcu, M. COVID-19 in third trimester may not be as scary as you think, it can be innocent: Evaluating vertical transmission from a COVID-19 positive asymptomatic pregnant woman with early membrane rupture. J. Obstet. Gynaecol. Res. 2021, 47, 838–842. [Google Scholar] [CrossRef]
- Palmrich, P.; Roessler, B.; Wisgrill, L.; Kampf, S.; Gattinger, P.; Valenta, R.; Fleischmann, E.; Berger, A.; Kiss, H.; Farr, A. Multiprofessional perinatal care in a pregnant patient with acute respiratory distress syndrome due to COVID-19. BMC Pregnancy Childbirth 2021, 21, 587. [Google Scholar] [CrossRef] [PubMed]
- Pikovsky, M.; Tan, M.Y.; Ahmed, A.; Sykes, L.; Agha-Jaffar, R.; Yu, C.K.H. Euglycaemic ketoacidosis in pregnant women with COVID-19: Two case reports. BMC Pregnancy Childbirth 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Ravn, A.; Eysturoy, A.; Reynstind, D.; Nielsen, C.M.; Walther-Larsen, S. Præterm forløsning ved sectio hos en kvinde med svær COVID-19-pneumoni. Ugeskr Læger 2021, 183, V10200779. [Google Scholar] [PubMed]
- Reindorf, M.; Newman, J.; Ingle, T. Successful use of CPAP in a pregnant patient with COVID-19 pneumonia. BMJ Case Rep. 2021, 14, e238055. [Google Scholar] [CrossRef]
- Garcia Rodriguez, A.; Marcos Contreras, S.; Fernandez Manovel, S.M.; Marcos Vidal, J.M.; Diez Buron, F.; Fernandez Fernandez, C.; Riveira Gonzalez, M.D.C. SARS-COV-2 infection during pregnancy, a risk factor for eclampsia or neurological manifestations of COVID-19? Case report. BMC Pregnancy Childbirth 2020, 20, 587. [Google Scholar] [CrossRef]
- Rosner-Tenerowicz, A.; Fuchs, T.; Zimmer-Stelmach, A.; Pomorski, M.; Trzeszcz, M.; Zwierzchowski, J.; Zimmer, M. Placental pathology in a pregnant woman with severe COVID-19 and successful ECMO treatment: A case report. BMC Pregnancy Childbirth 2021, 21, 760. [Google Scholar] [CrossRef]
- Sileo, F.G.; Tramontano, A.L.; Leone, C.; Meacci, M.; Gennari, W.; Ternelli, G.; La Marca, A.; Lugli, L.; Berardi, A.; Facchinetti, F.; et al. Pregnant woman infected by Coronavirus disease (COVID-19) and calcifications of the fetal bowel and gallbladder. Minerva Obstet. Gynecol. 2021, 73, 121–124. [Google Scholar] [CrossRef]
- Smeele, H.T.; Perez-Garcia, L.F.; Grimminck, K.; Schoenmakers, S.; Mulders, A.G.; Dolhain, R.J. Systemic lupus erythematosus and COVID-19 during pregnancy. Lupus 2021, 30, 1188–1191. [Google Scholar] [CrossRef]
- Socolov, R.; Akad, M.; Păvăleanu, M.; Popovici, D.; Ciuhodaru, M.; Covali, R.; Akad, F.; Păvăleanu, I. The Rare Case of a COVID-19 Pregnant Patient with Quadruplets and Postpartum Severe Pneumonia. Case Report and Review of the Literature. Medicina 2021, 57, 1186. [Google Scholar] [CrossRef]
- Stout, A.; Crichton, R.; Tahmasebi, F. Maternal death secondary to COVID-19 infection: A case report and review of the literature. Obstet. Med. 2021, 14, 248–252. [Google Scholar] [CrossRef]
- Van Amesfoort, J.E.; E Werter, D.; Painter, R.C.; Hermans, F.J.R. Severe metabolic ketoacidosis as a primary manifestation of SARS-CoV-2 infection in non-diabetic pregnancy. BMJ Case Rep. 2021, 14, e241745. [Google Scholar] [CrossRef] [PubMed]
- Vibert, F.; Kretz, M.; Thuet, V.; Barthel, F.; De Marcillac, F.; Deruelle, P.; Lecointre, L. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Yassa, M.; Birol, P.; Mutlu, A.M.; Tekin, A.B.; Sandal, K.; Tug, N. Lung Ultrasound Can Influence the Clinical Treatment of Pregnant Women With COVID-19. J. Ultrasound Med. 2021, 40, 191–203. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/on (accessed on 20 February 2025).
