Mesenchymal Stem Cells in Oral and Maxillofacial Surgery: A Systematic Review of Clinical Applications and Regenerative Outcomes
Abstract
1. Introduction
1.1. Regeneration with Mesenchymal Stem Cells
1.2. Stem Cells: Classification and Biological Properties
1.3. Mesenchymal Stem Cells (MSCs)
1.4. Bone Marrow-Derived Stem Cells (BMSCs)
1.5. Adipose-Derived Stem Cells (ADSCs)
1.6. Stem Cell-Based Approaches in Maxillofacial and Oral Surgery
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Inclusion Criteria
- Focus on stem cell applications in maxillofacial and oral surgery;
- Types of studies: randomized controlled trials, retrospective research, case–control studies, case series, and prospective studies;
- Studies published in English;
- Full-text availability.
- -
- Participants: Human patients receiving MSC-based regenerative therapies specifically within the field of oral and maxillofacial surgery;
- -
- Interventions: Application of MSCs derived from various sources (e.g., bone marrow, dental pulp, adipose tissue);
- -
- Comparison: conventional regenerative approaches or no treatment controls, when applicable;
- -
- Outcomes: Quantitative and qualitative evaluation of regenerative outcomes, including bone/soft tissue formation, clinical integration, and safety/adverse events;
- -
- Study Design: Prospective and retrospective clinical studies, including RCTs and controlled case series.
2.4. Exclusion Criteria
- Animal studies;
- Studies on unrelated topics;
- Review articles, letters, or commentaries;
- Studies published in languages other than English.
2.5. Data Processing
2.6. Quality Assessment
- Confounding bias;
- Bias related to exposure measurement;
- Bias in participant selection;
- Bias from post-exposure interventions;
- Bias resulting from missing data;
- Bias from outcome measurement;
- Bias in reporting the results.
3. Results
3.1. Study Selection and Methodological Features
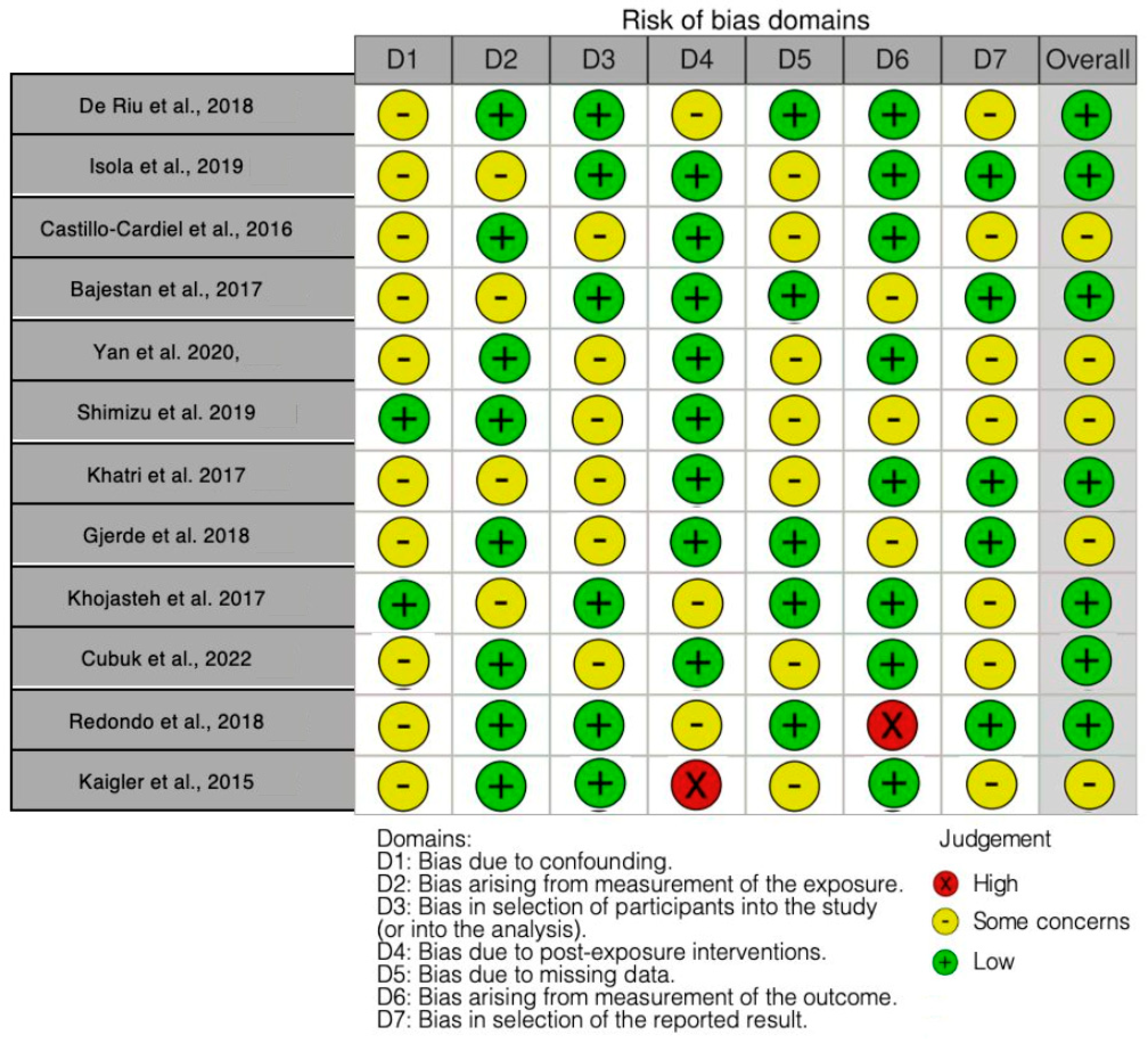
3.2. Quality Assessment and Risk of Bias
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose-derived stem cells |
| AMSCs | Autologous mesenchymal stem cells |
| BFSCs | Buccal fat pad-derived MSCs |
| BMNc | Bone marrow nucleated cells |
| BMSCs | Bone marrow stem cells |
| CD133+/KDR+ | Cell surface markers for endothelial progenitor cells (CD133 and KDR) |
| DMSO | Dimethyl sulfoxide |
| EPC | Endothelial progenitor cell |
| HA | Hyaluronic acid |
| L-PRF | Platelet-rich fibrin |
| MPCs | Multopotent progenitor cells |
| MSCs | Mesenchymal stem cell |
| TMJ | Temporomandibular joint |
References
- Pendharkar, S.S. Therapeutic Potential of Stem Cells in Regenerative Maxillofacial Surgery—A Review. RGUHS J. Dent. Sci. 2022, 14, 41–45. [Google Scholar] [CrossRef]
- Entezami, S.; Sam, M.R. The Role of Mesenchymal Stem Cells-derived from Oral and Teeth in Regenerative and Reconstructive Medicine. Tissue Cell 2025, 93, 102766. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory Properties of Mesenchymal Stem Cells/Dental Stem Cells and Their Therapeutic Applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Ma, L.; Yuan, Y.; Ye, X.; Montagne, A.; He, J.; Ho, T.-V.; Wu, Y.; Zhao, Z.; Sta Maria, N.; et al. Cranial Suture Regeneration Mitigates Skull and Neurocognitive Defects in Craniosynostosis. Cell 2021, 184, 243–256.e18. [Google Scholar] [CrossRef]
- Feinberg, S.E.; Aghaloo, T.L.; Cunningham, L.L. Role of Tissue Engineering in Oral and Maxillofacial Reconstruction: Findings of the 2005 AAOMS Research Summit. J. Oral Maxillofac. Surg. 2005, 63, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Apablaza, J.; Prieto, R.; Rojas, M.; Fuentes, R. Potential of Oral Cavity Stem Cells for Bone Regeneration: A Scoping Review. Cells 2023, 12, 1392. [Google Scholar] [CrossRef]
- Mayo, V.; Sawatari, Y.; Huang, C.-Y.C.; Garcia-Godoy, F. Neural Crest-Derived Dental Stem Cells—Where We Are and Where We Are Going. J. Dent. 2014, 42, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Cedar, S.H. The Function of Stem Cells and Their Future Roles in Healthcare. Br. J. Nurs. 2006, 15, 104–107. [Google Scholar] [CrossRef]
