Strategies to Reduce Rebound Pain and Facilitate Early Recovery After Transforaminal Endoscopic Lumbar Discectomy
Abstract
1. Introduction
2. Definition of Rebound Pain After TELD
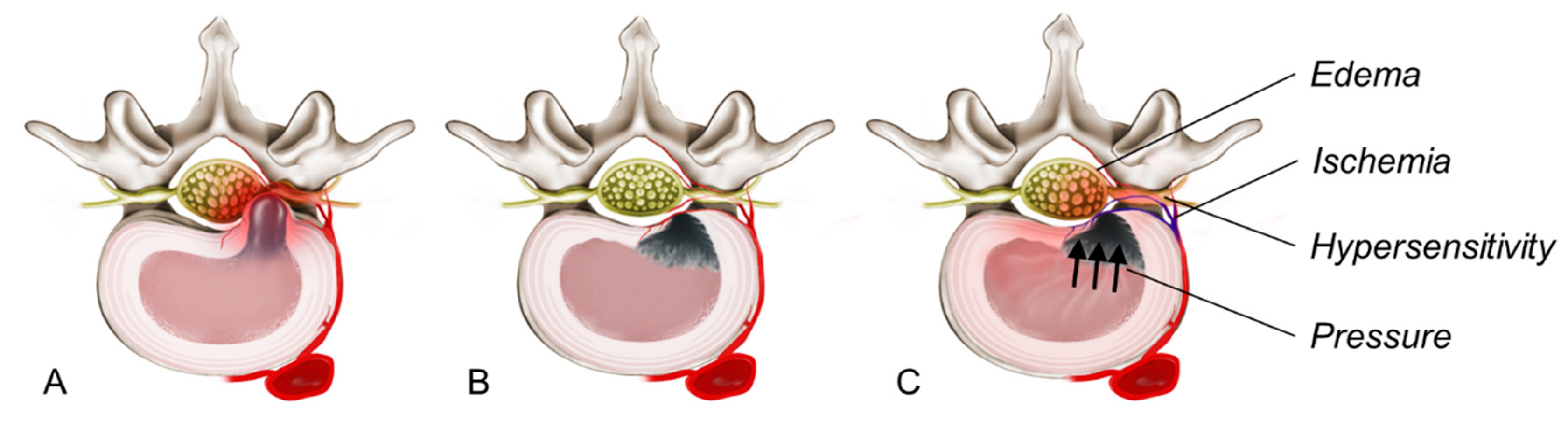
3. Pathogenesis of Rebound Pain After TELD
3.1. Inflammatory Edema of the Nerve Root
3.2. Vascular and Microcirculatory Changes
3.3. Neural Hypersensitivity Due to the Disruption of Pain Pathways
3.4. High Intradiscal and Epidural Pressure After Discectomy
4. Incidence of and Risk Factors for Rebound Pain After TELD
5. Differential Diagnosis of Rebound Pain
6. TELD Versus Open Lumbar Discectomy: Differences in Rebound Pain
7. Effect of Rebound Pain on Rehabilitation and Patient Satisfaction
8. Strategies to Prevent Rebound Pain After TELD
8.1. Preoperative Strategies
8.2. Intraoperative Strategies
8.3. Postoperative Strategies
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TELD | Transforaminal endoscopic lumbar discectomy |
| MISS | Minimally invasive spine surgery |
| VAS | Visual analogue scale |
| IL | Interleukin |
| TNF | Tumour necrosis factor |
| MRI | Magnetic resonance imaging |
References
- Ishimoto, Y.; Yoshimura, N.; Muraki, S.; Yamada, H.; Nagata, K.; Hashizume, H.; Takiguchi, N.; Minamide, A.; Oka, H.; Kawaguchi, H.; et al. Prevalence of symptomatic lumbar spinal stenosis and its association with physical performance in a population-based cohort in Japan: The Wakayama Spine Study. Osteoarthr. Cartil. 2012, 20, 1103–1108. [Google Scholar] [CrossRef]
- Ju, C.I.; Lee, S.M. Complications and management of endoscopic spinal surgery. Neurospine 2023, 20, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.M.; Brock, M. Percutaneous endoscopic lumbar discectomy (PELD). Neurosurg. Rev. 1993, 16, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Kambin, P.; O’Brien, E.; Zhou, L.; Schaffer, J.L. Arthroscopic microdiscectomy and selective fragmentectomy. Clin. Orthop. Relat. Res. 1998, 347, 150–167. [Google Scholar] [CrossRef]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27, 722–731. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: A prospective, randomized, controlled study. J. Neurosurg. Spine 2009, 10, 476–485. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.K.; Kim, H.; Lee, S.H.; Lee, H.N. Percutaneous endoscopic lumbar foraminotomy: An advanced surgical technique and clinical outcomes. Neurosurgery 2014, 75, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Kambin, P.; Sampson, S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin. Orthop. Relat. Res. 1986, 207, 37–43. [Google Scholar] [CrossRef]
- Hijikata, S. Percutaneous nucleotomy. A new concept technique and 12 years’ experience. Clin. Orthop. Relat. Res. 1989, 238, 9–23. [Google Scholar] [CrossRef]
- Hermantin, F.U.; Peters, T.; Quartararo, L.; Kambin, P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J. Bone Jt. Surg. Am. 1999, 81, 958–965. [Google Scholar] [CrossRef]
- Hoogland, T.; Schubert, M.; Miklitz, B.; Ramirez, A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: A prospective randomized study in 280 consecutive cases. Spine 2006, 31, E890–E897. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.M.; Brock, M. Percutaneous endoscopic discectomy: Surgical technique and preliminary results compared to microsurgical discectomy. J. Neurosurg. 1993, 78, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: A prospective, randomized, controlled study. Spine 2008, 33, 931–939. [Google Scholar] [CrossRef]
