The Antimicrobial Effect of a Low-Frequency Square Wave Compared to Chlorhexidine
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Tooth Specimens
2.2. Experimental Groups
2.2.1. Chemical Antimicrobial Agents
2.2.2. Electric Stimulation
2.2.3. Brushing
2.3. Biofilm Formation
2.3.1. Saliva Collection
2.3.2. Biofilm Culture
2.3.3. Standard Strains
2.4. Colony-Forming Unit (CFU) Measurement
2.5. Absorbance Measurement
2.6. High-Resolution Field Emission Scanning Electron Microscopy (HR FE-SEM) Observation
2.7. Statistical Analysis
3. Results
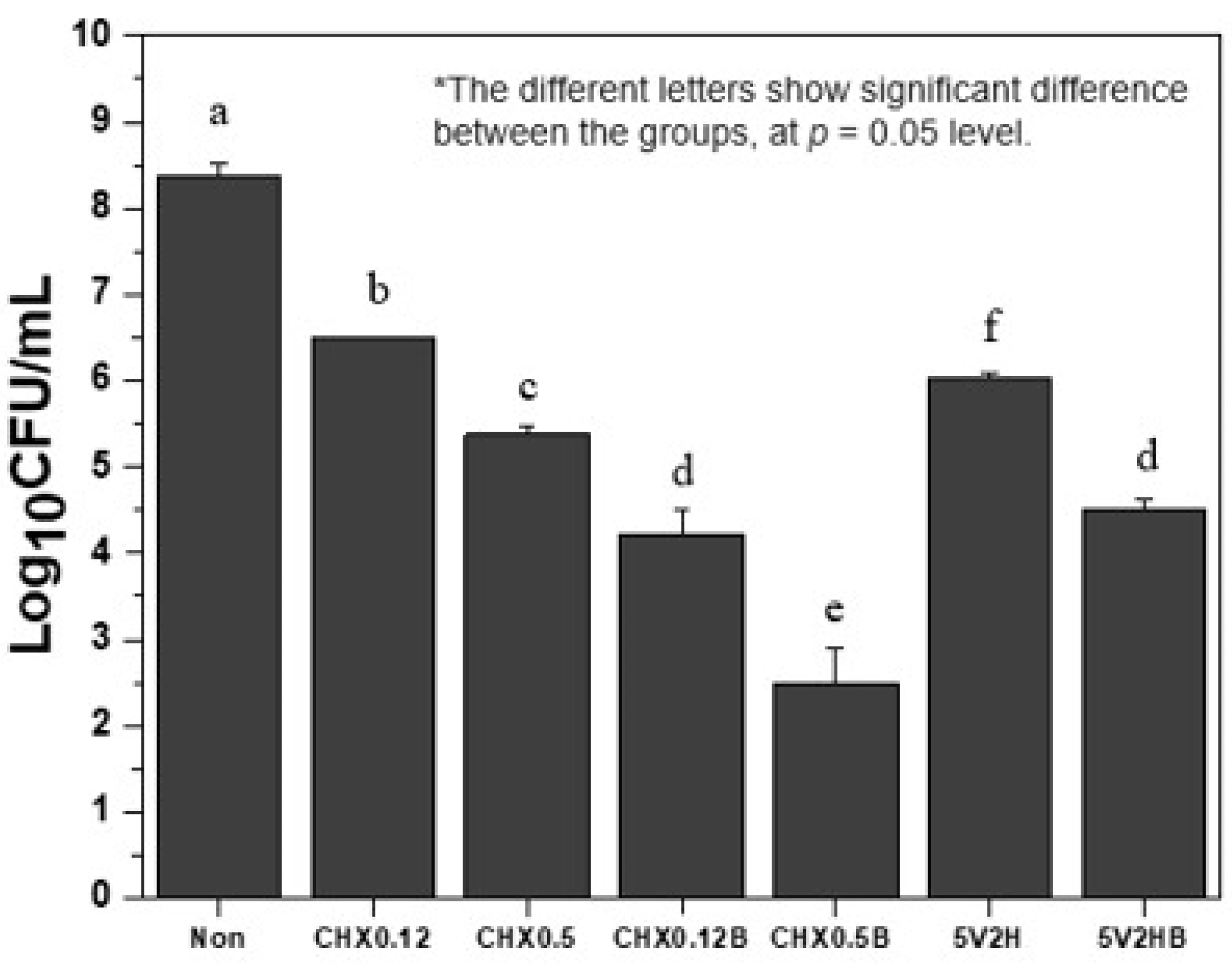
3.1. Bacterial Colony Counts
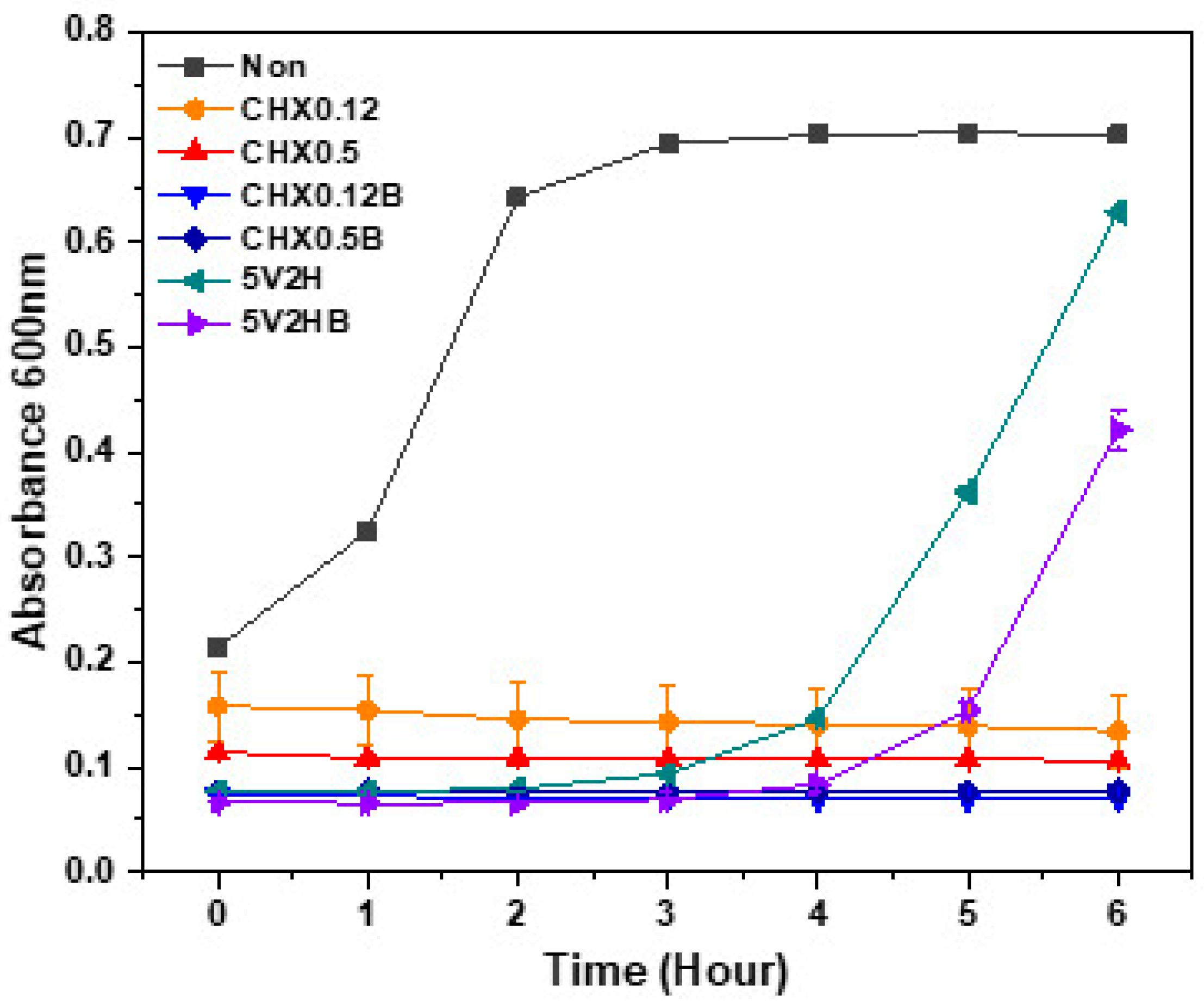
3.2. Absorbance Changes
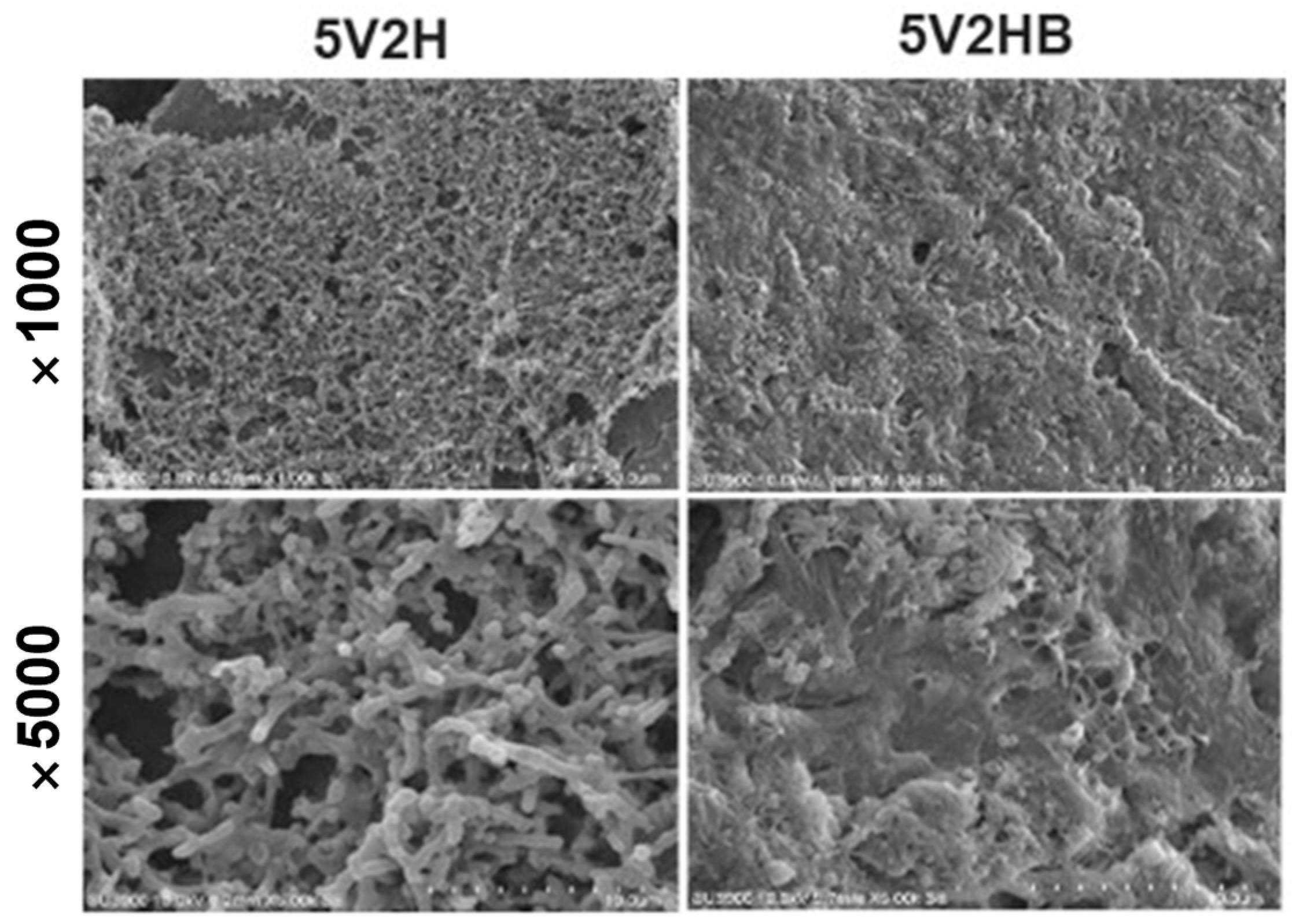
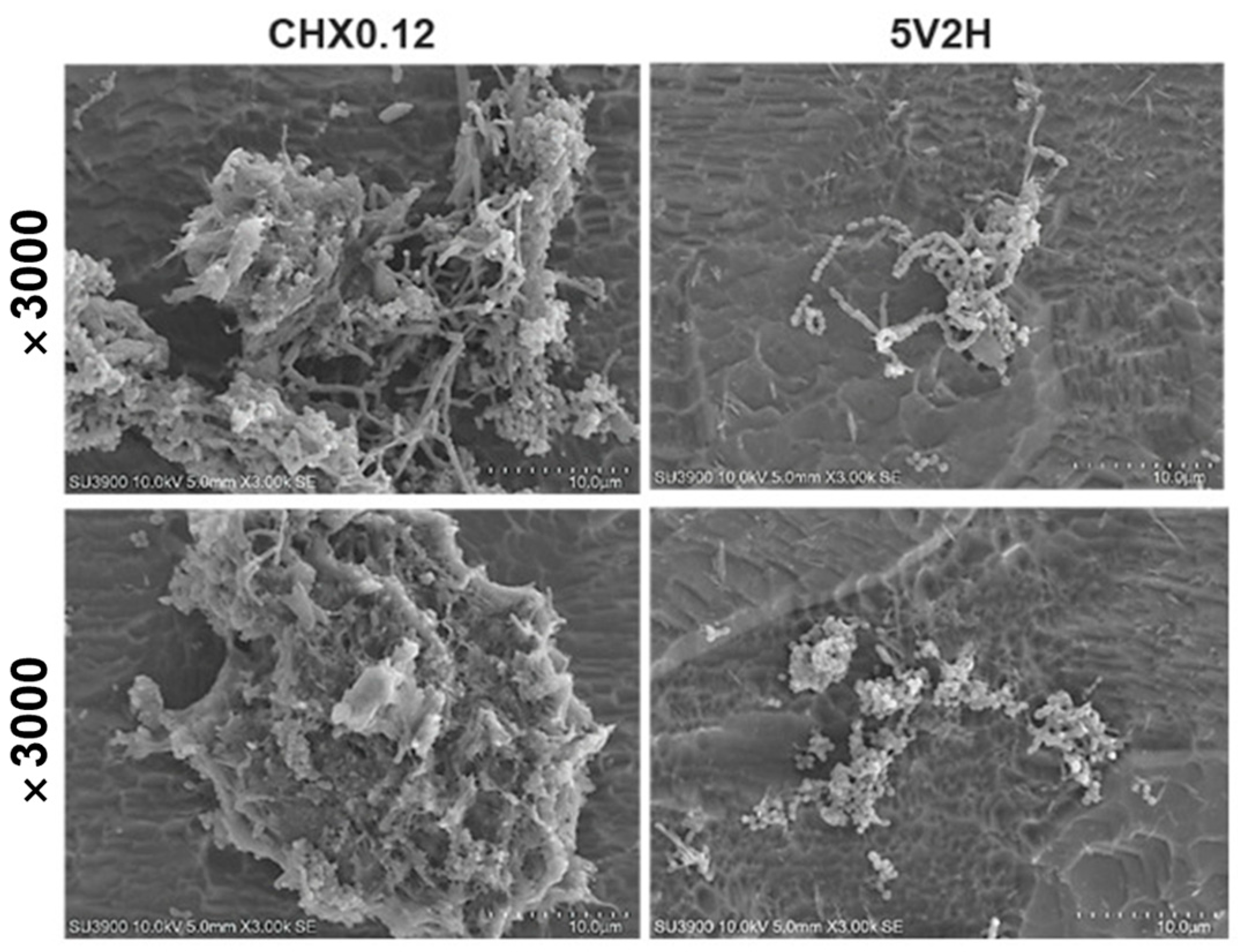
3.3. Scanning Electron Microscopy (SEM) Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- de Souza, I.S.; Santaella, N.G.; da Silva Santos, P.S. The practice of hospital dentistry in Brazil: An integrative literature review. Rev. Bras. Odontol. 2017, 74, 232–239. [Google Scholar]
- Jun, M.K.; Ku, J.K.; Kim, I.H.; Park, S.Y.; Hong, J.; Kim, J.Y.; Lee, J.K. Hospital Dentistry for Intensive Care Unit Patients: A Comprehensive Review. J. Clin. Med. 2021, 10, 3681. [Google Scholar] [CrossRef]
- Amaral, C.O.F.d.; Belon, L.M.R.; Silva, E.A.d.; Nadai, A.d.; Amaral Filho, M.S.P.d.; Straioto, F.G. The importance of hospital dentistry: Oral health status in hospitalized patients. RGO Rev. Gaúcha Odontol. 2018, 66, 35–41. [Google Scholar] [CrossRef]
