Maladaptive Compensatory Neural Mechanisms Associated with Activity-Related Osteoarthritis Pain: Dissociation of Psychological and Activity-Related Neural Mechanisms of WOMAC Pain and VAS Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Procedures
2.4. Demographic and Clinical Assessments
2.5. Pain Intensity
2.6. Static and Dynamic Quantitative Sensory Testing (QST)
2.7. Pressure Pain Threshold (PPT)
2.8. Conditioned Pain Modulation (CPM)
2.9. Transcranial Magnetic Stimulation (TMS)
2.10. Resting-State Electroencephalography (EEG)
EEG Acquisition
2.11. Resting-State Spectral Power Analysis
2.12. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Univariate Analysis
3.3. Multivariate Analysis
3.3.1. Predictors of WOMAC Pain Score
General Findings
3.4. Findings Summary
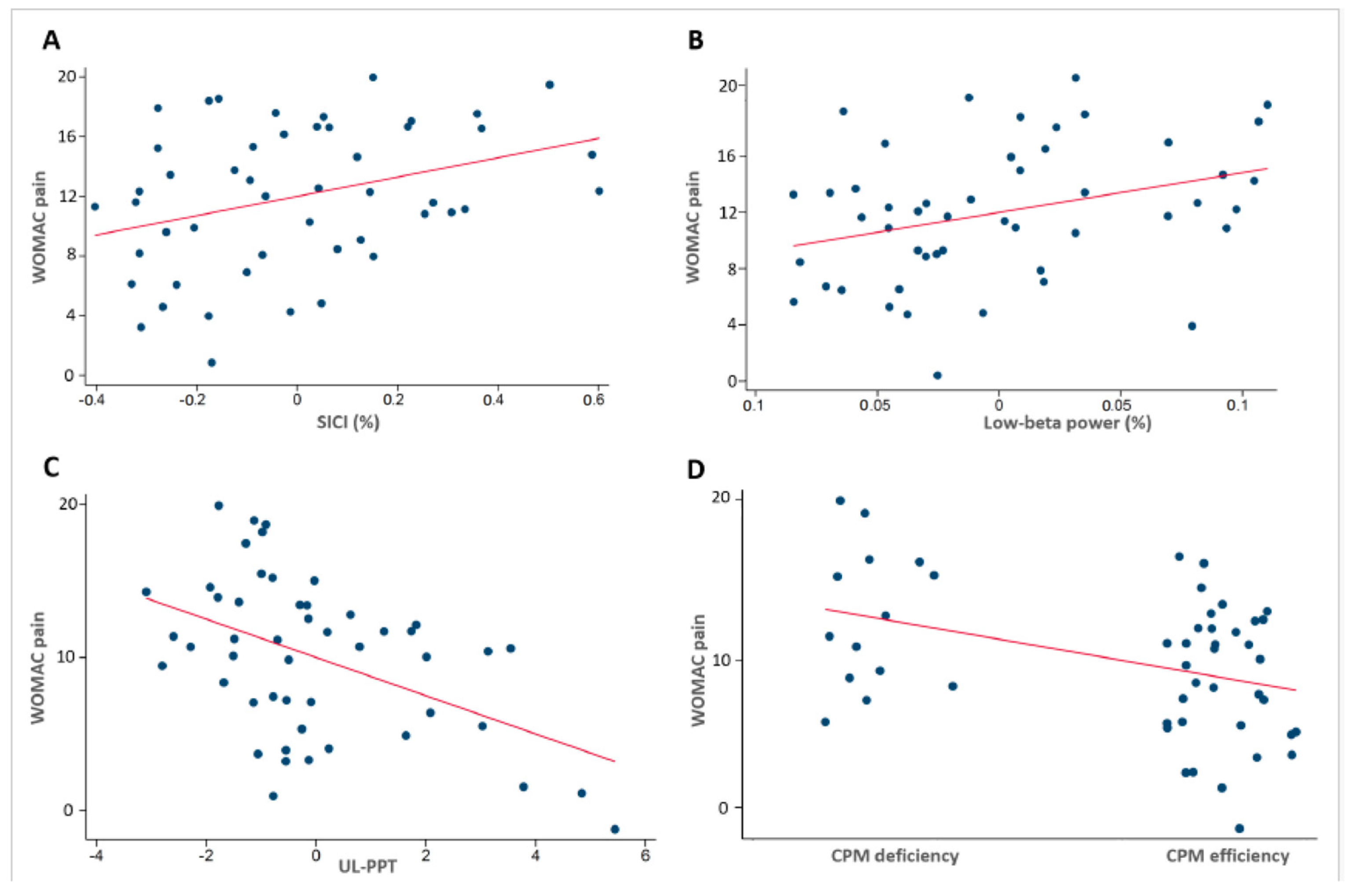
3.5. Neurophysiological Association Findings
3.6. Model Variance Explanation
3.7. Predictors of VAS Pain Scale
3.7.1. General Findings
3.7.2. Neurophysiological Findings
3.7.3. Model Variance Explanation
4. Discussion
4.1. Main Findings
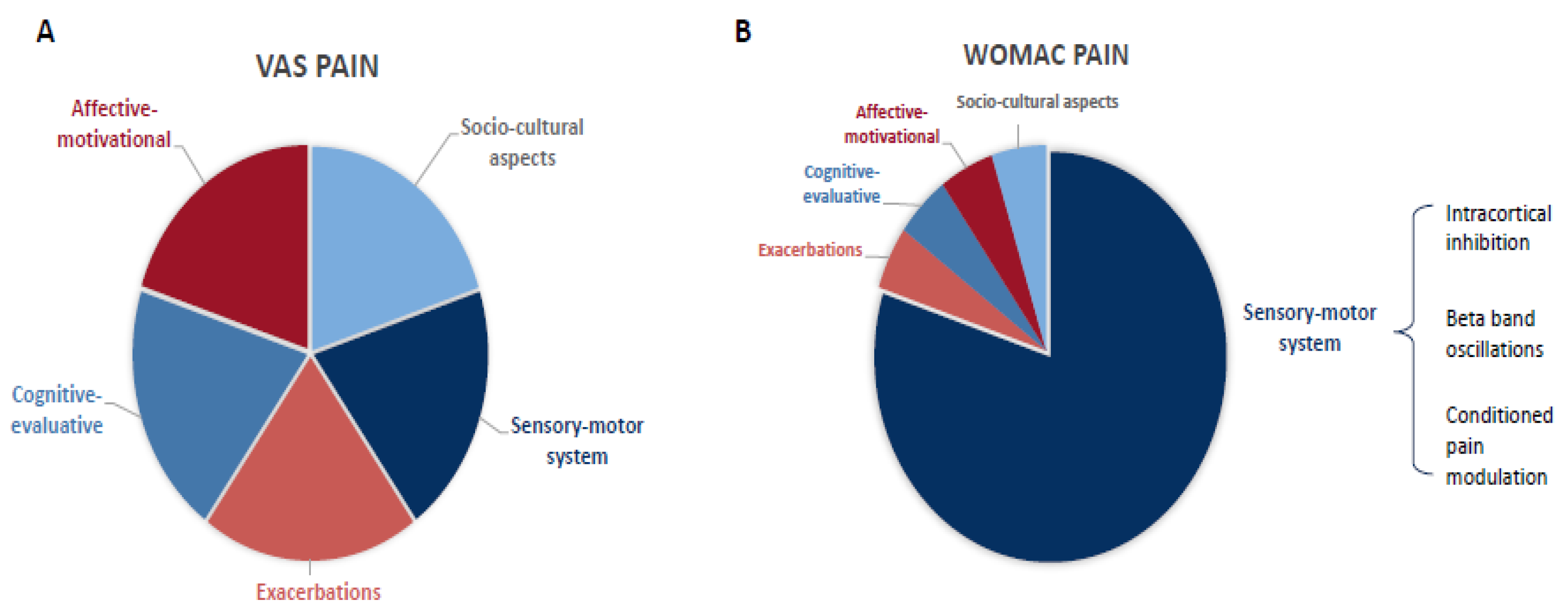
4.2. Dissociation of Neural Mechanisms Associated with WOMAC and VAS Pain Scores
4.3. Cortical Inhibitory Markers: Intracortical Inhibition and Frontal Beta Oscillations
4.4. Quantitative Sensory Testing (QST)
4.5. Depression
4.6. Pain Catastrophizing
4.7. Negative Findings
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidd, B.L. Osteoarthritis and joint pain. Pain 2006, 123, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Abbate, L.M.; Callahan, L.F.; et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2007, 34, 172–180. [Google Scholar] [PubMed]
- Courties, A.; Kouki, I.; Soliman, N.; Mathieu, S.; Sellam, J. Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthr. Cartil. 2024, 32, 1397–1404. [Google Scholar] [CrossRef]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee osteoarthritis: Epidemiology, pathogenesis, and mesenchymal stem cells: What else is new? An update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L. Osteoarthritis of the knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of Persons with Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.F.; Cleveland, R.J.; Allen, K.D.; Golightly, Y. Racial/Ethnic, Socioeconomic, and Geographic Disparities in the Epidemiology of Knee and Hip Osteoarthritis. Rheum. Dis. Clin. N. Am. 2021, 47, 1–20. [Google Scholar] [CrossRef]
- Losina, E.; Weinstein, A.M.; Reichmann, W.M.; Burbine, S.A.; Solomon, D.H.; Daigle, M.E.; Rome, B.N.; Chen, S.P.; Hunter, D.J.; Suter, L.G.; et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care Res. 2013, 65, 703–711. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- McDougall, J.J.; Andruski, B.; Schuelert, N.; Hallgrímsson, B.; Matyas, J.R. Unravelling the relationship between age, nociception and joint destruction in naturally occurring osteoarthritis of Dunkin Hartley guinea pigs. Pain 2009, 141, 222–232. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.K.; Hanlon, J.T.; Marcum, Z.A. Adverse effects of analgesics commonly used by older adults with osteoarthritis: Focus on non-opioid and opioid analgesics. Am. J. Geriatr. Pharmacother. 2012, 10, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.C.; Henderson, C.R., Jr.; Papaleontiou, M.; Amanfo, L.; Olkhovskaya, Y.; Moore, A.A.; Parikh, S.S.; Turner, B.J. Characteristics of older adults receiving opioids in primary care: Treatment duration and outcomes. Pain Med. 2010, 11, 1063–1071. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.R.; Dworkin, R.H.; Sullivan, M.D.; Turk, D.C.; Wasan, A.D. The Role of Psychosocial Processes in the Development and Maintenance of Chronic Pain. J. Pain 2016, 17 (Suppl. S9), T70–T92. [Google Scholar] [CrossRef] [PubMed]
