Evaluating Virtual Planning Accuracy in Bimaxillary Advancement Surgery: A Retrospective Study Introducing the Planning Accuracy Coefficient
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Requirements
2.2. Treatment
2.3. Data Acquisition
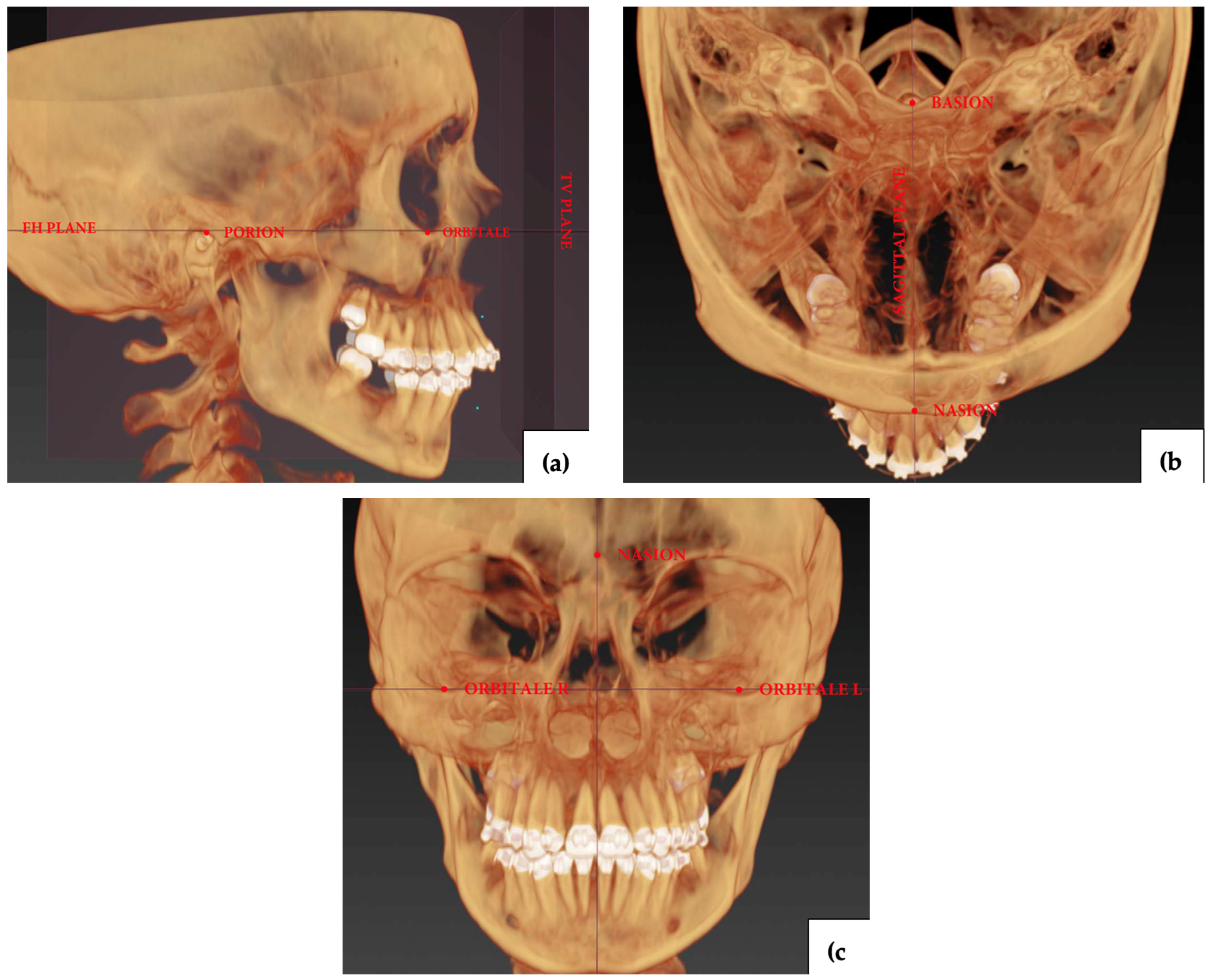
2.4. Measurements
2.5. Statistical Analysis
2.6. Ethical Approval and Consent
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zammit, D.; Ettinger, R.E.; Sanati-Mehrizy, P.; Susarla, S.M. Current Trends in Orthognathic Surgery. Medicina 2023, 59, 2100. [Google Scholar] [CrossRef]
- Cariati, P.; Martínez, R.; Martínez-Lara, I. Psycho-social impact of orthogathic sugery. J. Clin. Exp. Dent. 2016, 8, e540–e545. [Google Scholar] [CrossRef]
- Sahu, G.R.; Kaur, A.; Rattan, V.; Singh, S.P.; Rai, S. Effect of Orthognathic Surgery on Temporomandibular Disorders: A Prospective Study. J. Maxillofac. Oral Surg. 2022, 21, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, L.; Stokbro, K.; Aagaard, E.; Torkov, P.; Thygesen, T. Changes in Upper Airway Volume Following Orthognathic Surgery. J. Craniofac. Surg. 2017, 28, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Alkaabi, S.; Maningky, M.; Helder, M.N.; Alsabri, G. Virtual and traditional surgical planning in orthognathic surgery—Systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Grab, P.P.; Szałwiński, M.; Rot, P.; Chloupek, A.; Sobol, M.; Jurkiewicz, D. Changes in Maxillary Sinus Volume and Mucosal Thickness Post Bimaxillary Advancement Procedures: A Retrospective Study. J. Clin. Med. 2024, 13, 3425. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, S.G. Redefining Precision and Efficiency in Orthognathic Surgery through Virtual Surgical Planning and 3D Printing: A Narrative Review. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 42. [Google Scholar] [CrossRef]
- Modabber, A.; Baron, T.; Peters, F.; Kniha, K.; Danesh, G.; Hölzle, F.; Ayoub, N.; Möhlhenrich, S.C. Comparison of soft tissue simulations between two planning software programs for orthognathic surgery. Sci. Rep. 2022, 12, 5013. [Google Scholar] [CrossRef]
- Swennen, G. Benefits and limitations of three-dimensional virtual planning of orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2009, 38, 415. [Google Scholar] [CrossRef]
- Kwon, T.G. Accuracy and reliability of three-dimensional computer-assisted planning for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 14. [Google Scholar] [CrossRef]