- de Carvalho Baptista, I.M.; Martinho, F.C.; Nascimento, G.G.; da Rocha Santos, C.E.; Prado, R.F.D.; Valera, M.C. Colonization of oropharynx and lower respiratory tract in critical patients: Risk of ventilator-associated pneumonia. Arch. Oral Biol. 2018, 85, 64–69. [Google Scholar] [CrossRef]
- Kalanuria, A.A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.H.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.W.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Liao, Y.M.; Tsai, J.R.; Chou, F.H. The effectiveness of an oral health care program for preventing ventilator-associated pneumonia. Nurs. Crit. Care 2015, 20, 89–97. [Google Scholar] [CrossRef]
- Pisano, M.; Giordano, F.; Sangiovanni, G.; Capuano, N.; Acerra, A.; D’Ambrosio, F. The Interaction between the Oral Microbiome and Systemic Diseases: A Narrative Review. Microbiol. Res. 2023, 14, 1862–1878. [Google Scholar] [CrossRef]
- Winning, L.; Lundy, F.T.; Blackwood, B.; McAuley, D.F.; El Karim, I. Oral health care for the critically ill: A narrative review. Crit. Care 2021, 25, 353. [Google Scholar] [CrossRef]
- Jeronimo, L.S.; Abreu, L.G.; Cunha, F.A.; Esteves Lima, R.P. Association Between Periodontitis and Nosocomial Pneumonia: A Systematic Review and Meta-analysis of Observational Studies. Oral Health Prev. Dent. 2020, 18, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, D.; Li, A.; Yue, J. Oral topical decontamination for preventing ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. J. Hosp. Infect. 2013, 84, 283–293. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Buxser, S. Has resistance to chlorhexidine increased among clinically-relevant bacteria? A systematic review of time course and subpopulation data. PLoS ONE 2021, 16, e0256336. [Google Scholar] [CrossRef]
- Deshmukh, J.; Vandana, K.L.; Chandrashekar, K.T.; Savitha, B. Clinical evaluation of an ionic tooth brush on oral hygiene status, gingival status, and microbial parameter. Indian. J. Dent. Res. 2006, 17, 74–77. [Google Scholar] [CrossRef]
- Busscher, H.; Jager, D.; Finger, G.; Schaefer, N.; Van der Mei, H. Energy transfer, volumetric expansion, and removal of oral biofilms by non-contact brushing. Eur. J. Oral Sci. 2010, 118, 177–182. [Google Scholar]
- Schmidt, J.C.; Zaugg, C.; Weiger, R.; Walter, C. Brushing without brushing?--a review of the efficacy of powered toothbrushes in noncontact biofilm removal. Clin. Oral Investig. 2013, 17, 687–709. [Google Scholar] [CrossRef]
- Sen, C.K.; Mathew-Steiner, S.S.; Das, A.; Sundaresan, V.B.; Roy, S. Electroceutical Management of Bacterial Biofilms and Surgical Infection. Antioxid. Redox Signal 2020, 33, 713–724. [Google Scholar] [CrossRef]
- Asadi, M.R.; Torkaman, G. Bacterial Inhibition by Electrical Stimulation. Adv. Wound Care 2014, 3, 91–97. [Google Scholar] [CrossRef]
- El-Kaliuoby, M.I.; Amer, M.; Shehata, N. Enhancement of Nano-Biopolymer Antibacterial Activity by Pulsed Electric Fields. Polymers 2021, 13, 1869. [Google Scholar] [CrossRef]