- Mosquera-Perez, R.; Fernández-Olavarria, A.; Diaz-Sanchez, R.-M.; Gutierrez-Perez, J.-L.; Serrera-Figallo, M.-Á.; Torres-Lagares, D. Stem Cells and Oral Surgery: A Systematic Review. J. Clin. Exp. Dent. 2019, 11, e1181–e1189. [Google Scholar] [CrossRef]
- Volarevic, V.; Ljujic, B.; Stojkovic, P.; Lukic, A.; Arsenijevic, N.; Stojkovic, M. Human Stem Cell Research and Regenerative Medicine--Present and Future. Br. Med. Bull. 2011, 99, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem Cell-Based Biological Tooth Repair and Regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal Cells and Stem Cells in Clinical Bone Regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Lazzaro, R.; Pham, V.H.; Tran, T.C.; Dipalma, G.; Inchingolo, F.; Serlenga, E.M.; Aityan, S.K.; Ballini, A.; et al. The Anti-Viral Activity of Stem Cells: A Rational Explanation for Their Use in Clinical Application. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.A.; El-Alami, M.; Gambini, J.; Borras, C.; Viña, J.; Peñarrocha, M.A. Application of Mesenchymal Stem Cells in Bone Regenerative Procedures in Oral Implantology. A Literature Review. J. Clin. Exp. Dent. 2014, 6, e60–e65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inchingolo, A.M.; Inchingolo, A.D.; Nardelli, P.; Latini, G.; Trilli, I.; Ferrante, L.; Malcangi, G.; Palermo, A.; Inchingolo, F.; Dipalma, G. Stem Cells: Present Understanding and Prospects for Regenerative Dentistry. J. Funct. Biomater. 2024, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, T.; Zhou, Y. Interplay between Craniofacial Stem Cells and Immune Stimulus. Stem. Cell Res. Ther. 2017, 8, 147. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reichert, T.E. Dental Stem Cells in Tooth Regeneration and Repair in the Future. Expert Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef]
- Götz, C.; Warnke, P.H.; Kolk, A. Current and Future Options of Regeneration Methods and Reconstructive Surgery of the Facial Skeleton. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 120, 315–323. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Dipalma, G.; Postiglione, F.; Fulle, S.; Scarano, A. Synthetic Scaffold/Dental Pulp Stem Cell (DPSC) Tissue Engineering Constructs for Bone Defect Treatment: An Animal Studies Literature Review. Int. J. Mol. Sci. 2020, 21, 9765. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of Mesenchymal Stem Cell: Machinery, Markers, and Strategies of Fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef]
- Dominici, M.; Blanc, K.L.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Yan, X.; Yan, F.; Mohammed, H.A.G.; Liu, O. Maxillofacial-Derived Mesenchymal Stem Cells: Characteristics and Progress in Tissue Regeneration. Stem Cells Int. 2021, 2021, 5516521. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Z. Gingiva-Derived Mesenchymal Stem Cells and Their Potential Applications in Oral and Maxillofacial Diseases. Curr. Stem Cell Res. Ther. 2020, 15, 43–53. [Google Scholar] [CrossRef]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Curr. Stem Cell Res. Ther. 2022, 17, 606–620. [Google Scholar] [CrossRef]
- Di Domenico, M.; Feola, A.; Ambrosio, P.; Pinto, F.; Galasso, G.; Zarrelli, A.; Di Fabio, G.; Porcelli, M.; Scacco, S.; Inchingolo, F.; et al. Antioxidant Effect of Beer Polyphenols and Their Bioavailability in Dental-Derived Stem Cells (D-dSCs) and Human Intestinal Epithelial Lines (Caco-2) Cells. Stem Cells Int. 2020, 2020, 8835813. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C. Dental Stem Cells for Tooth Regeneration: How Far Have We Come and Where Next? Expert Opin. Biol. Ther. 2023, 23, 527–537. [Google Scholar] [CrossRef]
- Peng, L.; Ye, L.; Zhou, X. Mesenchymal Stem Cells and Tooth Engineering. Int. J. Oral Sci. 2009, 1, 6–12. [Google Scholar] [CrossRef][Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human Mesenchymal Stem Cells—Current Trends and Future Prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chan, Y.-H.; Hsieh, S.-C.; Lew, W.-Z.; Feng, S.-W. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int. J. Mol. Sci. 2019, 20, 5015. [Google Scholar] [CrossRef]
