Apigenin Attenuates Hepatic Ischemia–Reperfusion-Induced Lung Injury via Downregulation of MMP-3 and MCP-1: An Experimental Study in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Protocol
- Sham group (n = 7): Underwent an open–close surgical procedure without vascular clamping.
- Control (C) group (n = 28): Underwent 45 min of hepatic ischemia.
- Apigenin (Ap) group (n = 28): Underwent 45 min of hepatic ischemia followed by intraperitoneal administration of 5 mg apigenin (dissolved in 0.3 mL of 0.9% NaCl and 0.2 mL dimethyl sulfoxide [DMSO]). This dosage was selected based on its previously demonstrated efficacy in similar models of I-R injury [23].
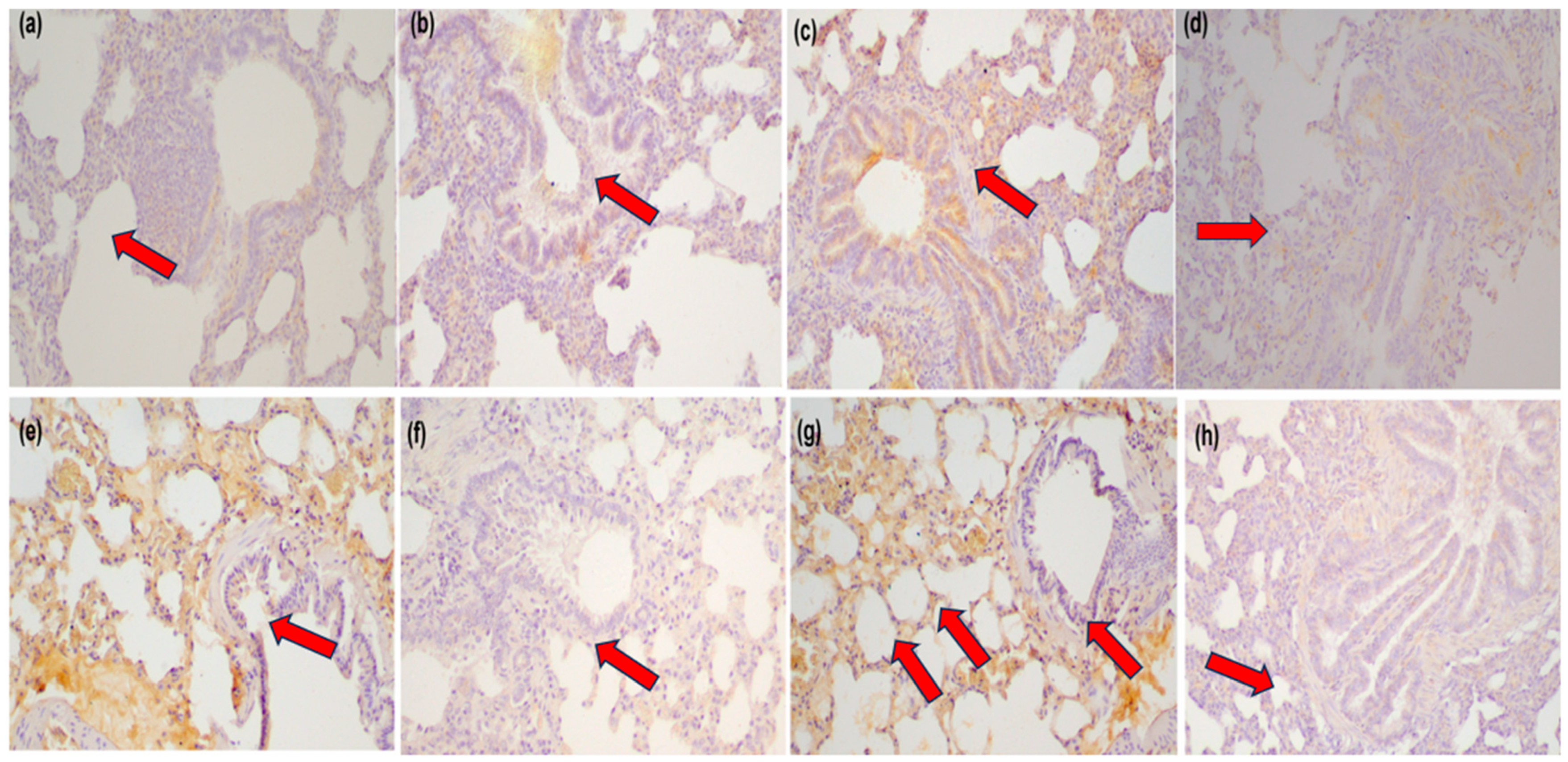
2.3. Immunohistochemistry
- MMP-3 (rabbit polyclonal, Novus Biologicals, Centennial, CO, USA; catalog no. NB100-91878), diluted 1:70.