- Oncul, S.; Cuce, E.M.; Aksu, B.; Inhan Garip, A. Effect of extremely low frequency electromagnetic fields on bacterial membrane. Int. J. Radiat. Biol. 2016, 92, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Park, J.-H.; Jeon, Y.M.; Kim, J.G.; Jang, Y.S.; Lee, M.H.; Bae, T.-S. Effects of low-frequency positive square wave voltage on putative periodontal pathogen Aggregatibactor actinomycetemcomitans. Korean J. Dent. Mater. 2024, 51, 5–28. [Google Scholar] [CrossRef]
- Danho, S.; Schoellhorn, W.; Aclan, M. Innovative technical implementation of the Schumann resonances and its influence on organisms and biological cells. IOP Conf. Ser. Mater. Sci. Eng. 2019, 564, 012081. [Google Scholar] [CrossRef]
- Tang, J.-Y.; Yeh, T.-W.; Huang, Y.-T.; Wang, M.-H.; Jang, L.-S. Effects of extremely low-frequency electromagnetic fields on B16F10 cancer cells. Electromagn. Biol. Med. 2019, 38, 149–157. [Google Scholar]
- Choi, J.-H.; Jung, E.-H.; Lee, E.-S.; Jung, H.-I.; Kim, B.-I. Anti-biofilm activity of chlorhexidine-releasing elastomerics against dental microcosm biofilms. J. Dent. 2022, 122, 104153. [Google Scholar]
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467. [Google Scholar] [CrossRef]
- Rajendran, S.B.; Challen, K.; Wright, K.L.; Hardy, J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021, 12, 40. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.; Housler, G.; Marcel, V.; Cross, S.; Izadjoo, M. An Overview of the Efficacy of a Next Generation Electroceutical Wound Care Device. Mil. Med. 2016, 181, 184–190. [Google Scholar] [CrossRef]
