Exploring the Prevalence and Risk Factors of MASLD in Patients with Newly Diagnosed Diabetes Mellitus: A Comprehensive Investigation
Abstract
1. Introduction
2. Methods
2.1. DefinitionsandCalculations
2.2. StatisticalAnalysis
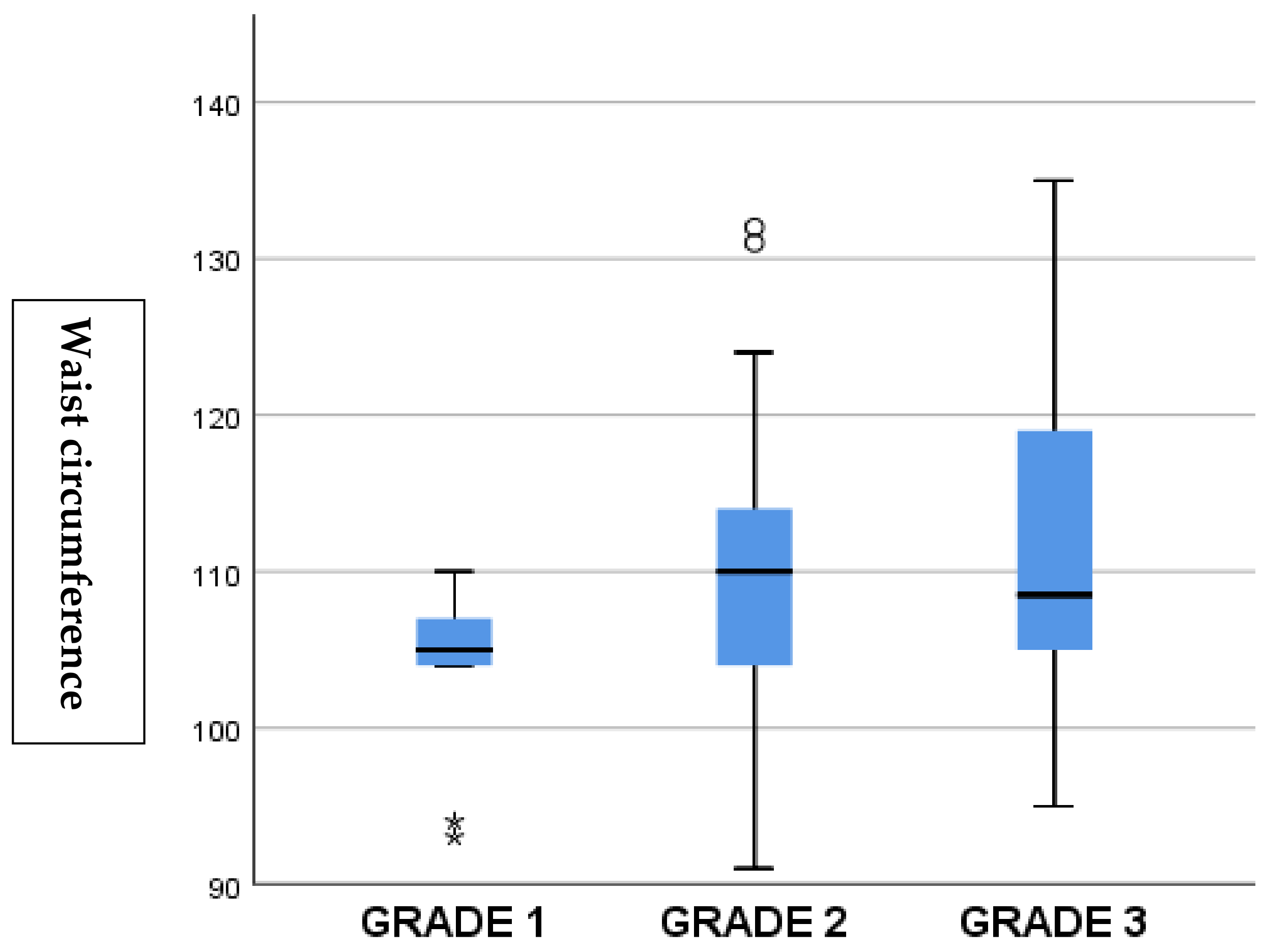
3. Results
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Śliwińska, A.; Drzewoski, J. Non-Alcoholic Fatty Liver Disease or Type 2 Diabetes Mellitus—The Chicken or the Egg Dilemma. Biomedicines 2023, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Diehl, A.M. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Gastroenterology Hepatology. Redefining non-alcoholic fatty liver disease: What’s in a name? Lancet Gastroenterol. Hepatol. 2020, 5, 419. [Google Scholar] [CrossRef]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29, S5–S16. [Google Scholar] [CrossRef]
- Chang, Y.; Jung, H.S.; Yun, K.E.; Cho, J.; Cho, Y.K.; Ryu, S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am. J. Gastroenterol. 2013, 108, 1861–1868. [Google Scholar] [CrossRef]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 2009, 13, 9–19. [Google Scholar]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation—A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef]
- Al Hashmi, K.; Giglio, R.V.; Pantea Stoian, A.; Patti, A.M.; Al Waili, K.; Al Rasadi, K.; Ciaccio, M.; Rizzo, M. Metabolic dysfunction-associated fatty liver disease: Current therapeutic strategies. Front. Nutr. 2024, 11, 1355732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Committee ADAPP. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S17–S38. [Google Scholar]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, K.; McGavin, D.D. Diabetic retinopathy: Clinical findings and management. Commun. Eye Health 2003, 16, 21–24. [Google Scholar]
- Nazar, C.M. Diabetic nephropathy; principles of diagnosis and treatment of diabetic kidney disease. J. Nephropharmacol. 2014, 3, 15–20. [Google Scholar] [PubMed]
- Thomas, P.K. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997, 46 (Suppl. S2), S54–S57. [Google Scholar] [CrossRef]
- Neglia, D.; Rovai, D.; Caselli, C.; Pietila, M.; Teresinska, A.; Aguadé-Bruix, S.; Pizzi, M.N.; Todiere, G.; Gimelli, A.; Schroeder, S.; et al. Detection of Significant Coronary Artery Disease by Noninvasive Anatomical and Functional Imaging. Circ. Cardiovasc. Imaging 2015, 8, e002179. [Google Scholar] [CrossRef]