- Shanti, R.M.; Li, W.-J.; Nesti, L.J.; Wang, X.; Tuan, R.S. Adult Mesenchymal Stem Cells: Biological Properties, Characteristics, and Applications in Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2007, 65, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Malcangi, G.; Patano, A.; Guglielmo, M.; Sardano, R.; Palmieri, G.; Di Pede, C.; de Ruvo, E.; Inchingolo, A.D.; Mancini, A.; Inchingolo, F.; et al. Precision Medicine in Oral Health and Diseases: A Systematic Review. J. Pers. Med. 2023, 13, 725. [Google Scholar] [CrossRef]
- Shuai, Y.; Ma, Y.; Guo, T.; Zhang, L.; Yang, R.; Qi, M.; Liu, W.; Jin, Y. Dental Stem Cells and Tooth Regeneration. Adv. Exp. Med. Biol. 2018, 1107, 41–52. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Paduano, F. The Regenerative Medicine in Oral and Maxillofacial Surgery: The Most Important Innovations in the Clinical Application of Mesenchymal Stem Cells. Int. J. Med. Sci. 2015, 12, 72–77. [Google Scholar] [CrossRef]
- Liu, X.; Fang, T.; Shi, T.; Wang, Y.; Liu, G. Hydrogels Provide Microenvironments to Mesenchymal Stem Cells for Craniofacial Bone Regeneration: Review. J. Biomater. Appl. 2023, 38, 3–24. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Piras, F.; Palmieri, G.; Settanni, V.; Riccaldo, L.; Morolla, R.; Buongiorno, S.; de Ruvo, E.; Inchingolo, A.D.; et al. Precision Medicine on the Effects of Microbiota on Head-Neck Diseases and Biomarkers Diagnosis. J. Pers. Med. 2023, 13, 933. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem Cell-Based Tooth and Periodontal Regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Bueno, C.; Insausti, C.L.; Meseguer, L.; Ramírez, M.C.; Blanquer, M.; Marín, N.; Martínez, S.; Moraleda, J.M. Mesenchymal Stem Cells Derived from Dental Tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef]
- Chaudhary, D.; Trivedi, R.N.; Kathuria, A.; Goswami, T.K.; Khandia, R.; Munjal, A. In Vitro And In Vivo Immunomodulating Properties of Mesenchymal Stem Cells. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of Adipose-Derived Mesenchymal Stem Cells-Based Biomaterial Scaffolds for Oral and Maxillofacial Tissue Engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Shakoori, P.; Zhang, Q.; Le, A.D. Applications of Mesenchymal Stem Cells in Oral and Craniofacial Regeneration. Oral Maxillofac. Surg. Clin. N. Am. 2017, 29, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.T.; Huynh, T.D.; Inchingolo, F.; Dipalma, G.; Inchingolo, A.D.; Cantore, S.; Paduanelli, G.; Nguyen, K.C.D.; Ballini, A.; Isacco, C.G.; et al. Human Chondrocytes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Seeded on a Dermal-Derived Collagen Matrix Sheet: Our Preliminary Results for a Ready to Go Biotechnological Cartilage Graft in Clinical Practice. Stem Cells Int. 2021, 2021, 6664697. [Google Scholar] [CrossRef]
- Kadar, K.; Kiraly, M.; Porcsalmy, B.; Molnar, B.; Racz, G.Z.; Blazsek, J.; Kallo, K.; Szabo, E.L.; Gera, I.; Gerber, G.; et al. Differentiation Potential of Stem Cells from Human Dental Origin—Promise for Tissue Engineering. J. Physiol. Pharmacol. 2009, 60 (Suppl. S7), 167–175. [Google Scholar]
- Dave, J.R.; Tomar, G.B. Dental Tissue-Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit. Rev. Biomed. Eng. 2018, 46, 429–468. [Google Scholar] [CrossRef]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental Stem Cells: Recent Progresses in Tissue Engineering and Regenerative Medicine. Ann. Med. 2017, 49, 644–651. [Google Scholar] [CrossRef]
- Al-Hamad, K.A.; Shanab, H.G.; Gaballah, O.M.; Moawad, A.A.R.; Alayfan, G.; Alshebel, A.; Alqahtani, A.M.A.; Almaziad, M. Potential Therapeutic Applications of Mesenchymal Stem Cells in the Oral and Maxillofacial Tissues. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6006–6017. [Google Scholar] [CrossRef]