- Rowley, B.A. Electrical current effects on E. coli growth rates. Proc. Soc. Exp. Biol. Med. 1972, 139, 929–934. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Mohseni, S.; Dillow, A.M.; Abdul Azees, P.A.; Movaghari Pour, F.; Kataoka, Y.; Restrepo, M.C. Investigation of the effects of Bipolar Radiofrequency Energy on the Structural Morphology of Dental Plaque. Eur. J. Dent. 2024, 18, 243–252. [Google Scholar] [CrossRef]
- Milleman, K.R.; Levi, L.; Grahovac, T.L.; Milleman, J.L. Safety and efficacy of a novel toothbrush utilizing RF energy for the reduction of plaque, calculus and gingivitis. Am. J. Dent. 2020, 33, 151–156. [Google Scholar] [PubMed]
- Lasserre, J.F.; Toma, S.; Bourgeois, T.; El Khatmaoui, H.; Marichal, E.; Brecx, M.C. Influence of low direct electric currents and chlorhexidine upon human dental biofilms. Clin. Exp. Dent. Res. 2016, 2, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.F.; Leprince, J.G.; Toma, S.; Brecx, M.C. Electrical enhancement of chlorhexidine efficacy against the periodontal pathogen Porphyromonas gingivalis within a biofilm. New Microbiol. 2015, 38, 511–519. [Google Scholar] [PubMed]
- Del Pozo, J.; Rouse, M.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [CrossRef]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of chlorhexidine rinses after periodontal or implant surgery: A systematic review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef]
- Łukomska-Szymańska, M.; Sokołowski, J.; Łapińska, B. Chlorhexidine–mechanism of action and its application to dentistry. J. Stomatol. 2017, 70, 405–417. [Google Scholar]