- Lui, G.; Guaraldi, G. Drug treatment of COVID-19 infection. Curr. Opin. Pulm. Med. 2023, 29, 174–183. [Google Scholar] [CrossRef]
- Mullins, E.; Evans, D.; Viner, R.M.; O’Brien, P.; Morris, E. Coronavirus in pregnancy and delivery: Rapid review. Ultrasound Obstet. Gynecol. 2020, 55, 586–592. [Google Scholar] [CrossRef]
- Reis, H.L.B.D.; Boldrini, N.A.T.; Caldas, J.V.J.; Paz, A.P.C.D.; Ferrugini, C.L.P.; Miranda, A.E. Severe coronavirus infection in pregnancy: Challenging cases report. Rev. Inst. Med. Trop. 2020, 62, e49. [Google Scholar] [CrossRef]
- Hammad, W.A.B.; Al Beloushi, M.; Ahmed, B.; Konje, J.C. Severe acute respiratory syndrome (SARS) coronavirus-2 infection (COVID-19) in pregnancy—An overview. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 106–116. [Google Scholar] [CrossRef]
- Dashraath, P.; Wong, J.L.J.; Lim, M.X.K.; Lim, L.M.; Li, S.; Biswas, A.; Choolani, M.; Mattar, C.; Su, L.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020, 222, 521–531. [Google Scholar] [CrossRef]
- Sison, S.M.; Sivakumar, G.K.; Caufield-Noll, C.; Greenough, W.B.; Oh, E.S.; Galiatsatos, P. Mortality outcomes of patients on chronic mechanical ventilation in different care settings: A systematic review. Heliyon 2021, 7, e06230. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sun, W.; Yang, S.; Liu, T.; Hou, N. The impact of COVID-19 infections on pregnancy outcomes in women. BMC Pregnancy Childbirth 2024, 24, 562. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Cheng, S.-F.; Kuo, C.-L.; Huang, C.-Y.; Wu, C.-H. Gestational weight gain patterns as predictors of cesarean deliveries in women diagnosed with gestational diabetes mellitus. BMC Pregnancy Childbirth 2025, 25, 79. [Google Scholar] [CrossRef]
- Berton, D.; Kalil, A.C.; Teixeira, P. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2014, 10, CD006482. [Google Scholar] [CrossRef]
- Prasad, V.; Haslam, A. COVID-19 vaccines: History of the pandemic’s great scientific success and flawed policy implementation. Monash Bioeth. Rev. 2024, 42, 28–54. [Google Scholar] [CrossRef]
- Manchanda, K.; Singh, J.; Bhagat, R.; Tiwana, I.K.; Singh, H. Safety of pharmacological options for the management of COVID-19 in pregnant women: An Indian perspective. Int. J. Risk Saf. Medicine 2021, 32, 3–17. [Google Scholar] [CrossRef]
- Huybrechts, K.F.; Bateman, B.T.; Zhu, Y.; Straub, L.; Mogun, H.; Kim, S.C.; Desai, R.J.; Hernandez-Diaz, S. Hydroxychloroquine early in pregnancy and risk of birth defects. Am. J. Obstet. Gynecol. 2021, 224, 290.e1–290.e22. [Google Scholar] [CrossRef]
- Yao, T.T.; Quian, J.D.; Zhu, W.Y.; Wang, Y.; Wang, G.Q. A systematic review of lopinavir therapy of SARS coronavirus and MERS coronavirus—A possible reference for coronavirus disease 19 treatment option. J. Med. Virol. 2020, 92, 556–563. [Google Scholar] [CrossRef]
- Salvatore, M.A.; Corsi Decenti, E.; Bonasoni, M.P.; Botta, G.; Castiglione, F.; D’Armiento, M.; Fulcheri, E.; Nebuloni, M.; Donati, S. The ItOSS Covid-Working Group. Placental Characteristics of a Large Italian Cohort of SARS-CoV-2-Positive Pregnant Women. Microorganisms 2022, 10, 1435. [Google Scholar] [CrossRef]