- Zong, C.; Zhao, L.; Huang, C.; Chen, Y.; Tian, L. Isolation and Culture of Bone Marrow Mesenchymal Stem Cells from the Human Mandible. J. Vis. Exp. 2022, 63, e63811. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Huynh, T.D.; Nguyen, H.K.; Hoang, H.K.; Tran, T.T.T.; Nhan, H.N.; Nguyen, H.T.T.; Ngo, Q.M.T.; Nguyen, K.T.; Inchingolo, F.; et al. Human-like Skin Tissue Graft Generated Using Human UmbilicalCordBlood Mesenchymal Stem Cells and Acellular Amniotic Membrane: A New Approach in Reconstructive Medicine. Endocr. Metab. Immune Disord. Drug Targets 2025, 2. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.; Negruțiu, M.L.; Rusu, L.C.; Riviș, M. Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine. Biomedicines 2023, 11, 2436. [Google Scholar] [CrossRef] [PubMed]
- Đokić, J.; Tomić, S.; Cerović, S.; Todorović, V.; Rudolf, R.; Čolić, M. Characterization and Immunosuppressive Properties of Mesenchymal Stem Cells from Periapical Lesions. J. Clin. Periodontol. 2012, 39, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P.; Horwat, R.; Ray, H.L.; Garlet, T.P.; Silveira, E.M.; Campanelli, A.P.; Trombone, A.P.F.; Letra, A.; Silva, R.M. Expression Analysis of Wound Healing Genes in Human Periapical Granulomas of Progressive and Stable Nature. J. Endod. 2012, 38, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.A.; Farshidfar, N.; Hamedani, S. The Feasibility of Craniofacial-Derived Bone Marrow Stem Cells for the Treatment of Oral and Maxillofacial Hard Tissue Defects. J. Dent. Sci. 2022, 17, 1445–1447. [Google Scholar] [CrossRef]
- Pavan Kumar, B.; Ram Mohan, S.; Mohan, A.P.; Jeevan Kumar, K.A.; Yashwanth Yadav, B. Versatility of Pleuripotent Undifferentiated Stem Cells Aspirated from Bone Marrow and Its Applications in Oral and Maxillofacial Surgery. J. Maxillofac. Oral Surg. 2016, 15, 1–11. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Botero, T.M. Dental Stem Cells and Their Sources. Dent. Clin. N. Am. 2012, 56, 549–561. [Google Scholar] [CrossRef]
- Gerth, D.J.; Thaller, S.R. Adipose-Derived Mesenchymal Stem Cells: Current and Future Applications in Craniofacial Surgery. J. Craniofacial Surg. 2019, 30, 636–638. [Google Scholar] [CrossRef]
- Tobita, M.; Orbay, H.; Mizuno, H. Adipose-Derived Stem Cells: Current Findings and Future Perspectives. Discov. Med. 2011, 11, 160–170. [Google Scholar]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Stosich, M.S.; Mao, J.J. Adipose Tissue Engineering from Human Adult Stem Cells: Clinical Implications in Plastic and Reconstructive Surgery. Plast. Reconstr. Surg. 2007, 119, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H. Adipose-Derived Stem and Stromal Cells for Cell-Based Therapy: Current Status of Preclinical Studies and Clinical Trials. Curr. Opin. Mol. Ther. 2010, 12, 442–449. [Google Scholar] [PubMed]
- Baer, P.C. Adipose-Derived Stem Cells and Their Potential to Differentiate into the Epithelial Lineage. Stem Cells Dev. 2011, 20, 1805–1816. [Google Scholar] [CrossRef]
- Rada, T.; Santos, T.C.; Marques, A.P.; Correlo, V.M.; Frias, A.M.; Castro, A.G.; Neves, N.M.; Gomes, M.E.; Reis, R.L. Osteogenic Differentiation of Two Distinct Subpopulations of Human Adipose-Derived Stem Cells: An In Vitro and In Vivo Study. J. Tissue Eng. Regen. Med. 2012, 6, 1–11. [Google Scholar] [CrossRef]
- Rada, T.; Gomes, M.E.; Reis, R.L. A Novel Method for the Isolation of Subpopulations of Rat Adipose Stem Cells with Different Proliferation and Osteogenic Differentiation Potentials. J. Tissue Eng. Regen. Med. 2011, 5, 655–664. [Google Scholar] [CrossRef]
- Gomillion, C.T.; Burg, K.J.L. Stem Cells and Adipose Tissue Engineering. Biomaterials 2006, 27, 6052–6063. [Google Scholar] [CrossRef]
- Tanzi, M.C.; Farè, S. Adipose Tissue Engineering: State of the Art, Recent Advances and Innovative Approaches. Expert Rev. Med. Devices 2009, 6, 533–551. [Google Scholar] [CrossRef]
- Vallée, M.; Côté, J.-F.; Fradette, J. Adipose-Tissue Engineering: Taking Advantage of the Properties of Human Adipose-Derived Stem/Stromal Cells. Pathol. Biol. 2009, 57, 309–317. [Google Scholar] [CrossRef]
- Pikuła, M.; Marek-Trzonkowska, N.; Wardowska, A.; Renkielska, A.; Trzonkowski, P. Adipose Tissue-Derived Stem Cells in Clinical Applications. Expert Opin. Biol. Ther. 2013, 13, 1357–1370. [Google Scholar] [CrossRef]