- Przybyszewski, E.M.; Targher, G.; Roden, M.; Corey, K.E. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease. Clin. Liver Dis. 2021, 17, 19–22. [Google Scholar] [CrossRef]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Indian J. Endocrinol. Metab. 2018, 22, 421–428. [Google Scholar] [CrossRef]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.E.L.; Ang, C.Z.; Quek, J.; Fu, C.E.; Lim, L.K.E.; Heng, Z.E.Q.; Tan, D.J.H.; Lim, W.H.; Yong, J.N.; Zeng, R.; et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: An updated systematic review and meta-analysis. Gut 2023, 72, 2138–2148. [Google Scholar] [CrossRef]
- Atan, N.A.D.; Koushki, M.; Motedayen, M.; Dousti, M.; Sayehmiri, F.; Vafaee, R.; Norouzinia, M.; Gholami, R. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Gastroenterol. Hepatol. Bed. Bench. 2017, 10 (Suppl. S11), S1–S7. [Google Scholar]
- Butt, A.S.; Hamid, S.; Haider, Z.; Sharif, F.; Salih, M.; Awan, S.; A Khan, A.; Akhter, J. Nonalcoholic Fatty Liver Diseases among Recently Diagnosed Patients with Diabetes Mellitus and Risk Factors. Euroasian J. Hepato-Gastroenterol. 2019, 9, 9–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Yu, L.; Ji, L.; Li, M.; Hu, F. Establishment of a Risk Prediction Model for Non-alcoholic Fatty Liver Disease in Type 2 Diabetes. Diabetes Ther. 2020, 11, 2057–2073. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Birt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. S1), S47–S64. [Google Scholar] [CrossRef]
- Tsochatzis, E.; Coilly, A.; Nadalin, S.; Levistky, J.; Tokat, Y.; Ghobrial, M.; Klinck, J.M.; Berenguer, M. International Liver Transplantation Consensus Statement on End-stage Liver Disease Due to Nonalcoholic Steatohepatitis and Liver Transplantation. Transplantation 2019, 103, 45–56. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 32–42. [Google Scholar] [CrossRef]
- Oliveros-Montiel, A.; Santos-López, G.; Sedeño-Monge, V. Proteins involved in lipid metabolism as possible biomarkers or predisposing factors for non-alcoholic fatty liver disease. Acta Gastroenterol. Belg. 2020, 83, 622–630. [Google Scholar] [PubMed]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]


| Without MASLD | With MASLD | p Value | |
|---|---|---|---|
| (n = 25) | (n = 103) | ||
| Age (years) | 57.8 | 54.9 | |
| Sex, female | 11 (34.3) | 39 (40.6) | 0.631 |
| BMI (kg/m2) | 30.6 ± 4.2 | 33.4 ± 5.3 | 0.130 |
| Waist circumference (cm) | 109.9 ± 7.8 | 109.7 ± 9.6 | 0.884 |
| Retinopathy, n (%) | 7 (21.8) | 27 (28.1) | 0.753 |
| Nephropathy, n (%) | 8 (25) | 31 (32.2) | 0.530 |
| Neuropathy, n (%) | 5 (15.6) | 23 (23.9) | 0.355 |
| CAD, n (%) | 4 (12.5) | 32 (33.3) | 0.128 |
| Hypertension, n (%) | 16 (50.3) | 55 (57.2) | 0.648 |
| Smoking, n (%) | 11 (34.3) | 35 (34.4) | 0.650 |
| cIMT (mm) | 0.72 ± 0.21 | 0.81 ± 0.27 | 0.259 |