- Turk, D.C.; Fillingim, R.B.; Ohrbach, R.; Patel, K.V. Assessment of Psychosocial and Functional Impact of Chronic Pain. J. Pain 2016, 17 (Suppl. S9), T21–T49. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Uchida, K.; Fukushima, K.; Inoue, G.; Takaso, M. Mechanisms of Peripheral and Central Sensitization in Osteoarthritis Pain. Cureus 2023, 15, e35331. [Google Scholar] [CrossRef] [PubMed]
- Maria da Graca, L.T.; Deitos, A.; Brietzke, A.P.; Vercelino, R.; Torres, I.L.; Fregni, F.; Caumo, W. Descending Control of Nociceptive Processing in Knee Osteoarthritis Is Associated with Intracortical Disinhibition: An Exploratory Study. Medicine 2016, 95, e3353. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Romero, E.A.; Fernández-Carnero, J.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos-Martín, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef]
- Sánchez-Romero, E.A.; González-Zamorano, Y.; Arribas-Romano, A.; Martínez-Pozas, O.; Fernández Espinar, E.; Pedersini, P.; Villafañe, J.H.; Alonso Pérez, J.L.; Fernández-Carnero, J. Efficacy of Manual Therapy on Facilitatory Nociception and Endogenous Pain Modulation in Older Adults with Knee Osteoarthritis: A Case Series. Appl. Sci. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Sinatti, P.; Sánchez Romero, E.A.; Martínez-Pozas, O.; Villafañe, J.H. Effects of Patient Education on Pain and Function and Its Impact on Conservative Treatment in Elderly Patients with Pain Related to Hip and Knee Osteoarthritis: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 6194. [Google Scholar] [CrossRef]
- Youngcharoen, P.; Hershberger, P.E.; Aree-Ue, S. Pain in elderly patients with knee osteoarthritis: An integrative review of psychosocial factors. Int. J. Orthop. Trauma Nurs. 2017, 25, 19–28. [Google Scholar] [CrossRef]
- Somers, T.J.; Keefe, F.J.; Godiwala, N.; Hoyler, G.H. Psychosocial factors and the pain experience of osteoarthritis patients: New findings and new directions. Curr. Opin. Rheumatol. 2009, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Helminen, E.E.; Sinikallio, S.H.; Valjakka, A.L.; Väisänen-Rouvali, R.H.; Arokoski, J.P. Determinants of pain and functioning in knee osteoarthritis: A one-year prospective study. Clin. Rehabil. 2016, 30, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Rayahin, J.E.; Chmiel, J.S.; Hayes, K.W.; Almagor, O.; Belisle, L.; Chang, A.H.; Moisio, K.; Zhang, Y.; Sharma, L. Factors associated with pain experience outcome in knee osteoarthritis. Arthritis Care Res. 2014, 66, 1828–1835. [Google Scholar] [CrossRef]
- George, S.Z.; Allen, K.D.; Alvarez, C.; Arbeeva, L.; Callahan, L.F.; Nelson, A.E.; Schwartz, T.A.; Golightly, Y.M. Prevalence and Factors Associated with High-Impact Chronic Pain in Knee Osteoarthritis: The Johnston County Health Study. J. Pain 2024, 25, 104687. [Google Scholar] [CrossRef]
- Costa, D.; Cruz, E.B.; Lopes, D.G.; da Silva, C.N.; Henriques, A.R.; Luis, D.; Branco, J.; Canhão, H.; Rodrigues, A.M. Prevalence of and factors associated with unmanageable pain levels in people with knee or hip osteoarthritis: A cross-sectional population-based study. BMC Musculoskelet. Disord. 2023, 24, 60. [Google Scholar] [CrossRef]