- Rada, T.; Reis, R.L.; Gomes, M.E. Novel Method for the Isolation of Adipose Stem Cells (ASCs). J. Tissue Eng. Regen. Med. 2009, 3, 158–159. [Google Scholar] [CrossRef]
- Marra, K.G.; Rubin, J.P. The Potential of Adipose-Derived Stem Cells in Craniofacial Repair and Regeneration. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Tang, Q.; Li, L.; Chen, Y. Optimization and Implication of Adipose-Derived Stem Cells in Craniofacial Bone Regeneration and Repair. Bioengineering 2024, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Pourlak, T.; Pourlak, T.; Ghodrati, M.; Mortazavi, A.; Dolati, S.; Yousefi, M. Usage of Stem Cells in Oral and Maxillofacial Region. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Bone Regeneration by Stem Cell and Tissue Engineering in Oral and Maxillofacial Region. Front. Med. 2011, 5, 401–413. [Google Scholar] [CrossRef]
- Okić-Đorđević, I.; Obradović, H.; Kukolj, T.; Petrović, A.; Mojsilović, S.; Bugarski, D.; Jauković, A. Dental Mesenchymal Stromal/Stem Cells in Different Microenvironments—Implications in Regenerative Therapy. World J. Stem Cells 2021, 13, 1863–1880. [Google Scholar] [CrossRef]
- Niño-Sandoval, T.C.; Vasconcelos, B.C.; Moraes, S.L.D.; Lemos, C.A.A.; Pellizzer, E.P. Efficacy of Stem Cells in Maxillary Sinus Floor Augmentation: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 1355–1366. [Google Scholar] [CrossRef]
- Zuk, P.A. Tissue Engineering Craniofacial Defects with Adult Stem Cells? Are We Ready Yet? Pediatr. Res. 2008, 63, 478–486. [Google Scholar] [CrossRef]
- Si, J.-W.; Wang, X.-D.; Shen, S.G. Perinatal Stem Cells: A Promising Cell Resource for Tissue Engineering of Craniofacial Bone. World J. Stem Cells 2015, 7, 149–159. [Google Scholar] [CrossRef]
- Tang, W.; Huo, F.; Long, J.; Zhang, S.; Tian, W. Cellular Senescence in Craniofacial Tissue Regeneration: Inducers, Biomarkers, and Interventions. Tissue Eng. Part B Rev. 2024, 30, 128–141. [Google Scholar] [CrossRef]
- Block, M.S. Oral and Maxillofacial Surgery. Int. J. Oral Maxillofac. Implant. 2011, 26, 107–108. [Google Scholar]
- Costello, B.J.; Kumta, P.; Sfeir, C.S. Regenerative Technologies for Craniomaxillofacial Surgery. J. Oral Maxillofac. Surg. 2015, 73, S116–S125. [Google Scholar] [CrossRef]
- Engstrand, T. Biomaterials and Biologics in Craniofacial Reconstruction. J. Craniofacial Surg. 2012, 23, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Miura, Y.; Sonoyama, W.; Yamaza, T.; Gronthos, S.; Shi, S. Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine in Craniofacial Region. Oral Dis. 2006, 12, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental Stem Cell and Dental Tissue Regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Ansari, S.; Seagroves, J.T.; Chen, C.; Shah, K.; Aghaloo, T.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. Dental and Orofacial Mesenchymal Stem Cells in Craniofacial Regeneration: The Prosthodontist’s Point of View. J. Prosthet. Dent. 2017, 118, 455–461. [Google Scholar] [CrossRef]
- Saltz, A.; Kandalam, U. Mesenchymal Stem Cells and Alginate Microcarriers for Craniofacial Bone Tissue Engineering: A Review. J. Biomed. Mater. Res. A 2016, 104, 1276–1284. [Google Scholar] [CrossRef]
- Farré-Guasch, E.; Wolff, J.; Helder, M.N.; Schulten, E.A.J.M.; Forouzanfar, T.; Klein-Nulend, J. Application of Additive Manufacturing in Oral and Maxillofacial Surgery. J. Oral Maxillofac. Surg. 2015, 73, 2408–2418. [Google Scholar] [CrossRef]
- Tan, J.; Xu, X.; Lin, J.; Fan, L.; Zheng, Y.; Kuang, W. Dental Stem Cell in Tooth Development and Advances of Adult Dental Stem Cell in Regenerative Therapies. Curr. Stem Cell Res. Ther. 2015, 10, 375–383. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Pezzolla, C.; Patano, A.; Ceci, S.; Ciocia, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Cardarelli, F.; Piras, F.; et al. Experimental Analysis of the Use of Cranial Electromyography in Athletes and Clinical Implications. Int. J. Environ. Res. Public Health 2022, 19, 7975. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Mohammadi, M.; Alam, M.; Abbasi, K.; Gharibian Bajestani, S.; Khanmohammad, R.; Haseli, M.; Yazdanian, M.; Esmaeili Fard Barzegar, P.; Tebyaniyan, H. The Current Regenerative Medicine Approaches of Craniofacial Diseases: A Narrative Review. Front. Cell Dev. Biol. 2023, 11, 1112378. [Google Scholar] [CrossRef]