- Otranto de Britto Teixeira, A.; de Oliveira Almeida, M.A.; da Cunha Almeida, R.C.; Maues, C.P.; Pimentel, T.; Ribeiro, D.P.B.; de Medeiros, P.J.; Quintão, C.C.A.; de Assis Ribeiro Carvalho, F. Three-dimensional accuracy of virtual planning in orthognathic surgery. Am. J. Orthod. Dentofac. Orthop. 2020, 158, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ritto, F.G.; Schmitt, A.R.M.; Pimentel, T.; Canellas, J.V.; Medeiros, P.J. Comparison of the accuracy of maxillary position between conventional model surgery and virtual surgical planning. Int. J. Oral Maxillofac. Surg. 2018, 47, 160–166. [Google Scholar] [CrossRef]
- Van Hemelen, G.; Van Genechten, M.; Renier, L.; Desmedt, M.; Verbruggen, E.; Nadjmi, N. Three-dimensional virtual planning in orthognathic surgery enhances the accuracy of soft tissue prediction. J. Craniomaxillofac. Surg. 2015, 43, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.J.; Denadai, R.; Pai, B.C.J.; Lin, H.H.; Lo, L.J. Outcome of facial contour asymmetry after conventional two-dimensional versus computer-assisted three-dimensional planning in cleft orthognathic surgery. Sci. Rep. 2020, 10, 2346. [Google Scholar] [CrossRef]
- Donaldson, C.D.; Manisali, M.; Naini, F.B. Three-dimensional virtual surgical planning (3D-VSP) in orthognathic surgery: Advantages, disadvantages and pitfalls. J. Orthod. 2021, 48, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Zoabi, A.; Redenski, I.; Oren, D.; Kasem, A.; Zigron, A.; Daoud, S.; Moskovich, L.; Kablan, F.; Srouji, S. 3D Printing and Virtual Surgical Planning in Oral and Maxillofacial Surgery. J. Clin. Med. 2022, 11, 2385. [Google Scholar] [CrossRef]
- Awad, D.; Reinert, S.; Kluba, S. Accuracy of Three-Dimensional Soft-Tissue Prediction Considering the Facial Aesthetic Units Using a Virtual Planning System in Orthognathic Surgery. J. Pers. Med. 2022, 12, 1379. [Google Scholar] [CrossRef]
- Alkhayer, A.; Piffkó, J.; Lippold, C.; Segatto, E. Accuracy of virtual planning in orthognathic surgery: A systematic review. Head Face Med. 2020, 16, 34. [Google Scholar] [CrossRef]
- Tondin, G.M.; Dutra Leal, M.O.C.; Costa, S.T.; Grillo, R.; Jodas, C.R.P.; Teixeira, R.G. Evaluation of the accuracy of virtual planning in bimaxillary orthognathic surgery: A systematic review. Br. J. Oral Maxillofac. Surg. 2022, 60, 412–421. [Google Scholar] [CrossRef]
- Beek, D.M.; Visser, D.J.; Chen, Y.H.; Baan, F.; Nienhuijs, M.; Xi, T. Is there a difference in surgical accuracy following bimaxillary surgery between cleft and non-cleft patients? Clin. Oral Investig. 2024, 28, 112. [Google Scholar] [CrossRef]
- Liebregts, J.; Baan, F.; de Koning, M.; Ongkosuwito, E.; Bergé, S.; Maal, T.; Xi, T. Achievability of 3D planned bimaxillary osteotomies: Maxilla-first versus mandible-first surgery. Sci. Rep. 2017, 7, 9314. [Google Scholar] [CrossRef] [PubMed]