- MCP-1 (mouse monoclonal, Merck Millipore, Burlington, MA, USA; catalog no. MABN712), uMABN712), diluted 1:200.
2.4. Evaluation of Antibody Expression
- Negative (0): <10% of cells stained.
- Low (1): 10–30% of cells stained.
- Moderate (2): 30–70% of cells stained.
- High (3): >70% of cells stained.
2.5. Statistical Analysis
- BF = 1–3: Weak evidence.
- BF = 3–20: Positive evidence supporting H1.
- BF = 20–150: Strong evidence.
- BF > 150: Very strong evidence.
3. Results
3.1. Immunohistochemical Analysis
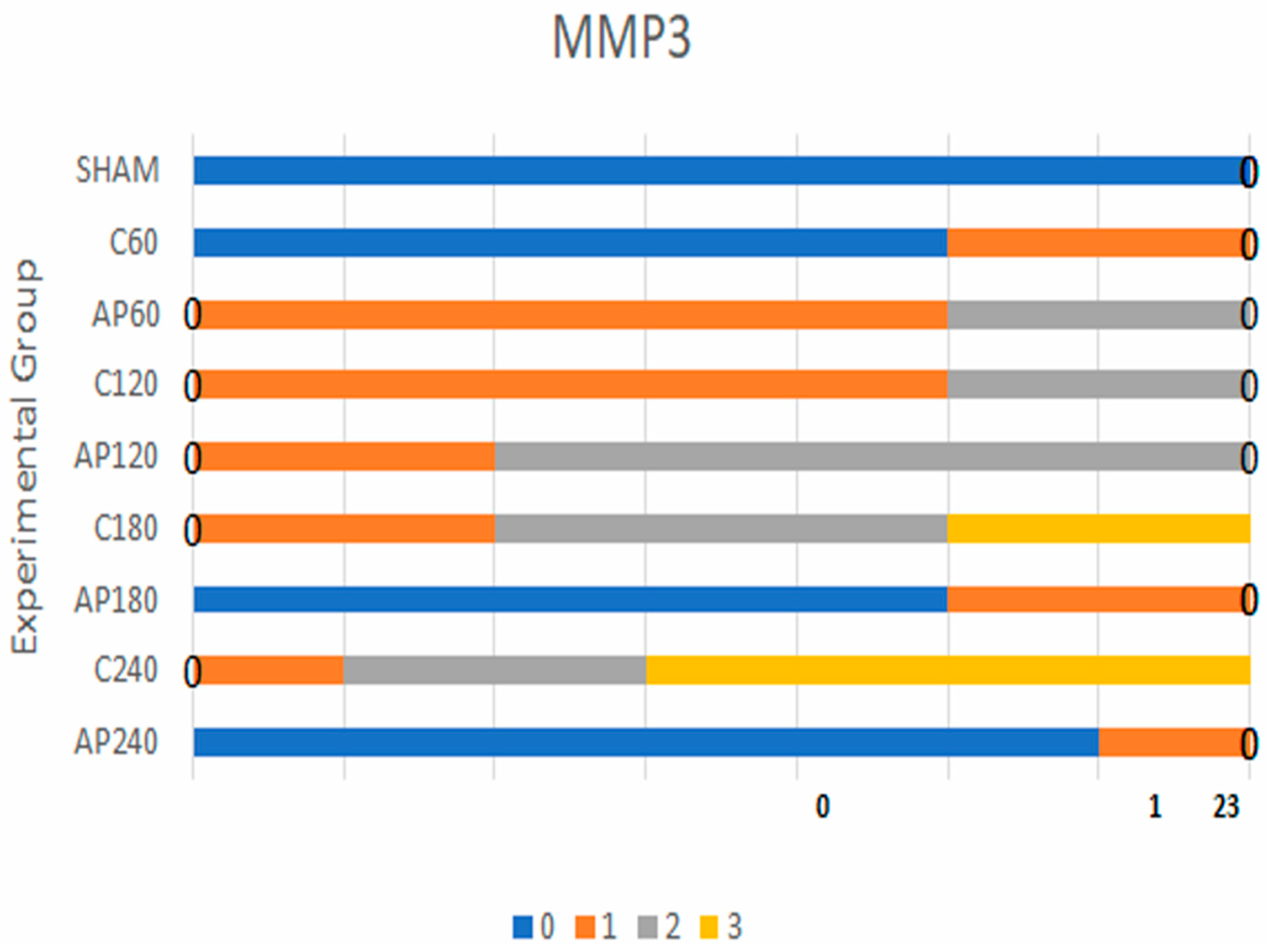
3.1.1. MMP-3 Expression
- C120 vs. Ap120: BF = 30
- C180 vs. Ap180: BF = 43
- C240 vs. Ap240: BF = 85
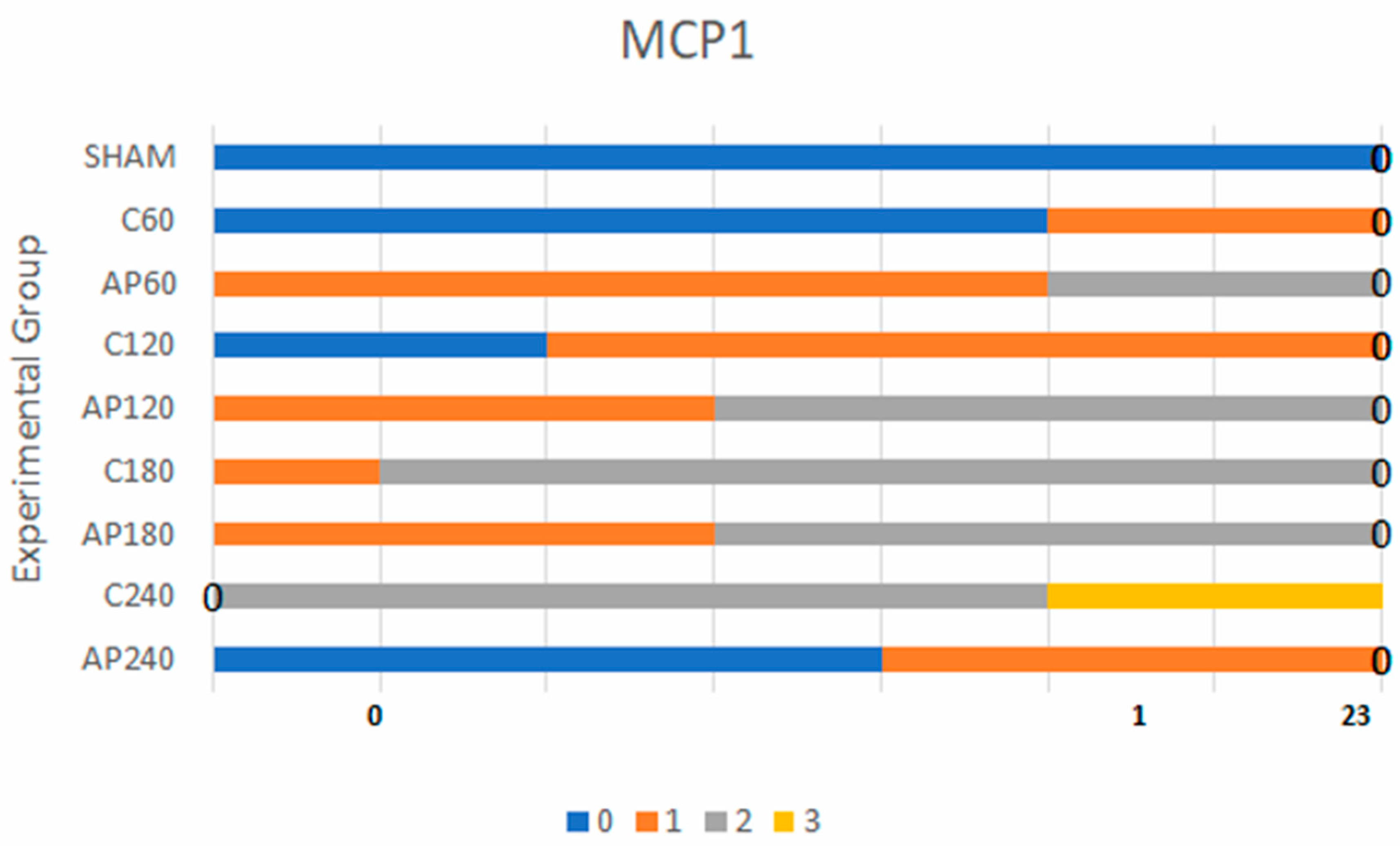
3.1.2. MCP-1 Expression
- C180 vs. Ap180: BF = 470 (very strong)
- C240 vs. Ap240: BF = 90 (strong)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| ALI | Acute Lung Injury |
| Ap | Apigenin-treated group |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| BF | Bayes Factor |
| C | Control group |
| CD40L | CD40 Ligand |
| DAB | Diaminobenzidine |
| DMSO | Dimethyl Sulfoxide |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| INF-γ | Interferon-gamma |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-18 | Interleukin-18 |
| I-R | Ischemia-Reperfusion |
| IRI | Ischemia-Reperfusion Injury |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MMP-3 | Matrix Metalloproteinase-3 |
| NaCl | Sodium Chloride |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| ROS | Reactive Oxygen Species |
| Sham | Sham-operated group |
| TNF-α | Tumor Necrosis Factor-alpha |
References
- Smyrniotis, V.; Kostopanagiotou, G.; Lolis, E.; Theodoraki, K.; Farantos, C.; Andreadou, I.; Polymeneas, G.; Genatas, C.; Contis, J. Effects of Hepatovenous Back Flow on Ischemic-Reperfusion Injuries in Liver Resections with the Pringle Maneuver. J. Am. Coll. Surg. 2003, 197, 949–954. [Google Scholar] [CrossRef]
- Bilzer, M.; Gerbes, A.L. Preservation Injury of the Liver: Mechanisms and Novel Therapeutic Strategies. J. Hepatol. 2000, 32, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Lentsch, A.B.; Kato, A.; Yoshidome, H.; McMasters, K.M.; Edwards, M.J. Inflammatory Mechanisms and Therapeutic Strategies for Warm Hepatic Ischemia/Reperfusion Injury. Hepatology 2000, 32, 169–173. [Google Scholar] [CrossRef]