- Mantesso, A.; Nör, J.E. Stem Cells in Clinical Dentistry. J. Am. Dent. Assoc. 2023, 154, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Fujii, S.; Seki, Y.; Mizoguchi, T.; Shimo, T.; Iijima, M.; Irie, K. Stem Cell Properties of Gli1-Positive Cells in the Periodontal Ligament. J. Oral Biosci. 2020, 62, 299–305. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Ma, Y.; Tan, S.; Ren, B.; Liu, S.; Dai, H.; Xu, Z. Application of Dental Pulp Stem Cells in Oral Maxillofacial Tissue Engineering. Int. J. Med. Sci. 2022, 19, 310–320. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Derivatives—Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Dipalma, G.; Inchingolo, A.D.; Palumbo, I.; Guglielmo, M.; Morolla, R.; Mancini, A.; Inchingolo, F. Advancing Postoperative Pain Management in Oral Cancer Patients: A Systematic Review. Pharmaceuticals 2024, 17, 542. [Google Scholar] [CrossRef]
- Sybil, D.; Jain, V.; Mohanty, S.; Husain, S.A. Oral Stem Cells in Intraoral Bone Formation. J. Oral Biosci. 2020, 62, 36–43. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Hu, M.S.; Longaker, M.T.; Lorenz, H.P. Tissue Engineering and Regenerative Medicine in Craniofacial Reconstruction and Facial Aesthetics. J. Craniofacial Surg. 2020, 31, 15–27. [Google Scholar] [CrossRef]
- Oliver, J.D.; Madhoun, W.; Graham, E.M.; Hendrycks, R.; Renouard, M.; Hu, M.S. Stem Cells Regenerating the Craniofacial Skeleton: Current State-Of-The-Art and Future Directions. J. Clin. Med. 2020, 9, 3307. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Tarullo, A.; Inchingolo, A.D.; Dipalma, G.; Podo Brunetti, S.; Tarullo, A.; Cagiano, R. Combined Occlusal and Pharmacological Therapy in the Treatment of Temporo-Mandibular Disorders. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1296–1300. [Google Scholar]
- Van Bellinghen, X.; Idoux-Gillet, Y.; Pugliano, M.; Strub, M.; Bornert, F.; Clauss, F.; Schwinté, P.; Keller, L.; Benkirane-Jessel, N.; Kuchler-Bopp, S.; et al. Temporomandibular Joint Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 446. [Google Scholar] [CrossRef] [PubMed]
- Helgeland, E.; Shanbhag, S.; Pedersen, T.O.; Mustafa, K.; Rosén, A. Scaffold-Based Temporomandibular Joint Tissue Regeneration in Experimental Animal Models: A Systematic Review. Tissue Eng. Part B Rev. 2018, 24, 300–316. [Google Scholar] [CrossRef]
- Gaihre, B.; Uswatta, S.; Jayasuriya, A.C. Reconstruction of Craniomaxillofacial Bone Defects Using Tissue-Engineering Strategies with Injectable and Non-Injectable Scaffolds. J. Funct. Biomater. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.R.; Gao, J.; Wang, Y.; Taboas, J.M.; Almarza, A.J. Regenerative Potential of Various Soft Polymeric Scaffolds in the Temporomandibular Joint Condyle. J. Oral Maxillofac. Surg. 2018, 76, 2019–2026. [Google Scholar] [CrossRef] [PubMed]
- Hagandora, C.K.; Gao, J.; Wang, Y.; Almarza, A.J. Poly (Glycerol Sebacate): A Novel Scaffold Material for Temporomandibular Joint Disc Engineering. Tissue Eng. Part A 2013, 19, 729–737. [Google Scholar] [CrossRef]
- Garland, C.B.; Pomerantz, J.H. Regenerative Strategies for Craniofacial Disorders. Front. Physiol. 2012, 3, 453. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Cazzolla, A.P.; Di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; Topi, S.; Malcangi, G.; Hazballa, D.; Scarano, A.; et al. The Integumentary System and Its Microbiota between Health and Disease. J. Biol. Regul. Homeost. Agents 2021, 35, 303–321. [Google Scholar] [CrossRef]
- Estrela, C.; de Alencar, A.H.G.; Kitten, G.T.; Vencio, E.F.; Gava, E. Mesenchymal Stem Cells in the Dental Tissues: Perspectives for Tissue Regeneration. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Di Cosola, M.; Inchingolo, A.M.; Greco Lucchina, A.; Malcangi, G.; Pettini, F.; Scarano, A.; Bordea, I.R.; Hazballa, D.; Lorusso, F.; et al. Correlation between Occlusal Trauma and Oral Microbiota: A Microbiological Investigation. J. Biol. Regul. Homeost. Agents 2021, 35, 295–302. [Google Scholar] [CrossRef]