- Franz, L.; Isola, M.; Bagatto, D.; Calzolari, F.; Travan, L.; Robiony, M.A. A Novel Protocol for Planning and Navigation in Craniofacial Surgery: A Preclinical Surgical Study. J. Oral Maxillofac. Surg. 2017, 75, 1971–1979. [Google Scholar] [CrossRef]
- Bengtsson, M.; Wall, G.; Miranda-Burgos, P.; Rasmusson, L. Treatment outcome in orthognathic surgery—A prospective comparison of accuracy in computer assisted two and three-dimensional prediction techniques. J. Craniomaxillofac. Surg. 2018, 46, 1867–1874. [Google Scholar] [CrossRef]
- Salazar, D.; Rossouw, P.E.; Javed, F.; Michelogiannakis, D. Artificial intelligence for treatment planning and soft tissue outcome prediction of orthognathic treatment: A systematic review. J. Orthod. 2024, 51, 107119. [Google Scholar] [CrossRef] [PubMed]
- Badiali, G.; Bevini, M.; Gulotta, C.; Lunari, O.; Incerti Parenti, S.; Pironi, M.; Bianchi, A.; Felice, P.; Marchetti, C. Three-dimensional cephalometric outcome predictability of virtual orthodontic-surgical planning in surgery-first approach. Prog. Orthod. 2022, 23, 51. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Ho, C.T.; Lin, H.H.; Lo, L.J. Three-dimensional cephalometry for orthognathic planning: Normative data and analyses. J. Formos. Med. Assoc. 2020, 119, 191–203. [Google Scholar] [CrossRef]
- Pascal, E.; Majoufre, C.; Bondaz, M.; Courtemanche, A.; Berger, M.; Bouletreau, P. Current status of surgical planning and transfer methods in orthognathic surgery. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 245–248. [Google Scholar] [CrossRef]
- Lechuga, L.; Weidlich, G. Cone Beam CT vs. Fan Beam CT: A Comparison of Image Quality and Dose Delivered Between Two Differing CT Imaging Modalities. Cureus 2016, 8, e778. [Google Scholar] [CrossRef]
- Perez, D.; Ellis, E. Sequencing bimaxillary surgery: Mandible first. J. Oral Maxillofac. Surg. 2011, 69, 2217–2224. [Google Scholar] [CrossRef]
- Serafin, M.; Baldini, B.; Cabitza, F.; Carrafiello, G.; Baselli, G.; Del Fabbro, M.; Sforza, C.; Caprioglio, A.; Tartaglia, G.M. Accuracy of automated 3D cephalometric landmarks by deep learning algorithms: Systematic review and meta-analysis. Radiol. Med. 2023, 128, 544–555. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, K.; Kang, S.H.; Lee, S.H. 3D cephalometric landmark detection by multiple stage deep reinforcement learning. Sci. Rep. 2021, 11, 17509. [Google Scholar] [CrossRef] [PubMed]
- Dallel, I.; Tobji, S.; Ben Amor, F. A Study of the Parameters That Influence Orthosurgical Treatments. J. Dentofac. Anom. Orthod. 2017, 20, 408. [Google Scholar] [CrossRef]