- Nastos, C.; Kalimeris, K.; Papoutsidakis, N.; Tasoulis, M.-K.; Lykoudis, P.M.; Theodoraki, K.; Nastou, D.; Smyrniotis, V.; Arkadopoulos, N. Global Consequences of Liver Ischemia/Reperfusion Injury. Oxid. Med. Cell. Longev. 2014, 2014, 906965. [Google Scholar] [CrossRef] [PubMed]
- Colletti, L.M.; Kunkel, S.L.; Walz, A.; Burdick, M.D.; Kunkel, R.G.; Wilke, C.A.; Strieter, R.M. Chemokine Expression during Hepatic Ischemia/Reperfusion-Induced Lung Injury in the Rat. The Role of Epithelial Neutrophil Activating Protein. J. Clin. Investig. 1995, 95, 134–141. [Google Scholar] [CrossRef]
- Witthaut, R.; Farhood, A.; Smith, C.W.; Jaeschke, H. Complement and Tumor Necrosis Factor-Alpha Contribute to Mac-1 (CD11b/CD18) up-Regulation and Systemic Neutrophil Activation during Endotoxemia in Vivo. J. Leukoc. Biol. 1994, 55, 105–111. [Google Scholar] [CrossRef]
- Knittel, T.; Mehde, M.; Kobold, D.; Saile, B.; Dinter, C.; Ramadori, G. Expression Patterns of Matrix Metalloproteinases and Their Inhibitors in Parenchymal and Non-Parenchymal Cells of Rat Liver: Regulation by TNF-Alpha and TGF-Beta1. J. Hepatol. 1999, 30, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Scott, T.L.; Rangaswamy, S.; Wicker, C.A.; Izumi, T. Repair of Oxidative DNA Damage and Cancer: Recent Progress in DNA Base Excision Repair. Antioxid. Redox Signal. 2014, 20, 708–726. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Cochran, B.H.; Reffel, A.C.; Stiles, C.D. Molecular Cloning of Gene Sequences Regulated by Platelet-Derived Growth Factor. Cell 1983, 33, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Docherty, A.J. The Matrix Metalloproteinases and Their Inhibitors. Am. J. Respir. Cell Mol. Biol. 1992, 7, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.P.; Thomas, P.J. A novel clan of zinc metallopeptidases with possible intramembrane cleavage properties. Protein Sci. 1999, 8, 439–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brinckerhoff, C.E.; Matrisian, L.M. Matrix Metalloproteinases: A Tail of a Frog That Became a Prince. Nat. Rev. Mol. Cell Biol. 2002, 3, 207–214. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of Matrix Metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix Metalloproteinases: Role in Arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Jung, U.J.; Cho, Y.-Y.; Choi, M.-S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; Tufail, T.; et al. Therapeutical Properties of Apigenin: A Review on the Experimental Evidence and Basic Mechanisms. Int. J. Food Prop. 2023, 26, 1914–1939. [Google Scholar] [CrossRef]
- Kupiec-Weglinski, J.W.; Busuttil, R.W. Ischemia and Reperfusion Injury in Liver Transplantation. Transplant. Proc. 2005, 37, 1653–1656. [Google Scholar] [CrossRef]
- Wang, C.; Feng, X.; Li, W.; Chen, L.; Wang, X.; Lan, Y.; Tang, R.; Jiang, T.; Zheng, L.; Liu, G. Apigenin as an Emerging Hepatoprotective Agent: Current Status and Future Perspectives. Front. Pharmacol. 2024, 15, 1508060. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Tsaroucha, A.K.; Tsiaousidou, A.; Ouzounidis, N.; Tsalkidou, E.; Lambropoulou, M.; Giakoustidis, D.; Chatzaki, E.; Simopoulos, C. Intraperitoneal Administration of Apigenin in Liver Ischemia/Reperfusion Injury Protective Effects. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 415–422. [Google Scholar] [CrossRef]
- Teoh, N.C.; Farrell, G.C. Hepatic Ischemia Reperfusion Injury: Pathogenic Mechanisms and Basis for Hepatoprotection. J. Gastroenterol. Hepatol. 2003, 18, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Kass, R.E.; Raftery, A.E. Bayes Factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Raza, S.L.; Cornelius, L.A. Matrix Metalloproteinases: Pro- and Anti-Angiogenic Activities. J. Investig. Dermatol. Symp. Proc. 2000, 5, 47–54. [Google Scholar] [CrossRef]
- Stan, F. Comparative Study of the Liver Anatomy in the Rat, Rabbit, Guinea Pig and Chinchilla. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Vet. Med. 2018, 75, 33. [Google Scholar] [CrossRef]
- Arii, S.; Teramoto, K.; Kawamura, T. Current Progress in the Understanding of and Therapeutic Strategies for Ischemia and Reperfusion Injury of the Liver. J. Hepatobiliary Pancreat. Surg. 2003, 10, 189–194. [Google Scholar] [CrossRef]