- Ohashi, Y.; Fukushima, K.; Inoue, G.; Uchida, K.; Koyama, T.; Tsuchiya, M.; Uchiyama, K.; Takahira, N.; Takaso, M. Central sensitization inventory scores correlate with pain at rest in patients with hip osteoarthritis: A retrospective study. BMC Musculoskelet. Disord. 2020, 21, 595. [Google Scholar] [CrossRef]
- López-Bravo, M.D.; Zamarrón-Cassinello, M.D.; Touche, R.L.; Muñoz-Plata, R.; Cuenca-Martínez, F.; Ramos-Toro, M. Psychological factors associated with functional disability in patients with hip and knee osteoarthritis. Behav. Med. 2020, 47, 285–295. [Google Scholar] [CrossRef]
- Glette, M.; Landmark, T.; Jensen, M.P.; Woodhouse, A.; Butler, S.; Borchgrevink, P.C.; Stiles, T.C. Catastrophizing, solicitous responses from significant others, and function in individuals with neuropathic pain, osteoarthritis, or spinal pain in the general population. J. Pain 2018, 19, 983–995. [Google Scholar] [CrossRef]
- Hruschak, V.; Cochran, G. Psychosocial predictors in the transition from acute to chronic pain: A systematic review. Psychol. Health Med. 2018, 23, 1151–1167. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.R.B.; Okazaki, J.E.F.; de Andrade Santana, M.V.; Pinto, A.C.P.N.; Tutiya, K.K.; Gazoni, F.M.; Pinto, C.B.; Santos, F.C.; Fregni, F.; Trevisani, V.F.M. Motor cortex transcranial direct current stimulation effects on knee osteoarthritis pain in elderly subjects with dysfunctional descending pain inhibitory system: A randomized controlled trial. Brain Stimul. 2021, 14, 477–487. [Google Scholar] [CrossRef]
- Simis, M.; Imamura, M.; de Melo, P.; Marduy, A.; Battistella, L.; Fregni, F. Deficit of inhibition as a marker of neuroplasticity (DEFINE study) in rehabilitation: A longitudinal cohort study protocol. Front. Neurol. 2021, 12, 1193. [Google Scholar] [CrossRef]
- Simis, M.; Pacheco-Barrios, K.; Uygur-Kucukseymen, E.; Castelo-Branco, L.; Battistella, L.R.; Fregni, F. Specific Electroencephalographic Signatures for Pain and Descending Pain Inhibitory System in Spinal Cord Injury. Pain Med. 2021, 23, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Simis, M.; Doruk, D.; Imamura, M.; Anghinah, R.; Morales-Quezada, L.; Fregni, F.; Battistella, L.R. Neurophysiologic predictors of motor function in stroke. Restor. Neurol. Neurosci. 2016, 34, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar] [PubMed]
- Reidler, J.S.; Mendonca, M.E.; Santana, M.B.; Wang, X.; Lenkinski, R.; Motta, A.F.; Marchand, S.; Latif, L.; Fregni, F. Effects of motor cortex modulation and descending inhibitory systems on pain thresholds in healthy subjects. J. Pain 2012, 13, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Mackey, I.G.; Dixon, E.A.; Johnson, K.; Kong, J.-T. Dynamic quantitative sensory testing to characterize central pain processing. JoVE (J. Vis. Exp.) 2017, 16, e54452. [Google Scholar]
- den Bandt, H.L.; Paulis, W.D.; Beckwée, D.; Ickmans, K.; Nijs, J.; Voogt, L. Pain Mechanisms in Low Back Pain: A Systematic Review with Meta-analysis of Mechanical Quantitative Sensory Testing Outcomes in People with Nonspecific Low Back Pain. J. Orthop. Sports Phys. Ther. 2019, 49, 698–715. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Kunz, M.; Burkhardt, S. The effects of DNIC-type inhibition on temporal summation compared to single pulse processing: Does sex matter? Pain 2008, 140, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Streff, A.; Michaux, G.; Anton, F. Internal validity of inter-digital web pinching as a model for perceptual diffuse noxious inhibitory controls-induced hypoalgesia in healthy humans. Eur. J. Pain 2011, 15, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, M.P.; Triggs, W.J.; Light, K.E.; Shechtman, O.; Khandekar, G.; Rothi, L.J.G. Reliability of motor cortex transcranial magnetic stimulation in four muscle representations. Clin. Neurophysiol. 