- Sotiriou, S.; Samara, A.A.; Tsiamalou, I.A.; Donoudis, C.; Seviloglou, E.; Skentou, C.; Garas, A.; Daponte, A. Placental Ultrasonographical Findings during SARS-CoV-2 Infection. Diagnostics 2022, 12, 974. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Alipour, Z.; Samadi, P.; Eskandari, N.; Ghaedrahmati, M.; Vahedian, M.; Khalajinia, Z.; Mastanijahroodi, A. Relationship between coronavirus disease 2019 in pregnancy and maternal and fetal outcomes: Retrospective analytical cohort study. Midwifery 2021, 102, 103128. [Google Scholar] [CrossRef] [PubMed]
- Faraz, S.; Aftab, N.; Ammar, A.; Al Mulai, I.; Paulose, L.; Fernandes, S. An insight on the maternal-fetal outcomes of critically ill pregnant women during the Second Wave of COVID-19. Cureus 2022, 14, e20998. [Google Scholar] [CrossRef] [PubMed]
| No of Case | Publication ID | Study Design | Attrition/Reporting Bias | Age and BMI | Length of Hospitalization (Days) | Week of Pregnancy When COVID-19 Was Diagnosed | Morphological Diagnosis of Pneumonia |
|---|---|---|---|---|---|---|---|
| 1 | Agbontaen et al., 2021 [14] | Case report | Low/Low | 42/27.6 | 54 | 24 | CT |
| 2 | Ambroz et al., 2022 [15] | Case report | High/High | 32/NA | NA | 32 | CT |
| 3 | Anness et al., 2020 [16] | Case report | Low/Low | 35/NA | 160 | 28 | RTG |
| 4 | Baglı et al., 2021 [17] | Case series | Low/Low | 34/26 | NA | 34 | CT |
| 5 | Baglı et al., 2021 [17] | Case series | Low/Low | 37/28 | NA | 36 | CT |
| 6 | Baglı et al., 2021 [17] | Case series | Low/Low | 33/33 | NA | 33 | CT |
| 7 | Baglı et al., 2021 [17] | Case series | Low/Low | 39/30 | NA | 35 | CT |
| 8 | Beric et al., 2023 [18] | Case report | High/Low | 35/NA | 47 | 28 | CT and RTG |
| 9 | Clemenza et al., 2022 [19] | Case series | Low/Low | 27/NA | 120 | 18 | LUS |
| 10 | Clemenza et al., 2022 [19] | Case series | Low/Low | 38/NA | 60 | 28 | LUS |
| 11 | Clemenza et al., 2022 [19] | Case series | Low/Low | 43/38 | 90 | 38 | LUS |
| 12 | Craina et al., 2022 [20] | Case series | Low/Low | 35/26 | NA | 38 | NA |
| 13 | Craina et al., 2022 [20] | Case series | Low/Low | 21/26 | NA | 39 | NA |
| 14 | Craina et al., 2022 [20] | Case series | Low/Low | 35/26 | NA | 39 | NA |
| 15 | De Nardo et al., 2021 [21] | Case report | High/High | 36/NA | 30 | 40 | NA |
| 16 | Esposito et al., 2023 [22] | Case report | Low/Low | 34/NA | 60 | 19 | CT |
| 17 | Federici et al., 2020 [23] | Case report | Low/Low | 33/NA | 20 | 23 | RTG |
| 18 | Figuerido et al., 2020 [24] | Case report | Low/Low | 35/39 | 20 | 39 | NA |
| 19 | Fiore et al., 2021 [25] | Case report | High/Low | 31/31 | NA | 31 | RTG and CT |
| 20 | Giavioli et al., 2021 [26] | Case report | High/Low | 39/35.2 | 35 | 36 | CT |
| 21 | Lyra et al., 2020 [27] | Case report | High/Low | 35/NA | 3 | 39 | None |
| 22 | Maier et al., 2020 [28] | Case report | Low/Low | 38 | NA | 38 | RTG and CT |
| 23 | Manau et al., 2020 [29] | Case series | High/High | 50/25 | 17 | 22 | RTG |
| 24 | Manau et al., 2020 [29] | Case series | High/High | 30/32 | 22 | 20 | RTG |
| 25 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 42/25.6 | NA | 29 | NA |
| 24 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 29/24 | NA | 27 | NA |