| Sex | Total | |||
|---|---|---|---|---|
| Female | Male | |||
| Skeletal malocclusion type | Type II | 11 (31%) | 1 (3%) | 12 (34%) |
| Type III | 14 (40%) | 9 (26%) | 23 (66%) | |
| Total | 25 (71%) | 10 (29%) | 35(100%) | |
| A-point—A | Anterior Nasal spine—ANS | B-point—B | Basion—Ba | Columella constructed point—c″ |
| Glabella—g | Gnathion—gn | Gnathion′—gn′ | Gonion left—Go(l) | Gonion right—Go(r) |
| Incisor midpoint—I(m) | Labiale inferius—li | Labiale superius—ls | Left molar midpoint—LM(m) | Lower incisor—LI(m) |
| Lower incisor apex—LIapex(m) | Lower incisor apex left—LIapex(l) | Lower incisor apex right—LIapex(r) | Lower incisor left—LI(l) | Lower incisor right—LI(r) |
| Lower molar cusp left—LMcusp(l) | Lower molar cusp right—LMcusp(r) | Menton—Men | Nasion—N | Nasion—n |
| Orbitale left—Or(l) | Orbitale right—Or(r) | Pogonion—pg | Pogonion—Pog | Porion left—Po(l) |
| Porion midpoint—Po(m) | Porion right—Po(r) | Posterior maxillary point left—PMP(l) | Posterior maxillary point right—PMP(r) | Posterior Nasal spine—PNS |
| Pronasale—prn | Right molar midpoint—RM(m) | Sella—S | Stomion inferius—st(i) | Stomion superius—st(s) |
| Sublabiale—sl | Subnasale—sn | Subspinale—ss | Upper canine left—UC(l) | Upper canine right—UC(r) |
| Upper incisor—UI(m) | Upper incisor apex—UIapex(m) | Upper incisor apex left—UIapex(l) | Upper incisor apex right—UIapex(r) | Upper incisor left—UI(l) |
| Upper incisor right—UI(r) | Upper molar cusp left—UMcusp(l) | Upper molar cusp right—UMcusp(r) | Zygion left—zy(l) | Zygion right—zy(r) |
| Data | ANB Angle | SNA Angle | SNB Angle | Occlusal Plane Angle to FH | Facial Angle | Skeletal Facial Angle | Height of the Face | Height of the Mandible | Height of the Maxilla | |
| Stat. | ||||||||||
| Delta (post-op—planned) | ||||||||||
| Mean ± SD | −0.8 ± 1.4 | −0.5 ± 1.1 | 0.2 ± 1.1 | −0.1 ± 1.6 | 4.1 ± 4.1 | 0.9 ± 3.0 | 0.1 ± 2.6 | 0.6 ± 1.3 | 0.3 ± 1.2 | |
| Median (Min–Max) | 0.9 (−3.8 to 1.9) | −0.6 (−2.0 to 1.5) | 0.5 (−2.5 to 1.8) | −0.1 (−4.2 to 4.0) | 3.7 (−2.6 to 10.7) | 1.2 (−10.7 to 6.6) | 0.4 (−6.0 to 7.8) | 0.6 (−1.9 to 3.7) | 0.3 (−2.0 to 2.8) | |
| MAE | 1.27 | 1.05 | 0.90 | 1.27 | 4.79 | 2.32 | 2.01 | 1.14 | 1.03 | |
| PAC | ||||||||||
| Mean ± SD | 0.65 ± 1.62 | 0.19 ± 0.13 | 1.29 ± 3.98 | 1.34 ± 2.16 | 1.34 ± 3.49 | 0.44 ± 0.42 | 5.25 ± 10.29 | 1.05 ± 1.58 | 1.31 ± 2.33 | |
| Median (Min–Max) | 0.23 (0.02 to 8.46) | 0.19 (0.01 to 0.74) | 0.39 (0.02 to 24) | 0.62 (0.01 to 10.05) | 0.53 (0.03 to 20.08) | 0.32 (0.01 to 2.1) | 1.36 (0.04 to 68.75) | 0.55 (0.01 to 8.06) | 0.66 (0.04 to 12.0) | |