- Ruetten, S.; Komp, M.; Merk, H.; Godolias, G. Recurrent lumbar disc herniation after conventional discectomy: A prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J. Spinal Disord. Tech. 2009, 22, 122–129. [Google Scholar] [CrossRef]
- Meyer, G.; DA Rocha, I.D.; Cristante, A.F.; Marcon, R.M.; Coutinho, T.P.; Torelli, A.G.; Petersen, P.A.; Letaif, O.B.; DE Barros Filho, T.E.P. Percutaneous endoscopic lumbar discectomy versus microdiscectomy for the treatment of lumbar disc herniation: Pain, disability, and complication rate—A randomized clinical trial. Int. J. Spine Surg. 2020, 14, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.N.A.; Subramanian, A.S.; Scott, C.E.H. A randomised controlled trial of transforaminal endoscopic discectomy vs microdiscectomy. Eur. Spine J. 2017, 26, 847–856. [Google Scholar] [CrossRef]
- Nellensteijn, J.; Ostelo, R.; Bartels, R.; Peul, W.; van Royen, B.; van Tulder, M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: A systematic review of the literature. Eur. Spine J. 2010, 19, 181–204. [Google Scholar] [CrossRef]
- Cong, L.; Zhu, Y.; Tu, G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur. Spine J. 2016, 25, 134–143. [Google Scholar] [CrossRef]
- Li, X.C.; Zhong, C.F.; Deng, G.B.; Liang, R.W.; Huang, C.M. Full-Endoscopic procedures versus traditional discectomy surgery for discectomy: A systematic review and meta-analysis of current global clinical trials. Pain Physician 2016, 19, 103–118. [Google Scholar] [CrossRef]
- Ruan, W.; Feng, F.; Liu, Z.; Xie, J.; Cai, L.; Ping, A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: A meta-analysis. Int. J. Surg. 2016, 31, 86–92. [Google Scholar] [CrossRef]
- Ding, W.; Yin, J.; Yan, T.; Nong, L.; Xu, N. Meta-analysis of percutaneous transforaminal endoscopic discectomy vs. fenestration discectomy in the treatment of lumbar disc herniation. Orthopade 2018, 47, 574–584. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, S.; Liu, J.; Yu, B.; Guo, W.; Li, Y.; Liu, Y.; Ruan, W.; Ning, G.; Feng, S. Transforaminal endoscopic discectomy versus conventional microdiscectomy for lumbar discherniation: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2018, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.M.; Nakhla, J.; Konakondla, S.; Fridley, J.S.; Oyelese, A.A.; Gokaslan, Z.L.; Telfeian, A.E. Outcomes of endoscopic discectomy compared with open microdiscectomy and tubular microdiscectomy for lumbar disc herniations: A meta-analysis. J. Neurosurg. Spine 2019, 31, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Gadjradj, P.S.; Harhangi, B.S.; Amelink, J.; van Susante, J.; Kamper, S.; van Tulder, M.; Peul, W.C.; Vleggeert-Lankamp, C.; Rubinstein, S.M. Percutaneous transforaminal endoscopic discectomy versus open microdiscectomy for lumbar disc herniation: A systematic review and meta-analysis. Spine 2021, 46, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.; Yan, Q.; Cong, L. Comparison of endoscopic discectomy versus non-endoscopic discectomy for symptomatic lumbar disc herniation: A systematic review and meta-analysis. Global Spine J. 2022, 12, 1012–1026. [Google Scholar] [CrossRef]
- Tsou, P.M.; Yeung, A.T. Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: Outcome and technique. Spine J. 2002, 2, 41–48. [Google Scholar] [CrossRef]