- Fadel, M.A.; Mohamed, S.A.; Abdelbacki, A.M.; El-Sharkawy, A.H. Inhibition of Salmonella typhi growth using extremely low frequency electromagnetic (ELF-EM) waves at resonance frequency. J. Appl. Microbiol. 2014, 117, 358–365. [Google Scholar] [CrossRef]
- Inhan-Garip, A.; Aksu, B.; Akan, Z.; Akakin, D.; Ozaydin, A.N.; San, T. Effect of extremely low frequency electromagnetic fields on growth rate and morphology of bacteria. Int. J. Radiat. Biol. 2011, 87, 1155–1161. [Google Scholar] [CrossRef]
- Del Re, B.; Bersani, F.; Agostini, C.; Mesirca, P.; Giorgi, G. Various effects on transposition activity and survival of Escherichia coli cells due to different ELF-MF signals. Radiat. Environ. Biophys. 2004, 43, 265–270. [Google Scholar] [CrossRef]
- Fojt, L.; Strašák, L.; Vetterl, V.r.; Šmarda, J. Comparison of the low-frequency magnetic field effects on bacteria Escherichia coli, Leclercia adecarboxylata and Staphylococcus aureus. Bioelectrochemistry 2004, 63, 337–341. [Google Scholar] [CrossRef]
- Bayır, E.; Bilgi, E.; Şendemir-Ürkmez, A.; Hameş-Kocabaş, E.E. The effects of different intensities, frequencies and exposure times of extremely low-frequency electromagnetic fields on the growth of Staphylococcus aureus and Escherichia coli O157: H7. Electromagn. Biol. Med. 2015, 34, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Ummer, F.; Dhivakar, C.P. Aggregatibacter actinomycetemcomitans—A tooth killer? J. Clin. Diagn. Res. 2014, 8, ZE13–ZE16. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Zhang, T.; He, K. Cariogenicity features of Streptococcus mutans in presence of rubusoside. BMC Oral Health 2016, 16, 54. [Google Scholar] [CrossRef] [PubMed]