| 27 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 32/30 | NA | 33.6 | NA |
| 28 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 38/32 | NA | 27 | NA |
| 29 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 33/27 | NA | 33 | NA |
| 30 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 26/46 | NA | 29.33 | NA |
| 31 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 30/32 | NA | 29.4 | NA |
| 32 | Mihajlovic et al., 2022 [30] | Case series | High/Low | 30/32 | NA | 14 | NA |
| 33 | Munoz et al., 2023 [31] | Case report | Low/High | 40/NA | NA | 23 | RTG |
| 34 | Palailoglu et al., 2021 [32] | Case report | Low/Low | 42/NA | NA | 37 | CT |
| 35 | Palmrich et al., 2021 [33] | Case report | Low/Low | 36/50 | 13 | 27 | RTG |
| 36 | Pikovsky et al., 2021 [34] | Case series | Low/Low | 34/25 | 12 | 35 | CT |
| 37 | Pikovsky et al., 2021 [34] | Case series | Low/Low | 34/28 | 5 | 36 | CT |
| 38 | Ravn et al., 2021 [35] | Case report | High/High | 30/NA | NA | 33 | CT |
| 39 | Reindorf et al., 2021 [36] | Case report | Low/Low | 35/NA | 5 | 27 | RTG |
| 40 | Rodrigues et al., 2020 [37] | Case report | High/High | 35/NA | 3 | 40 | CT |
| 41 | Rosner Tenerowich et al., 2021 [38] | Case report | Low/Low | 38/NA | 60 | 27 | NA |
| 42 | Sileo et al., 2021 [39] | Case report | High/Low | 30/NA | NA | 38 | None |
| 43 | Smeele et al., 2021 [40] | Case series | High/Low | 31/NA | 2 | 39 | None |
| 44 | Smeele et al., 2021 [40] | Case series | High/Low | 39/NA | 70 | 19 | None |
| 45 | Sokolov et al., 2022 [41] | Case report | High/Low | 37/NA | NA | 34 | RTG |
| 46 | Stout et al., 2020 [42] | Case report | Low/Low | 28/24 | 19 | 36 | RTG and CT |
| 47 | van Amesfort et al., 2021 [43] | Case report | Low/Low | 21/NA | 15 | 37 | CT |
| 48 | Vibert et al., 2020 [44] | Case report | High/High | 21/NA | NA | 23 | CT |
| 49 | Yassa et al., 2021 [45] | Case series | Low/High | 32/NA | NA | 39 | LUS |
| 50 | Yassa et al., 2021 [45] | Case series | Low/High | 32/NA | NA | 27 | LUS |
| 51 | Yassa et al., 2021 [45] | Case series | Low/High | 33/NA | NA | 20 | LUS and CT |
| 52 | Yassa et al., 2021 [45] | Case series | Low/High | 19/NA | NA | 9 | LUS |
| 53 | Yassa et al., 2021 [45] | Case series | Low/High | 41/NA | NA | 17 | LUS and CT |
| 54 | Yassa et al., 2021 [45] | Case series | Low/High | 40/NA | NA | 7 | LUS and CT |
| 55 | Yassa et al., 2021 [45] | Case series | Low/High | 23/NA | NA | 10 | LUS and RTG |
| 56 | Yassa et al., 2021 [45] | Case series | Low/High | 40/NA | NA | 38 | LUS and CT |
| No of Case | Publication ID | Antibiotic Treatment of the Patient BD | Antivirotic Treatment of the Patient BD | Respiratory Support; Method of Delivery | Outcome of the Mother and the Children (Weight and Apgar Score at 1 and 5 Min) |
|---|---|---|---|---|---|
| 1 | Agbontaen et al., 2021 [14] | Piperacilin tazobactam, clindamycin; meropenem and amikacin | NA | No; CS | Survived; survived 1164 g |
| 2 | Ambroz et al., 2022 [15] | None | None | MV; CS | Survived; death |
| 3 | Anness et al., 2020 [16] | NA | NA | No; ND | Survived; survived 3200 g |
| 4 | Baglı et al., 2021 [17] | Vancomycin | Lopinavir-ritonavir | No; CS | Died; survived 2500; 5, 8 |