2006, 117, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Schwenkreis, P.; Janssen, F.; Rommel, O.; Pleger, B.; Volker, B.; Hosbach, I.; Dertwinkel, R.; Maier, C.; Tegenthoff, M. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 2003, 61, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R.; Lehmann, D.; da Silva, F.L.; Matsuoka, S.; Sutherling, W.; Vibert, J.F. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 15–20. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Delorme, A.; Sejnowski, T.; Makeig, S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 2007, 34, 1443–1449. [Google Scholar] [CrossRef]
- Jensen, K.B.; Regenbogen, C.; Ohse, M.C.; Frasnelli, J.; Freiherr, J.; Lundström, J.N. Brain activations during pain: A neuroimaging meta-analysis of patients with pain and healthy controls. Pain 2016, 157, 1279–1286. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Shtatland, E.S.; Cain, E.; Barton, M.B. (Eds.) The Perils of Stepwise Logistic Regression and How to Escape Them Using Information Criteria and the Output Delivery System; Harvard Pilgrim Health Care, Harvard Medical School: Boston, MA, USA, 2001. [Google Scholar]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
- Osborne, J.W.; Waters, E. Four assumptions of multiple regression that researchers should always test. Pract. Assess. Res. Eval. 2002, 8, 2. [Google Scholar]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Spinazzola, L.; Pagliari, C.; Facchin, A.; Maravita, A. A new clinical evaluation of asomatognosia in right brain damaged patients using visual and reaching tasks. J. Clin. Exp. Neuropsychol. 2020, 42, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Chiarotto, A.; Terwee, C.B.; Ostelo, R.W. Choosing the right outcome measurement instruments for patients with low back pain. Best. Pract. Res. Clin. Rheumatol. 2016, 30, 1003–1020. [Google Scholar] [CrossRef]
- Teixeira, P.E.P.; Pacheco-Barrios, K.; Gunduz, M.E.; Gianlorenço, A.C.; Castelo-Branco, L.; Fregni, F. Understanding intracortical excitability in phantom limb pain: A multivariate analysis from a multicenter randomized clinical trial. Neurophysiol. Clin. 2021, 51, 161–173. [Google Scholar] [CrossRef]
- Uygur-Kucukseymen, E.; Castelo-Branco, L.; Pacheco-Barrios, K.; Luna-Cuadros, M.A.; Cardenas-Rojas, A.; Giannoni-Luza, S.; Zeng, H.; Gianlorenco, A.C.; Gnoatto-Medeiros, M.; Shaikh, E.S.; et al. Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiol. Clin. 2020, 50, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Barrios, K.; Pinto, C.B.; Velez, F.S.; Duarte, D.; Gunduz, M.E.; Simis, M.; Gianlorenco, A.L.; Barouh, J.L.; Crandell, D.; Guidetti, M.; et al. Structural and functional motor cortex asymmetry in unilateral lower limb amputation with phantom limb pain. Clin. Neurophysiol. 2020, 131, 2375–2382. [Google Scholar] [CrossRef]
- Fernandes, C.; Pidal-Miranda, M.; Samartin-Veiga, N.; Carrillo-de-la-Peña, M.T. Conditioned pain modulation as a biomarker of chronic pain: A systematic review of its concurrent validity. Pain 2019, 160, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Torrance, G.W.; Feeny, D.; Furlong, W. Visual analog scales: Do they have a role in the measurement of preferences for health states? Med. Decis. Mak. 2001, 21, 329–334. [Google Scholar] [CrossRef]