- Birkenmaier, C.; Komp, M.; Leu, H.F.; Wegener, B.; Ruetten, S. The current state of endoscopic disc surgery: Review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician 2013, 16, 335–344. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.G.; Son, S.; Keum, H.J. Transforaminal endoscopic lumbar discectomy versus open lumbar microdiscectomy: A comparative cohort study with a 5-year follow-up. Pain Physician 2019, 22, 295–304. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Ahn, Y.; Choi, G.; Gibson, J.N.A.; Ruetten, S.; Zhou, Y.; Li, Z.Z.; Siepe, C.J.; Wagner, R.; Lee, J.H.; et al. AOSpine consensus paper on nomenclature for working-channel endoscopic spinal procedures. Glob. Spine J. 2020, 10, 111S–121S. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Kerezoudis, P.; Alvi, M.A.; Yoon, J.W.; Eluchie, J.; Murad, M.H.; Wang, Z.; Chen, S.G.; Bydon, M. Open versus minimally invasive surgery for extraforaminal lumbar disk herniation: A systematic review and meta-analysis. World Neurosurg. 2017, 108, 924–938. [Google Scholar] [CrossRef]
- Gu, Y.T.; Cui, Z.; Shao, H.W.; Ye, Y.; Gu, A.Q. Percutaneous transforaminal endoscopic surgery (PTES) for symptomatic lumbar disc herniation: A surgical technique, outcome, and complications in 209 consecutive cases. J. Orthop. Surg. Res. 2017, 12, 25. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Yu, K.; Wang, Y. A postoperative phenomenon of percutaneous endoscopic lumbar discectomy: Rebound pain. Orthop. Surg. 2021, 13, 2196–2205. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.X.; Sun, L.W.; Jhang, S.W.; Chen, C.M.; Rui, G.; Hu, B.S. Postoperative pain management after full endoscopic lumbar discectomy: An observational study. Medicina 2022, 58, 1817. [Google Scholar] [CrossRef]
- Ahn, Y. Transforaminal percutaneous endoscopic lumbar discectomy: Technical tips to prevent complications. Expert Rev. Med. Devices 2012, 9, 361–366. [Google Scholar] [CrossRef]
- Cakir, B.; Richter, M.; Puhl, W.; Schmidt, R. Reliability of motion measurements after total disc replacement: The spike and the fin method. Eur. Spine J. 2006, 15, 165–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; He, X.; Chen, S.; Weng, Y.; Liu, Z.; Pan, Q.; Zhang, R.; Li, Y.; Wang, H.; Lin, S.; et al. Annulus fibrosus repair for lumbar disc herniation: A meta-analysis of clinical outcomes from controlled studies. Glob. Spine J. 2024, 14, 306–321. [Google Scholar] [CrossRef]
- Carragee, E.J.; Helms, E.; O’Sullivan, G.S. Are postoperative activity restrictions necessary after posterior lumbar discectomy? A prospective study of outcomes in 50 consecutive cases. Spine 1996, 21, 1893–1897. [Google Scholar] [CrossRef]
- Bono, C.M.; Leonard, D.A.; Cha, T.D.; Schwab, J.H.; Wood, K.B.; Harris, M.B.; Schoenfeld, A.J. The effect of short (2-weeks) versus long (6-weeks) post-operative restrictions following lumbar discectomy: A prospective randomized control trial. Eur. Spine J. 2017, 26, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, T.; Davis, J.; Nicassio, N.; Malik, I. Endoscopic lumbar discectomy under local anesthesia may be an alternative to microdiscectomy: A single centre’s experience using the far lateral approach. Clin. Neurol. Neurosurg. 2015, 139, 324–327. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, S.H. Transforaminal full-endoscopic lumbar discectomy in obese patients. Int. J. Spine Surg. 2016, 10, 18. [Google Scholar] [CrossRef]