| 5 | Baglı et al., 2021 [17] | None | None | MV; CS | Died; survived 3000; 6, 8 |
| 6 | Baglı et al., 2021 [17] | Klaritromicine; Carbapenems | Lopinavir-ritonavir | MV; CS | Died; survived 2000; 7, 8 |
| 7 | Baglı et al., 2021 [17] | Piperacillin tazobactame and teicoplanin | Lopinavinir-ritonavir | MV; CS | Died; survived 2250; 8, 9 |
| 8 | Beric et al., 2023 [18] | NA | NA | MV, ECMO; CS | Survived; NA |
| 9 | Clemenza et al., 2022 [19] | Piperacillin-tazobactam and azithromycin; vancomycin | NA | ECMO; CS | Survived; survived 9 and 92,670 g |
| 10 | Clemenza et al., 2022 [19] | Oxacilin; vancomycin; piperacillin tazobactam | NA | ECMO; ND | Survived; survived 1 and 7; weighed 1880 g |
| 11 | Clemenza et al., 2022 [19] | NA | NA | MV, ECMO; CS | Survived; survived 9 and 10; baby weighed 3080 g |
| 12 | Craina et al., 2022 [20] | None | None | No; ND | Survived; survived 2990 g, 8, 10 |
| 13 | Craina et al., 2022 [20] | None | None | No; CS | Survived; survived: 9, 10 |
| 14 | Craina et al., 2022 [20] | None | None | No; CS | Survived; Survived; 9, 10 |
| 15 | De Nardo et al., 2021 [21] | None | NA | No; ND | Survived; 9 and 10 and 3290 g |
| 16 | Esposito et al., 2023 [22] | NA | NA | MV; ND | Survived; Death |
| 17 | Federici et al., 2020 [23] | cefotaxime and spiramycin | NA | MV; ND | Survived; Apgar score NA, 3300 g |
| 18 | Figuerido et al., 2020 [24] | NA | NA | No; ND | Survived; 5-min Apgar Score of 10, 2870 g |
| 19 | Fiore et al., 2021 [25] | NA | NA | MV, ECMO; CS | Survived; survived 1680 g. |
| 20 | Giavioli et al., 2021 [26] | Azythromicin | NA | MV; CS | Survived; NA |
| 21 | Lyra et al., 2020 [27] | NA | NA | No; CS | Survived; survived Apgar score 8, 3110 g |
| 22 | Maier et al., 2020 [28] | Sultamicillin | None | MV; ND | Survived; NA |
| 23 | Manau et al., 2020 [29] | Azithromycin | Lopinavir-ritonavir | MV; OP | Survived; NA |
| 24 | Manau et al., 2020 [29] | None | None | No; OP | Survived; NA |
| 25 | Mihajlovic et al., 2022 [30] | NA | NA | MV; NA | Death; death |
| 24 | Mihajlovic et al., 2022 [30] | NA | NA | MV; CS | Survived; Survived |
| 27 | Mihajlovic et al., 2022 [30] | NA | NA | MV; NA | Death; Death |
| 28 | Mihajlovic et al., 2022 [30] | NA | NA | MV; NA | Death; Death |
| 29 | Mihajlovic et al., 2022 [30] | NA | NA | MV; CS | Survived; Survived |
| 30 | Mihajlovic et al., 2022 [30] | NA | NA | MV; NA | Death; Death |
| 31 | Mihajlovic et al., 2022 [30] | NA | NA | MV; CS | Survived; Survived |
| 32 | Mihajlovic et al., 2022 [30] | NA | NA | MV; NA | Death; Death |
| 33 | Munoz et al., 2023 [31] | NA | None | O2; ND | Survived; survived 2750 g, the Apgar score was 10, |
| 34 | Palailoglu et al., 2021 [32] | None | None | No; CS | Survived; 3580 g, 8, 10 |
| 35 | Palmrich et al., 2021 [33] | None | Remdesivir | MV; CS | Survived; survived Apgar score of 3/7/7 at 1, 5, and 10 min, 1445 g |
| 36 | Pikovsky et al., 2021 [34] | NA | NA | O2; CS | Survived; Apgars of 8/9/10 at 1, 5, and 10 min, 3100 g |
| 37 | Pikovsky et al., 2021 [34] | NA | NA | No; CS | Survived; survived Apgar score NA, 3200 g |
| 38 | Ravn et al., 2021 [35] | Cefuroxime | None | No; CS | Survived; NA |