- Vollmar, B.; Glasz, J.; Leiderer, R.; Post, S.; Menger, M.D. Hepatic Microcirculatory Perfusion Failure Is a Determinant of Liver Dysfunction in Warm Ischemia-Reperfusion. Am. J. Pathol. 1994, 145, 1421–1431. [Google Scholar]
- Qin, Y.; Zhao, D.; Zhou, H.-G.; Wang, X.-H.; Zhong, W.-L.; Chen, S.; Gu, W.-G.; Wang, W.; Zhang, C.-H.; Liu, Y.-R.; et al. Apigenin Inhibits NF-ΚB and Snail Signaling, EMT and Metastasis in Human Hepatocellular Carcinoma. Oncotarget 2016, 7, 41421–41431. [Google Scholar] [CrossRef]
- Palladini, G.; Ferrigno, A.; Rizzo, V.; Tarantola, E.; Bertone, V.; Freitas, I.; Perlini, S.; Richelmi, P.; Vairetti, M. Lung Matrix Metalloproteinase Activation Following Partial Hepatic Ischemia/Reperfusion Injury in Rats. Sci. World J. 2014, 2014, 867548. [Google Scholar] [CrossRef] [PubMed]
- Moench, C.; Uhrig, A.; Lohse, A.W.; Otto, G. The Role of Monocyte Chemoattractant Protein-1 in Orthotopic Liver Transplantation. Transplant. Proc. 2003, 35, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Yu, Z.; Zhang, R.; Ding, X.; Chen, Z.; Li, Q. WISP1 Mediates Lung Injury Following Hepatic Ischemia Reperfusion Dependent on TLR4 in Mice. BMC Pulm. Med. 2018, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Vries, D.K.; Kortekaas, K.A.; Tsikas, D.; Wijermars, L.G.M.; van Noorden, C.J.F.; Suchy, M.-T.; Cobbaert, C.M.; Klautz, R.J.M.; Schaapherder, A.F.M.; Lindeman, J.H.N. Oxidative Damage in Clinical Ischemia/Reperfusion Injury: A Reappraisal. Antioxid. Redox Signal. 2013, 19, 535–545. [Google Scholar] [CrossRef]
- Abu-Amara, M.; Yang, S.Y.; Seifalian, A.; Davidson, B.; Fuller, B. The Nitric Oxide Pathway--Evidence and Mechanisms for Protection against Liver Ischaemia Reperfusion Injury. Liver Int. Off. J. Int. Assoc. Study Liver 2012, 32, 531–543. [Google Scholar] [CrossRef]
- Clemens, M.G. Nitric Oxide in Liver Injury. Hepatology 1999, 30, 1–5. [Google Scholar] [CrossRef]
- Kollaras, V.; Valsami, G.; Lambropoulou, M.; Konstandi, O.; Kostomistsopoulos, N.; Pikoulis, E.; Simopoulos, C.; Tsaroucha, A. Effect of Silibinin on the Expression of MMP2, MMP3, MMP9 and TIMP2 in Kidney and Lung after Hepatic Ischemia/Reperfusion Injury in an Experimental Rat Model. Acta Cir. Bras. 2021, 36, e360904. [Google Scholar] [CrossRef]