- Parker, R.S.; Lewis, G.N.; Rice, D.A.; McNair, P.J. Is Motor Cortical Excitability Altered in People with Chronic Pain? A Systematic Review and Meta-Analysis. Brain Stimul. 2016, 9, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Zolio, L.; Lim, K.Y.; McKenzie, J.E.; Yan, M.K.; Estee, M.; Hussain, S.M.; Cicuttini, F.; Wluka, A. Systematic review and meta-analysis of the prevalence of neuropathic-like pain and/or pain sensitisation in people with knee and hip osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1096–1116. [Google Scholar] [CrossRef] [PubMed]
- Simis, M.; Uygur-Kucukseymen, E.; Pacheco-Barrios, K.; Battistella, L.R.; Fregni, F. Beta-band oscillations as a biomarker of gait recovery in spinal cord injury patients: A quantitative electroencephalography analysis. Clin. Neurophysiol. 2020, 131, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Simis, M.; Battistella, L.R.; Fanciullacci, C.; Bertolucci, F.; Huerta-Gutierrez, R.; Chisari, C.; Fregni, F. Using Brain Oscillations and Corticospinal Excitability to Understand and Predict Post-Stroke Motor Function. Front. Neurol. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Cook, I.A.; O’Hara, R.; Uijtdehaage, S.H.; Mandelkern, M.; Leuchter, A.F. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J. Quantitative EEG, Event-Related Potentials and Neurotherapy; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Gram, M.; Erlenwein, J.; Petzke, F.; Falla, D.; Przemeck, M.; Emons, M.I.; Reuster, M.; Olesen, S.S.; Drewes, A.M. The cortical responses to evoked clinical pain in patients with hip osteoarthritis. PLoS ONE 2017, 12, e0186400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- May, E.S.; Nickel, M.M.; Ta Dinh, S.; Tiemann, L.; Heitmann, H.; Voth, I.; Tölle, T.R.; Gross, J.; Ploner, M. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum. Brain Mapp. 2019, 40, 293–305. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 1991, 251, 944–947. [Google Scholar] [CrossRef]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.R.B.; Trevisani, V.F.M.; Okazaki, J.E.F.; de Andrade Santana, M.V.; Pinto, A.C.P.N.; Tutiya, K.K.; Gazoni, F.M.; Pinto, C.B.; Dos Santos, F.C.; Fregni, F. Risk factors of pain, physical function, and health-related quality of life in elderly people with knee osteoarthritis: A cross-sectional study. Heliyon 2020, 6, e05723. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.E.P.; Zehry, H.I.; Chaudhari, S.; Dipietro, L.; Fregni, F. Pain perception in chronic knee osteoarthritis with varying levels of pain inhibitory control: An exploratory study. Scand. J. Pain. 2020, 20, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr. Opin. Anaesthesiol. 2010, 23, 611–615. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.T.; El-Hagrassy, M.M.; Rafferty, H.; Sanchez, P.; Huerta, R.; Chaudhari, S.; Conde, S.; Rosa, G.; Fregni, F. Impact of Therapeutic Interventions on Pain Intensity and Endogenous Pain Modulation in Knee Osteoarthritis: A Systematic Review and Meta-analysis. Pain Med. 2019, 20, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Wodehouse, T. Conditioned pain modulation—A comprehensive review. Neurophysiol. Clin. 2020, 51, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Arendt-Nielsen, L.; Graven-Nielsen, T. Translational musculoskeletal pain research. Best. Pr. Res. Clin. Rheumatol. 2011, 25, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Imamura, M.; Imamura, S.T.; Kaziyama, H.H.; Targino, R.A.; Hsing, W.T.; De Souza, L.P.M.; Cutait, M.M.; Fregni, F.; Camanho, G.L. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: A controlled analysis. Arthritis Care Res. Off. J. Am. Coll. Rheumatol. 2008, 59, 1424–1431. [Google Scholar] [CrossRef]