- Kamson, S.; Trescot, A.M.; Sampson, P.D.; Zhang, Y. Full-endoscopic assisted lumbar decompressive surgery performed in an outpatient, ambulatory facility: Report of 5 years of complications and risk factors. Pain Physician 2017, 20, E221–E231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Bao, J.; Su, J.; Tan, P.; Xie, W.; Huang, Z.; Xia, H. Novel targeted puncture technique for percutaneous transforaminal endoscopic lumbar discectomy reduces X-ray exposure. Exp. Ther. Med. 2017, 14, 2960–2968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosztowski, T.A.; Choi, D.; Fridley, J.; Galgano, M.; Gokaslan, Z.; Oyelese, A.; Telfeian, A.E. Lumbar disc reherniation after transforaminal lumbar endoscopic discectomy. Ann. Transl. Med. 2018, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Park, E.S.; Lee, S.H.; Lee, K.K.; Kwon, Y.K.; Kang, M.S.; Lee, S.Y.; Shin, Y.H. Risk factors for early recurrence after transforaminal endoscopic lumbar disc decompression. Pain Physician 2019, 22, E133–E138. [Google Scholar] [CrossRef]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T., Jr.; Shekelle, P.; Owens, D.K. Clinical efficacy assessment subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern Med. 2007, 147, 478–491. [Google Scholar] [CrossRef]
- Hadgaonkar, S.; Tomer, D. Analogy of lumbar disc: Retained, residual, or recurrent disc? J. Orthop. Case Rep. 2023, 13, 1–4. [Google Scholar] [CrossRef]
- Mueller, K.; Altshuler, M.; Voyadzis, J.M.; Sandhu, F.A. The incidence of symptomatic postoperative epidural hematoma after minimally invasive lumbar decompression: A single institution retrospective review. Clin. Neurol. Neurosurg. 2020, 195, 105868. [Google Scholar] [CrossRef]
- Basu, S.; Ghosh, J.D.; Malik, F.H.; Tikoo, A. Postoperative discitis following single-level lumbar discectomy: Our experience of 17 cases. Indian J. Orthop. 2012, 46, 427–433. [Google Scholar] [CrossRef]
- Ostelo, R.W.; Costa, L.O.; Maher, C.G.; de Vet, H.C.; van Tulder, M.W. Rehabilitation after lumbar disc surgery: An update Cochrane review. Spine 2009, 34, 1839–1848. [Google Scholar] [CrossRef]
- Ziegler, D.S.; Jensen, R.K.; Storm, L.; Carreon, L.; Andersen, M.O. The association between early postoperative leg pain intensity and disability at 1-year and 2-year follow-up after first-time lumbar discectomy. Glob. Spine J. 2021, 11, 81–88. [Google Scholar] [CrossRef]
- Ahn, Y.; Choi, J.E.; Lee, S. Access pain during transforaminal endoscopic lumbar discectomy for foraminal or extraforaminal disc herniation. Diagnostics 2024, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, C.; Guan, J.; Li, C.; Wu, H.; Cheng, X.; Ling, B.; Zhang, J. Outcomes of epidural steroids following percutaneous transforaminal endoscopic discectomy: A meta-analysis and systematic review. Korean J. Pain 2022, 35, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Alsiaf, H.; O’Neill, T.W.; Callaghan, M.J.; Goodwin, P.C. Physical therapy of patients undergoing first-time lumbar discectomy: A survey of current UK practice. BMC. Musculoskelet. Disord. 2022, 23, 503. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y. Strategies to Reduce Rebound Pain and Facilitate Early Recovery After Transforaminal Endoscopic Lumbar Discectomy. J. Clin. Med. 2025, 14, 3529. https://doi.org/10.3390/jcm14103529
Ahn Y. Strategies to Reduce Rebound Pain and Facilitate Early Recovery After Transforaminal Endoscopic Lumbar Discectomy. Journal of Clinical Medicine. 2025; 14(10):3529. https://doi.org/10.3390/jcm14103529
Chicago/Turabian StyleAhn, Yong. 2025. "Strategies to Reduce Rebound Pain and Facilitate Early Recovery After Transforaminal Endoscopic Lumbar Discectomy" Journal of Clinical Medicine 14, no. 10: 3529. https://doi.org/10.3390/jcm14103529
APA StyleAhn, Y. (2025). Strategies to Reduce Rebound Pain and Facilitate Early Recovery After Transforaminal Endoscopic Lumbar Discectomy. Journal of Clinical Medicine, 14(10), 3529. https://doi.org/10.3390/jcm14103529