- Tsalkidou, E.; Tsaroucha, A.; Chatzaki, E.; Lambropoulou, M.; Papachristou, F.; Trypsianis, G.; Pitiakoudis, M.; Vaos, G.; Simopoulos, C. The Effects of Apigenin on the Expression of Fas/FasL Apoptotic Pathway in Warm Liver Ischemia-Reperfusion Injury in Rats. BioMed Res. Int. 2014, 2014, 157216. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X.; Maskos, K.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; Bourenkov, G.P.; et al. Mechanism of Inhibition of the Human Matrix Metalloproteinase Stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef]
- Skondras, I.; Lambropoulou, M.; Tsaroucha, A.; Gardikis, S.; Tripsianis, G.; Simopoulos, C.; Vaos, G. The Role of Apigenin in Testicular Damage in Experimental Ischemia-Reperfusion Injury in Rats. Hippokratia 2015, 19, 225–230. [Google Scholar]
- Zhou, L.; Zhao, D.; An, H.; Zhang, H.; Jiang, C.; Yang, B. Melatonin Prevents Lung Injury Induced by Hepatic Ischemia-Reperfusion through Anti-Inflammatory and Anti-Apoptosis Effects. Int. Immunopharmacol. 2015, 29, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Roller, J.; Zhang, S.; Syk, I.; Menger, M.D.; Jeppsson, B.; Thorlacius, H. Metalloproteinases Regulate CD40L Shedding from Platelets and Pulmonary Recruitment of Neutrophils in Abdominal Sepsis. Inflamm. Res. 2012, 61, 571–579. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Shu, S.; Wang, S.; Guo, F.; Yin, Y.; Zhou, W.; Han, H.; Chai, X. Sufentanil Protects the Liver from Ischemia/Reperfusion-Induced Inflammation and Apoptosis by Inhibiting ATF4-Induced TP53BP2 Expression. Inflammation 2021, 44, 1160–1174. [Google Scholar] [CrossRef]
- Basios, N.; Lampropoulos, P.; Papalois, A.; Pitiakoudis, M.K.; Kotini, A.; Tsaroucha, A.K.; Basios, N.; Lampropoulos, P.; Papalois, A.; Lambropoulou, M.; et al. Apigenin Attenuates Inflammation in Experimentally Induced Acute Pancreatitis-Associated Lung Injury Apigenin Attenuates Inflammation in Experimentally Induced Acute Pancreatitis-Associated Lung Injury. J. Investig. Surg. 2016, 29, 121–127. [Google Scholar] [CrossRef]