- Braun, M.; Bello, C.; Riva, T.; Hönemann, C.; Doll, D.; Urman, R.D.; Luedi, M.M. Quantitative Sensory Testing to Predict Postoperative Pain. Curr. Pain. Headache Rep. 2021, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Manniche, C.; Graven-Nielsen, T.; Arendt-Nielsen, L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur. J. Pain 2007, 11, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.K.; Vaegter, H.B.; Stubhaug, A.; Wolff, A.; Scammell, B.E.; Arendt-Nielsen, L.; Larsen, D.B. The predictive value of quantitative sensory testing: A systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain 2021, 162, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Schliessbach, J.; Siegenthaler, A.; Bütikofer, L.; Vuilleumier, P.; Jüni, P.; Stamer, U.; Arendt-Nielsen, L.; Curatolo, M. Predicting drug efficacy in chronic low back pain by quantitative sensory tests. Eur. J. Pain 2018, 22, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.C.; Wegener, S.T.; Heins, S.E.; Haythornthwaite, J.A.; MacKenzie, E.J.; Bosse, M.J. Longitudinal relationships between anxiety, depression, and pain: Results from a two-year cohort study of lower extremity trauma patients. Pain 2013, 154, 2860–2866. [Google Scholar] [CrossRef] [PubMed]
- Lerman, S.F.; Rudich, Z.; Brill, S.; Shalev, H.; Shahar, G. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom. Med. 2015, 77, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, B.I.; Mackay, D.; Cullen, B.; Martin, D.J.; Ul-Haq, Z.; Mair, F.S.; Evans, J.; McIntosh, A.M.; Gallagher, J.; Roberts, B.; et al. Chronic multisite pain in major depression and bipolar disorder: Cross-sectional study of 149,611 participants in UK Biobank. BMC Psychiatry 2014, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Furlough, K.; Goldberg, T.; Ring, D.; Koenig, K. Factors associated with pain intensity and magnitude of limitations among people with hip and knee arthritis. J. Orthop. 2021, 25, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Peskoe, S.; Zhao, C.; Friedman, B.W.; George, S.Z.; Eucker, S.A. Depression and Functional Outcomes in Patients Presenting to the Emergency Department with Low Back Pain. Acad. Emerg. Med. 2020, 27, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Seng, E.K.; Kuka, A.J.; Mayson, S.J.; Smitherman, T.A.; Buse, D.C. Acceptance, Psychiatric Symptoms, and Migraine Disability: An Observational Study in a Headache Center. Headache 2018, 58, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, J.; Liu, Y. Depression as a mediator of quality of life in patients with neuropathic pain: A cross-sectional study. J. Adv. Nurs. 2019, 75, 2719–2726. [Google Scholar] [CrossRef]
- Eldufani, J.; Elahmer, N.; Blaise, G. A medical mystery of complex regional pain syndrome. Heliyon 2020, 6, e03329. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jang, Y.E.; Oh, S.; Lee, P.B. Psychological Characteristics in Patients with Chronic Complex Regional Pain Syndrome: Comparisons with Patients with Major Depressive Disorder and Other Types of Chronic Pain. J. Pain Res. 2020, 13, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, M.E.; Dunbar, M.J.; Hennigar, A.W.; Sullivan, M.J.; Gross, M. Prospective relation between catastrophizing and residual pain following knee arthroplasty: Two-year follow-up. Pain Res. Manag. 