| 39 | Reindorf et al., 2021 [36] | Co-amoxicllav | NA | O2; ND | Survived; NA |
| 40 | Rodrigues et al., 2020 [37] | NA | NA | No; CS | Survived; NA |
| 41 | Rosner Tenerowich et al., 2021 [38] | Piperacillin tazobactam and azithromycin | Remdesivir | ECMO; CS | Survived; Apgar score of 7, 1440 g |
| 42 | Sileo et al., 2021 [39] | None | None | No; CS | Survived; survived 8, 8: 2680 g |
| 43 | Smeele et al., 2021 [40] | NA | NA | No; ND | Survived; survived Apgar score 9, 2880 g |
| 44 | Smeele et al., 2021 [40] | NA | NA | No; CS | Survived; survived Apgar score 10, 4205 g |
| 45 | Sokolov et al., 2022 [41] | None | None | O2; CS | Survived; survived the first baby 1400 g, 7 and 8; the second baby 1600 g, 8; the third baby was 1820 g, 7 and 8; the fourth baby was 1520 g, 6 and 8 |
| 46 | Stout et al., 2020 [42] | NA | NA | ECMO; CS | Death; survived, Apgar score 8 |
| 47 | van Amesfort et al., 2021 [43] | NA | NA | No; CS | Survived; survived 2930 g, 7 and 8 |
| 48 | Vibert et al., 2020 [44] | NA | NA | O2; OP | Survived; NA |
| 49 | Yassa et al., 2021 [45] | NA | None | No; CS | Survived; survived 3070-g, 8 and 9. |
| 50 | Yassa et al., 2021 [45] | Azithromycin | Oseltamivir | No; OP | Survived; NA |
| 51 | Yassa et al., 2021 [45] | Azithromycin; meropenem | Lopinavir/ritonavir; favipiravir | No; OP | Survived; NA |
| 52 | Yassa et al., 2021 [45] | None | Ritonavir/lopinavir | No; OP | Survived; NA |
| 53 | Yassa et al., 2021 [45] | Azithromycin | Ritonavir/lopinavir | No; OP | Survived; NA |
| 54 | Yassa et al., 2021 [45] | Azithromycin | None | No; Abortion | Survived |
| 55 | Yassa et al., 2021 [45] | Azithromycin | Ritonavir/lopinavir | No; OP | Survived; NA |
| 56 | Yassa et al., 2021 [45] | None | None | No; CS | Survived; NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živković Zarić, R.; Zarić, M.; Protrka, S.; Andrić, V.; Arsenijević, N.; Čanović, P.; Mladenović, V.; Jakovljević, S.; Adamović, M.; Glišić, M. Treatment and Outcomes of COVID-19 Infection in Pregnant Women: Systematic Review of Cases Reported in Europe. J. Clin. Med. 2025, 14, 3743. https://doi.org/10.3390/jcm14113743
Živković Zarić R, Zarić M, Protrka S, Andrić V, Arsenijević N, Čanović P, Mladenović V, Jakovljević S, Adamović M, Glišić M. Treatment and Outcomes of COVID-19 Infection in Pregnant Women: Systematic Review of Cases Reported in Europe. Journal of Clinical Medicine. 2025; 14(11):3743. https://doi.org/10.3390/jcm14113743
Chicago/Turabian StyleŽivković Zarić, Radica, Milan Zarić, Simona Protrka, Veljko Andrić, Neda Arsenijević, Petar Čanović, Violeta Mladenović, Stefan Jakovljević, Miljan Adamović, and Miona Glišić. 2025. "Treatment and Outcomes of COVID-19 Infection in Pregnant Women: Systematic Review of Cases Reported in Europe" Journal of Clinical Medicine 14, no. 11: 3743. https://doi.org/10.3390/jcm14113743
APA StyleŽivković Zarić, R., Zarić, M., Protrka, S., Andrić, V., Arsenijević, N., Čanović, P., Mladenović, V., Jakovljević, S., Adamović, M., & Glišić, M. (2025). Treatment and Outcomes of COVID-19 Infection in Pregnant Women: Systematic Review of Cases Reported in Europe. Journal of Clinical Medicine, 14(11), 3743. https://doi.org/10.3390/jcm14113743