| Trimmed Mean | 0.28 | 0.18 | 0.60 | 0.97 | 0.59 | 0.40 | 3.25 | 0.75 | 1.00 | |
| Data | Overbite | Overjet | Mentolabial angle | Nasolabial angle | Z angle | Facial index | Lower incisor mean projection towards the TV-Pl | Upper incisor mean projection towards the TV-Pl | Chin projection | |
| Stat. | ||||||||||
| Delta (post-op—planned) | ||||||||||
| Mean ± SD | −0.8 ± 1.2 | −0.1 ± 1.3 | 2.1 ± 11.0 | −13.7 ± 13.1 | 2.3 ± 2.0 | −6.5 ± 8.3 | −1.6 ± 2.6 | −1.7 ± 2.3 | −2.2 ± 4.0 | |
| Median (Min–Max) | −0.7 (−3.6 to 2.5) | −0.1 (−2.8 to 3.0) | 3.7 (−29.7 to 26.6) | −14.4 (−46.1 to 7.3) | 2.7 (−3.1 to 7.6) | −5.9 (−24.5 to 13.6) | −1.5 (−8.8 to 4.3) | −1.5 (−9.0 to 3.2) | −2.7 (−11.7 to 7.7) | |
| MAE | 1.14 | 1.04 | 8.71 | 14.86 | 2.53 | 8.11 | 2.35 | 2.08 | 3.68 | |
| PAC | ||||||||||
| Mean ± SD | 0.84 ± 1.79 | 0.34 ± 0.73 | 1.55 ± 2.38 | 1.48 ± 1.69 | 1.07 ± 1.7 | 3.22 ± 4.17 | 0.79 ± 1.11 | 4.03 ± 7.33 | 0.96 ± 1.05 | |
| Median (Min–Max) | 0.52 (0.05 to 10.96) | 0.19 (0.01 to 4.39) | 0.75 (0.01 to 11.9) | 1.14 (0.09 to 10.05) | 0.65 0.01 to 9.61) | 2.01 (0.07 to 21.65) | 0.39 0.01 to 3.04) | 1.73 0.01 to 30.33) | 0.62 0.05 to 5.79) | |
| Trimmed Mean | 0.53 | 0.21 | 1.09 | 1.22 | 0.74 | 2.58 | 0.59 | 3.31 | 0.80 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grab, P.P.; Szałwiński, M.; Jagielak, M.; Rożko, J.; Jurkiewicz, D.; Chloupek, A.; Sobol, M.; Rot, P. Evaluating Virtual Planning Accuracy in Bimaxillary Advancement Surgery: A Retrospective Study Introducing the Planning Accuracy Coefficient. J. Clin. Med. 2025, 14, 3527. https://doi.org/10.3390/jcm14103527
Grab PP, Szałwiński M, Jagielak M, Rożko J, Jurkiewicz D, Chloupek A, Sobol M, Rot P. Evaluating Virtual Planning Accuracy in Bimaxillary Advancement Surgery: A Retrospective Study Introducing the Planning Accuracy Coefficient. Journal of Clinical Medicine. 2025; 14(10):3527. https://doi.org/10.3390/jcm14103527
Chicago/Turabian StyleGrab, Paweł Piotr, Michał Szałwiński, Maciej Jagielak, Jacek Rożko, Dariusz Jurkiewicz, Aldona Chloupek, Maria Sobol, and Piotr Rot. 2025. "Evaluating Virtual Planning Accuracy in Bimaxillary Advancement Surgery: A Retrospective Study Introducing the Planning Accuracy Coefficient" Journal of Clinical Medicine 14, no. 10: 3527. https://doi.org/10.3390/jcm14103527
APA StyleGrab, P. P., Szałwiński, M., Jagielak, M., Rożko, J., Jurkiewicz, D., Chloupek, A., Sobol, M., & Rot, P. (2025). Evaluating Virtual Planning Accuracy in Bimaxillary Advancement Surgery: A Retrospective Study Introducing the Planning Accuracy Coefficient. Journal of Clinical Medicine, 14(10), 3527. https://doi.org/10.3390/jcm14103527