- De Riu, G.; Vaira, L.A.; Carta, E.; Meloni, S.M.; Sembronio, S.; Robiony, M. Bone Marrow Nucleated Cell Concentrate Autograft in Temporomandibular Joint Degenerative Disorders: 1-Year Results of a Randomized Clinical Trial. J. Cranio-Maxillofac. Surg. 2019, 47, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Cardiel, G.; López-Echaury, A.C.; Saucedo-Ortiz, J.A.; Fuentes-Orozco, C.; Michel-Espinoza, L.R.; Irusteta-Jiménez, L.; Salazar-Parra, M.; González-Ojeda, A. Bone Regeneration in Mandibular Fractures after the Application of Autologous Mesenchymal Stem Cells, a Randomized Clinical Trial. Dent. Traumatol. 2017, 33, 38–44. [Google Scholar] [CrossRef]
- Bajestan, M.N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.H.S.; Polymeri, A.; Travan, S.; Kaigler, D. Stem Cell Therapy for Reconstruction of Alveolar Cleft and Trauma Defects in Adults: A Randomized Controlled, Clinical Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nada, O.A.; Kluwe, L.; Gosau, M.; Smeets, R.; Friedrich, R.E. Expansion of Human Dental Pulp Cells In Vitro Under Different Cryopreservation Conditions. In Vivo 2020, 34, 2363–2370. [Google Scholar] [CrossRef]
- Shimizu, S.; Tsuchiya, S.; Hirakawa, A.; Kato, K.; Ando, M.; Mizuno, M.; Osugi, M.; Okabe, K.; Katagiri, W.; Hibi, H. Design of a Randomized Controlled Clinical Study of Tissue-Engineered Osteogenic Materials Using Bone Marrow-Derived Mesenchymal Cells for Maxillomandibular Bone Defects in Japan: The TEOM Study Protocol. BMC Oral Health 2019, 19, 69. [Google Scholar] [CrossRef]
- Khatri, R.; Arad, M.; Ortlip, T.; Portney, B.A.; Meltzer, W.A.; Diaconu, S.; Silipino, L.E.; Wang, Y.; Kaetzel, D.M.; Taylor, R.J.; et al. Harvesting Multipotent Progenitor Cells from a Small Sample of Tonsillar Biopsy for Clinical Applications. Stem Cell Res. Ther. 2017, 8, 174. [Google Scholar] [CrossRef]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell Therapy Induced Regeneration of Severely Atrophied Mandibular Bone in a Clinical Trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Behnia, H.; Tehranchi, A.; Nazeman, P.; Nadjmi, N.; Soleimani, M. Lateral Ramus Cortical Bone Plate in Alveolar Cleft Osteoplasty with Concomitant Use of Buccal Fat Pad Derived Cells and Autogenous Bone: Phase I Clinical Trial. BioMed Res. Int. 2017, 2017, 6560234. [Google Scholar] [CrossRef]
- Cubuk, S.; Oduncuoglu, B.F.; Alaaddinoglu, E.E. The Effect of Dental Pulp Stem Cells and L-PRF When Placed into the Extraction Sockets of Impacted Mandibular Third Molars on the Periodontal Status of Adjacent Second Molars: A Split-Mouth, Randomized, Controlled Clinical Trial. Oral Maxillofac. Surg. 2023, 27, 59–68. [Google Scholar] [CrossRef]
- Redondo, L.M.; García, V.; Peral, B.; Verrier, A.; Becerra, J.; Sánchez, A.; García-Sancho, J. Repair of Maxillary Cystic Bone Defects with Mesenchymal Stem Cells Seeded on a Cross-Linked Serum Scaffold. J. Cranio-Maxillofac. Surg. 2018, 46, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Avila-Ortiz, G.; Travan, S.; Taut, A.D.; Padial-Molina, M.; Rudek, I.; Wang, F.; Lanis, A.; Giannobile, W.V. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90+ Stem Cell Therapy: A Randomized Clinical Trial. J. Bone Miner. Res. 2015, 30, 1206–1216. [Google Scholar] [CrossRef] [PubMed]

| Database | Search String |
|---|---|
| PubMed | (“stem cells” OR “mesenchymal stem cells” OR “bone marrow-derived stem cells” OR “adipose-derived stem cells”) AND (“maxillofacial” OR “craniofacial” OR “oral surgery”) |
| Scopus | (“stem cells” OR “mesenchymal stem cells” OR “bone marrow-derived stem cells” OR “adipose-derived stem cells”) AND (“maxillofacial” OR “craniofacial” OR “oral surgery”) |
| Web of Science | (“stem cells” OR “mesenchymal stem cells” OR “bone marrow-derived stem cells” OR “adipose-derived stem cells”) AND (“maxillofacial” OR “craniofacial” OR “oral surgery”) |