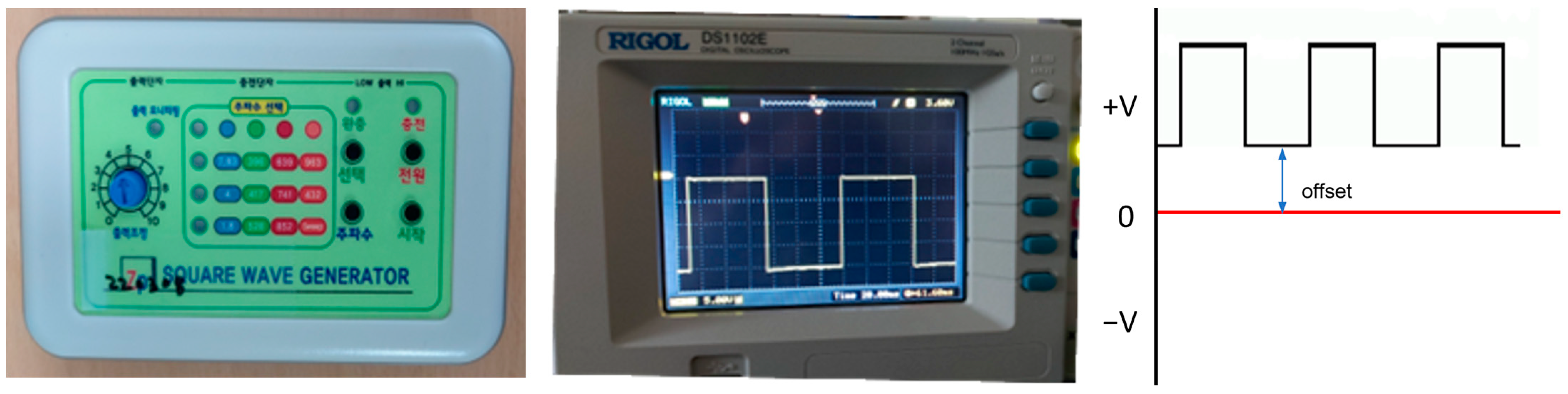


| Group | |
|---|---|
| Non | Untreated |
| CHX0.12 | Immerse in 0.12% chlorhexidine solution for 30 s |
| CHX0.12B | Immerse in 0.12% chlorhexidine solution for 30 s + Brushing 5 times |
| CHX0.5 | Immerse in 0.5% chlorhexidine solution for 30 s |
| CHX0.5B | Immerse in 0.5% chlorhexidine solution for 30 s + Brushing 5 times |
| 5V2H | Electrotherapy (Voltage of 5 V, for 2 h) |
| 5V2HB | Electrotherapy (Voltage of 5 V, for 2 h) + Brushing 5 times |
| Group | AVERAGE | SD |
|---|---|---|
| Non | 8.35758 | 0.16905 |
| CHX0.12 | 6.49114 | 0.01982 |
| CHX0.5 | 5.37409 | 0.10333 |
| CHX0.12B | 4.21568 | 0.30502 |
| CHX0.5B | 2.5 | 0.4 |
| 5V2H | 6.03959 | 0.05599 |
| 5V2HB | 4.50515 | 0.13705 |
| Time (Hours) | Non | CHX0.12 | CHX0.5 | CHX0.12B | CHX0.5B | 5V2H | 5V2HB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | |
| 0 | 0.21367 | 0.00208 | 0.15833 | 0.03361 | 0.11433 | 0.00057 | 0.07333 | 0.00057 | 0.07667 | 0.00055 | 0.077 | 0.0001 | 0.06667 | 0.00057 |
| 1 | 0.32467 | 0.00751 | 0.15433 | 0.03296 | 0.10867 | 0.00115 | 0.073 | 0 | 0.07733 | 0.00208 | 0.077 | 0.002 | 0.06567 | 0.00053 |
| 2 | 0.643 | 0.004 | 0.14567 | 0.03424 | 0.109 | 0.00173 | 0.07067 | 0.00057 | 0.076 | 0.00265 | 0.07967 | 0.00208 | 0.066 | 0.0001 |