- Lampropoulos, P.; Lambropoulou, M.; Papalois, A.; Basios, N.; Manousi, M.; Simopoulos, C.; Tsaroucha, A.K. The Role of Apigenin in an Experimental Model of Acute Pancreatitis. J. Surg. Res. 2012, 183, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Surgery, G.; Hospital, B.; Hospital, L.G. Apigenin Exerts Anti-Inflammatory Effects in an Experimental Model of Acute Pancreatitis by Down-Regulating TNF-α. In Vivo 2019, 1141, 1133–1141. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. cytokine Res. Off. J. Int. Soc. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Geng, J.; Zheng, Z.; Li, L.; Ren, Z.; Tian, G.; Qin, J.; Zhao, T.; Feng, X. Apigenin Attenuated Sepsis Induced Acute Lung Injury via Polarizing Macrophage towards M2 by Blocking MiR-146a→TLR7 Interaction. Int. Immunopharmacol. 2025, 152, 114446. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Liu, Y.; Meyer, C.; Xu, C.; Weng, H.; Hellerbrand, C.; ten Dijke, P.; Dooley, S. Animal Models of Chronic Liver Diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G449–G468. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; Huang, Y.; Chen, J.; Fu, H.; Yang, N.; et al. Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Comparison | Bayes Factor | Level 0 (n) | Level 1 (n) | Level 2 (n) | Level 3 (n) |
|---|---|---|---|---|---|---|
| MMP-3 | Sham vs. C60 | 1.8 | Sham: 7, C60: 5 | Sham: 0, C60: 2 | Sham: 0, C60: 0 | Sham: 0, C60: 0 |
| C60 vs. Ap60 | 3.0 | C60: 5, Ap60: 5 | C60: 2, Ap60: 2 | C60: 0, Ap60: 0 | C60: 0, Ap60: 0 | |
| C120 vs. Ap120 | 30 | C120: 0, Ap120: 0 | C120: 5, Ap120: 3 | C120: 2, Ap120: 4 | C120: 0, Ap120: 0 | |
| C180 vs. Ap180 | 43 | C180: 0, Ap180: 0 | C180: 2, Ap180: 3 | C180: 3, Ap180: 4 | C180: 2, Ap180: 0 | |
| C240 vs. Ap240 | 85 | C240: 0, Ap240: 1 | C240: 2, Ap240: 6 | C240: 4, Ap240: 0 | C240: 0, Ap240: 0 | |
| MCP-1 | Sham vs. C60 | 1.7 | Sham: 7, C60: 5 | Sham: 0, C60: 2 | Sham: 0, C60: 0 | Sham: 0, C60: 0 |
| C60 vs. Ap60 | 3.2 | C60: 5, Ap60: 5 | C60: 2, Ap60: 2 | C60: 0, Ap60: 0 | C60: 0, Ap60: 0 | |
| C120 vs. Ap120 | 9.0 | C120: 0, Ap120: 0 | C120: 5, Ap120: 3 | C120: 0, Ap120: 4 | C120: 0, Ap120: 0 | |