| Author (Year) | Study Design | Number of Patients | Average Age and Gender | Stem Cells Used | Outcomes |
|---|---|---|---|---|---|
| De Riu et al., 2018 [111] | RCT | 30 | Not Specified | BMNc | Pain relief, better chewing, increased mouth opening at 6–12 months. |
| Isola et al., 2019 [112] | Observational study | 167 | Not specified | Endothelial Progenitor Cells | Lower EPCs linked to worse periodontal disease. |
| Castillo-Cardiel et al. (2016) [113] | Randomized clinical trial | 20 (10 per group) | 31.2 ± 6.3 years (study group), 29.7 ± 7.2 years (control group), all male | Autologous MSCs (AMSCs) | Improved bone quality; 36.48% higher ossification at 12 weeks. |
| Bajestan et al. (2017) [114] | Randomized controlled clinical trial | 18 (10 with trauma, 8 with cleft palate) | Not specified | Bone marrow-derived MSCs | Less bone gain; 5/10 implant success in stem cell group. |
| Yan et al. (2020) [115] | Experimental laboratory study | 10 healthy children (aged 10–15 years) | Not specified | DPSCs isolated from dental pulp tissue of extracted third molars. | Cryopreservation slightly delayed cell outgrowth, no major functional impact. |
| Shimizu et al. (2019) [116] | Randomized controlled trial | 29 patients | Patients aged 20+ years | BM-MSCs derived from iliac crest bone marrow | Successful bone regeneration (CT ≥ 400, height > 10 mm). |
| Khatri et al., 2017 [117] | Sperimental study | 10 | 9 Female and 5 male | T-MPCs (tonsil-derived mesenchymal progenitor cells) | Tonsils as viable stem cell source for research/clinical use. |
| Gjerde et al., 2018 [118] | Clinical trial | 11 patients | 52–79 years | Bone marrow-derived MSCs | New bone formation without adverse effects. |
| Khojasteh et al. (2017) [119] | Prospective randomized clinical trial. | Ten patients | Four adult patients (20–29 years old) and six pediatric patients (8–14 years old) 3 female | MSCs derived from the buccal fat pad (BFP) | Enhanced regeneration, reduced resorption with scaffold. |
| Cubuk et al. (2022) [120] | Split-mouth RCT | 13 patients | 23.6 ± 4.4 years; 7F, 6M | DPSCs | Both groups improved; no difference with or without DPSCs. |
| Redondo et al., 2018 [121] | RCT | 9 patients | 38 ± 5 years (7F, 2M) | Autologous bone-derived mesenchymal stem cells | Bone density increased, no rejection, effective regeneration. |
| Kaigler et al., 2015 [122] | RCT | 26 patiens | Not specified | Autologous bone marrow-derived cells enriched with CD90+ stem cells and CD14+ monocytes | Higher bone volume; successful implants; no adverse events. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Marinelli, G.; Palumbo, I.; Guglielmo, M.; Riccaldo, L.; Morolla, R.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D.; Inchingolo, A.M. Mesenchymal Stem Cells in Oral and Maxillofacial Surgery: A Systematic Review of Clinical Applications and Regenerative Outcomes. J. Clin. Med. 2025, 14, 3623. https://doi.org/10.3390/jcm14113623
Dipalma G, Marinelli G, Palumbo I, Guglielmo M, Riccaldo L, Morolla R, Inchingolo F, Palermo A, Inchingolo AD, Inchingolo AM. Mesenchymal Stem Cells in Oral and Maxillofacial Surgery: A Systematic Review of Clinical Applications and Regenerative Outcomes. Journal of Clinical Medicine. 2025; 14(11):3623. https://doi.org/10.3390/jcm14113623
Chicago/Turabian StyleDipalma, Gianna, Grazia Marinelli, Irene Palumbo, Mariafrancesca Guglielmo, Lilla Riccaldo, Roberta Morolla, Francesco Inchingolo, Andrea Palermo, Alessio Danilo Inchingolo, and Angelo Michele Inchingolo. 2025. "Mesenchymal Stem Cells in Oral and Maxillofacial Surgery: A Systematic Review of Clinical Applications and Regenerative Outcomes" Journal of Clinical Medicine 14, no. 11: 3623. https://doi.org/10.3390/jcm14113623
APA StyleDipalma, G., Marinelli, G., Palumbo, I., Guglielmo, M., Riccaldo, L., Morolla, R., Inchingolo, F., Palermo, A., Inchingolo, A. D., & Inchingolo, A. M. (2025). Mesenchymal Stem Cells in Oral and Maxillofacial Surgery: A Systematic Review of Clinical Applications and Regenerative Outcomes. Journal of Clinical Medicine, 14(11), 3623. https://doi.org/10.3390/jcm14113623