2008, 13, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, Y.; Kao, M.-C.; Sturgeon, J.A.; Rico, T.J.; Mackey, S.; Darnall, B.D. Pain Catastrophizing Moderates Relationships between Pain Intensity and Opioid Prescription: Nonlinear Sex Differences Revealed Using a Learning Health System. Anesthesiology 2017, 127, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.M.; Nicholas, M.K.; Blyth, F.; Asghari, A.; Gibson, S. The mediating role of catastrophizing in the relationship between pain intensity and depressed mood in older adults with persistent pain: A longitudinal analysis. Scand. J. Pain 2016, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain catastrophizing: A critical review. Expert. Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Elboim-Gabyzon, M.; Rozen, N.; Laufer, Y. Gender differences in pain perception and functional ability in subjects with knee osteoarthritis. ISRN Orthop. 2012, 2012, 413105. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Capone, G.; Marchettini, P.; Candrian, C.; Zaffagnini, S.; Filardo, G. High Prevalence of Pain Sensitization in Knee Osteoarthritis: A Meta-Analysis with Meta-Regression. Cartilage 2022, 13, 19476035221087698. [Google Scholar] [CrossRef] [PubMed]
| Variables | Knee OA Subjects (N = 105) |
|---|---|
| Demographics | |
| Age | 68.65 (9.45) |
| Gender (%) | |
| Female | 90 (85.71) |
| Male | 15 (14.29) |
| Ethnicity | |
| White | 70 (66.66) |
| Black | 11 (10.47) |
| Mixed | 20 (19.04) |
| Asian | 4 (3.83) |
| Education level (%) | |
| Illiterate | 1 (0.95) |
| Elementary | 45 (42.85) |
| High school | 32 (30.47) |
| Superior | 27 (25.73) |
| Weight (kilograms) | 79.96 ± 15.56 |
| Height (meters) | 1.58 ± 0.09 |
| BMI | 31.99 ± 5.3 |
| Clinical assessments | |
| Bilateral knee OA (%) | 104 (99.02) |
| Time of ongoing pain (months) | 95.74 ± 98.75 |
| Total knee arthroplasty (%) | |
| Right | 3 (2.86) |
| Left | 3 (2.86) |
| Pain—visual analog scale | |
| Right | 5.69 ± 2.83 |
| Left | 5.38 ± 2.79 |
| Average | 5.54 ± 2.06 |
| WOMAC total score | 50.84 ± 19.49 |
| WOMAC pain | 10.77 ± 4.18 |
| WOMAC stiffness | 4.55 ± 2.07 |
| WOMAC physical function | 35.52 ± 14.51 |
| Kellgren–Lawrence classification | |
| Right | 2.5 ± 1.19 |
| Left | 2.33 ± 1.18 |
| Average | 2.43 ± 1.15 |
| Pain catastrophizing scale | 14.26 ± 11.04 |
| HAM-L scale | 9.36 ± 5.58 |
| Hospital anxiety and depression scale | |
| Anxiety | 5.92 ± 4.26 |
| Depression | 4.23 ± 3.55 |
| Montreal cognitive assessment | 21.02 ± 5.03 |
| Epworth sleepiness scale | 10.20 ± 5.96 |
| Quality of life (sf-36)—total score | 53.69 ± 20.00 |
| Quantitative sensory testing | |
| Pain pressure threshold | |
| Upper limb | |
| Right | 5.84 ± 2.05 |
| Left | 5.56 ± 2.12 |
| Average | 5.69 ± 2.02 |
| Knee | |
| Right | 4.84 ± 2.57 |
| Left | 4.79 ± 2.49 |
| Average | 4.82 ± 2.49 |
| Conditioned pain modulation | |
| Right | 0.93 ± 1.42 |
| Left | 1.05 ± 1.45 |
| Average | 1.01 ± 1.29 |
| Average (% of change from baseline) | 19.51 ± 25.11 |
| Transcranial magnetic stimulation | |
| Motor threshold | |
| Right | 52.73 ± 11.59 |
| Left | 50.97 ± 11.00 |
| Average | 51.36 ± 11.45 |
| Motor-evoked potential | |
| Right | 1.76 ± 1.30 |
| Left | 1.87 ± 2.02 |
| Average | 1.81 ± 1.41 |
| Cortico-silent period | |
| Right | 91.84 ± 35.82 |
| Left | 80.81 ± 31.67 |
| Average | 86.33 ± 31.46 |
| Short intracortical inhibition | |
| Right | 0.46 ± 0.32 |
| Left | 0.49 ± 0.32 |