| C180 vs. Ap180 | 470 | C180: 0, Ap180: 0 | C180: 1, Ap180: 0 | C180: 6, Ap180: 4 | C180: 0, Ap180: 0 | |
| C240 vs. Ap240 | 90 | C240: 0, Ap240: 0 | C240: 1, Ap240: 0 | C240: 2, Ap240: 3 | C240: 4, Ap240: 0 |
| Marker | Group Comparison | Bayes Factor | Interpretation |
|---|---|---|---|
| MMP-3 | C60 vs. Ap60 | 3.0 | Weak evidence |
| MMP-3 | C120 vs. Ap120 | 30 | Strong evidence |
| MMP-3 | C180 vs. Ap180 | 43 | Strong evidence |
| MMP-3 | C240 vs. Ap240 | 85 | Strong evidence |
| MCP-1 | C60 vs. Ap60 | 3.2 | Weak evidence |
| MCP-1 | C120 vs. Ap120 | 9.0 | Positive evidence |
| MCP-1 | C180 vs. Ap180 | 470 | Very strong evidence |
| MCP-1 | C240 vs. Ap240 | 90 | Strong evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariorakis, C.; Lambropoulou, M.; Oikonomou, P.; Tsalikidis, C.; Pitiakoudis, M.; Anestiadou, E.; Ioannidis, O.; Tsaroucha, A.K. Apigenin Attenuates Hepatic Ischemia–Reperfusion-Induced Lung Injury via Downregulation of MMP-3 and MCP-1: An Experimental Study in Rats. J. Clin. Med. 2025, 14, 3530. https://doi.org/10.3390/jcm14103530
Mariorakis C, Lambropoulou M, Oikonomou P, Tsalikidis C, Pitiakoudis M, Anestiadou E, Ioannidis O, Tsaroucha AK. Apigenin Attenuates Hepatic Ischemia–Reperfusion-Induced Lung Injury via Downregulation of MMP-3 and MCP-1: An Experimental Study in Rats. Journal of Clinical Medicine. 2025; 14(10):3530. https://doi.org/10.3390/jcm14103530
Chicago/Turabian StyleMariorakis, Chrysovalantis, Maria Lambropoulou, Panagoula Oikonomou, Christos Tsalikidis, Michail Pitiakoudis, Elissavet Anestiadou, Orestis Ioannidis, and Alexandra K. Tsaroucha. 2025. "Apigenin Attenuates Hepatic Ischemia–Reperfusion-Induced Lung Injury via Downregulation of MMP-3 and MCP-1: An Experimental Study in Rats" Journal of Clinical Medicine 14, no. 10: 3530. https://doi.org/10.3390/jcm14103530
APA StyleMariorakis, C., Lambropoulou, M., Oikonomou, P., Tsalikidis, C., Pitiakoudis, M., Anestiadou, E., Ioannidis, O., & Tsaroucha, A. K. (2025). Apigenin Attenuates Hepatic Ischemia–Reperfusion-Induced Lung Injury via Downregulation of MMP-3 and MCP-1: An Experimental Study in Rats. Journal of Clinical Medicine, 14(10), 3530. https://doi.org/10.3390/jcm14103530