| 3 | 0.694 | 0.00458 | 0.143 | 0.03559 | 0.108 | 0.00173 | 0.071 | 0.0001 | 0.07633 | 0.00416 | 0.09367 | 0.00208 | 0.06933 | 0.00115 |
| 4 | 0.702 | 0.00361 | 0.14033 | 0.03512 | 0.10733 | 0.00153 | 0.07 | 0 | 0.07667 | 0.00473 | 0.14667 | 0.00321 | 0.08367 | 0.00153 |
| 5 | 0.70433 | 0.00493 | 0.139 | 0.03451 | 0.107 | 0.00173 | 0.06967 | 0.00053 | 0.07733 | 0.00586 | 0.36233 | 0.00513 | 0.15467 | 0.00666 |
| 6 | 0.702 | 0.002 | 0.13467 | 0.03508 | 0.10667 | 0.00115 | 0.06967 | 0.00054 | 0.07667 | 0.00643 | 0.62833 | 0.00153 | 0.42133 | 0.0179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-W.; Byeon, S.-M.; Lee, D.-H.; Yun, P.-Y.; Ku, J.-K. The Antimicrobial Effect of a Low-Frequency Square Wave Compared to Chlorhexidine. J. Clin. Med. 2025, 14, 2429. https://doi.org/10.3390/jcm14072429
Choi J-W, Byeon S-M, Lee D-H, Yun P-Y, Ku J-K. The Antimicrobial Effect of a Low-Frequency Square Wave Compared to Chlorhexidine. Journal of Clinical Medicine. 2025; 14(7):2429. https://doi.org/10.3390/jcm14072429
Chicago/Turabian StyleChoi, Jin-Won, Seon-Mi Byeon, Da-Hyun Lee, Pil-Young Yun, and Jeong-Kui Ku. 2025. "The Antimicrobial Effect of a Low-Frequency Square Wave Compared to Chlorhexidine" Journal of Clinical Medicine 14, no. 7: 2429. https://doi.org/10.3390/jcm14072429
APA StyleChoi, J.-W., Byeon, S.-M., Lee, D.-H., Yun, P.-Y., & Ku, J.-K. (2025). The Antimicrobial Effect of a Low-Frequency Square Wave Compared to Chlorhexidine. Journal of Clinical Medicine, 14(7), 2429. https://doi.org/10.3390/jcm14072429