| Average | 0.47 ± 0.27 |
| Intracortical facilitation | |
| Right | 1.59 ± 0.65 |
| Left | 1.70 ± 0.82 |
| Average | 1.65 ± 0.58 |
| Electroencephalography (relative spectral power) (n = 66) | |
| Frontal | |
| Delta | 24.6% (IQR 17.1) |
| Theta | 18.8% (IQR 10.5) |
| Alpha | 25.2% (IQR 17.9) |
| Low beta | 12.7% (IQR 7.4) |
| Beta | 20.8% (IQR 13.7) |
| High beta | 7.1% (IQR 7.2) |
| Central | |
| Delta | 20.1% (IQR 15.5) |
| Theta | 18.3% (IQR 9.8) |
| Alpha | 26.6% (IQR 17.4) |
| Low beta | 14.9% (IQR 8.7) |
| Beta | 22.8% (IQR 14.7) |
| High beta | 7% (IQR 7.8) |
| Parietal | |
| Delta | 30.3% (IQR 24.0) |
| Theta | 28.2% (IQR 33.9) |
| Alpha | 57.8% (IQR 17.0) |
| Low beta | 22.3% (IQR 27.8) |
| Beta | 31.6% (IQR 35.7) |
| High beta | 8.0% (IQR 11.8) |
| Variables | Beta Coefficient | 95% CI | p-Value | Adjusted R2 |
|---|---|---|---|---|
| Model 1A: WOMAC pain score | 0.519 | |||
| Pain threshold—upper limb | −0.487 | −0.759 to −0.215 | 0.001 | |
| Hamilton depression scale | 0.275 | 0.174 to 0.376 | <0.001 | |
| Pain catastrophizing scale | 0.126 | 0.074 to 0.178 | <0.001 | |
| Patients with effective CPM response | −2.200 | −3.289 to −1.110 | <0.001 | |
| SICI | 1.067 | 0.887 to 3.022 | 0.021 | |
| Model 1B: WOMAC pain score | 0.572 | |||
| Frontal low-beta relative power | 14.149 | 1.679 to 26.619 | 0.027 | |
| Pain threshold—knee | −0.532 | −0.809 to −0.255 | <0.001 | |
| Hamilton depression scale | 0.296 | 0.158 to 0.433 | <0.001 | |
| Patients catastrophizing scale | 0.100 | 0.026 to 0.174 | 0.009 | |
| Patients with effective CPM response | −1.456 | −3.023 to 0.111 | 0.068 | |
| Model 2: VAS pain | 0.357 | |||
| Hamilton depression scale | 0.093 | 0.014 to 0.172 | 0.021 | |
| Berg balance scale | −0.112 | −0.156 to −0.068 | <0.001 | |
| Pain catastrophizing scale | 0.050 | 0.018 to 0.083 | 0.003 | |
| Pain threshold superior limb | −0.145 | −0.304 to −0.014 | 0.044 | |
| HAD anxiety scale | −0.078 | −0.186 to −0.030 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, M.; Pacheco-Barrios, K.; de Melo, P.S.; Marduy, A.; Battistella, L.; Fregni, F. Maladaptive Compensatory Neural Mechanisms Associated with Activity-Related Osteoarthritis Pain: Dissociation of Psychological and Activity-Related Neural Mechanisms of WOMAC Pain and VAS Pain. J. Clin. Med. 2025, 14, 3633. https://doi.org/10.3390/jcm14113633
Imamura M, Pacheco-Barrios K, de Melo PS, Marduy A, Battistella L, Fregni F. Maladaptive Compensatory Neural Mechanisms Associated with Activity-Related Osteoarthritis Pain: Dissociation of Psychological and Activity-Related Neural Mechanisms of WOMAC Pain and VAS Pain. Journal of Clinical Medicine. 2025; 14(11):3633. https://doi.org/10.3390/jcm14113633
Chicago/Turabian StyleImamura, Marta, Kevin Pacheco-Barrios, Paulo S. de Melo, Anna Marduy, Linamara Battistella, and Felipe Fregni. 2025. "Maladaptive Compensatory Neural Mechanisms Associated with Activity-Related Osteoarthritis Pain: Dissociation of Psychological and Activity-Related Neural Mechanisms of WOMAC Pain and VAS Pain" Journal of Clinical Medicine 14, no. 11: 3633. https://doi.org/10.3390/jcm14113633
APA StyleImamura, M., Pacheco-Barrios, K., de Melo, P. S., Marduy, A., Battistella, L., & Fregni, F. (2025). Maladaptive Compensatory Neural Mechanisms Associated with Activity-Related Osteoarthritis Pain: Dissociation of Psychological and Activity-Related Neural Mechanisms of WOMAC Pain and VAS Pain. Journal of Clinical Medicine, 14(11), 3633. https://doi.org/10.3390/jcm14113633