| Glucose (mg/dL) | 163.7 (92–437) | 167.6 (100–630) | 0.381 |
| HbA1c (%) | 8.6 ± 2.5 | 9.2 ± 3.7 | 0.341 |
| LDL cholesterol (mg/dL) | 145.8 ± 33.5 | 155.9 ± 49.8 | 0.457 |
| Triglyceride (mg/dL) | 170.6 (58–224) | 205.8 (58–1314) | 0.057 |
| Non-HDL cholesterol (mg/dL) | 167.9 ± 28.7 | 189.1 ± 57.5 | 0.048 |
| HDL cholesterol (mg/dL) | 47.8 ± 10.8 | 44.2 ± 10.6 | 0.492 |
| AST (U/L) | 23.42 (11–51) | 24.8 (8–176) | 0.165 |
| ALT (U/L) | 24.9 ± 8 | 47.1 ± 23 | <0.001 |
| Creatinine (mg/dL) | 0.87 (0.5–1.0) | 0.81 (0.5–6) | 0.091 |
| Platelet count (103/μL) | 246 ± 56 | 252 ± 62 | 0.672 |
| r | p-Value * | |
|---|---|---|
| cIMT | −0.017 | 0.867 |
| BMI | 0.216 | 0.077 |
| Waist circumference | 0.234 | 0.032 |
| Glucose | −0.030 | 0.788 |
| HbA1c | 0.102 | 0.346 |
| LDL cholesterol | 0.263 | 0.021 |
| Triglyceride | 0.142 | 0.222 |
| Non-HDL | 0.226 | 0.046 |
| AST | 0.182 | 0.121 |
| ALT | 0.187 | 0.119 |
| Creatinine | 0.112 | 0.354 |
| Platelet count | 0.226 | 0.040 |
| HDL cholesterol | −0.039 | 0.547 |
| Coefficients a | ||||
|---|---|---|---|---|
| Model | Unstandardized Coefficients | Standardized Coefficients | p-Value | |
| B | Std. Error | Beta | ||
| (Constant) | −0.354 | 0.660 | ||
| ALT | 0.00 | 0.002 | 0.332 | 0.001 |
| BMI | 0.051 | 0.021 | 0.256 | 0.013 |
| Excluded Variables b | ||||
| Model | B | Partial Correlation | Collinearity Statistics | p-Value |
| Tolerance | ||||
| cIMT | 0.051 | 0.055 | 0.974 | 0.611 |
| Waist circumference | −0.128 | −0.093 | 0.465 | 0.383 |
| Glucose | 0.081 | 0.097 | 0.988 | 0.371 |
| HbA1c | 0.169 | 0.191 | 0.972 | 0.080 |
| LDL cholesterol | 0.091 | 0.095 | 0.914 | 0.389 |
| Triglyceride | 0.149 | 0.164 | 0.993 | 0.136 |
| Non-HDL | 0.142 | 0.152 | 0.924 | 0.160 |
| AST | −0.259 | −0.120 | 0.173 | 0.269 |
| Creatinine | 0.159 | 0.162 | 0.976 | 0.140 |
| Platelet count | 0.104 | 0.114 | 0.957 | 0.321 |
| HDL cholesterol | −0.056 | −0.051 | 0.982 | 0.601 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyazal Polat, H.; Beyazal, M.; Arpa, M.; Kızılkaya, B.; Ayaz, T.; Gündoğdu, Ö.L.; Konur, K.; Polat, Z.; Beyazal Çeliker, F.; Atasoy, H. Exploring the Prevalence and Risk Factors of MASLD in Patients with Newly Diagnosed Diabetes Mellitus: A Comprehensive Investigation. J. Clin. Med. 2025, 14, 3513. https://doi.org/10.3390/jcm14103513
Beyazal Polat H, Beyazal M, Arpa M, Kızılkaya B, Ayaz T, Gündoğdu ÖL, Konur K, Polat Z, Beyazal Çeliker F, Atasoy H. Exploring the Prevalence and Risk Factors of MASLD in Patients with Newly Diagnosed Diabetes Mellitus: A Comprehensive Investigation. Journal of Clinical Medicine. 2025; 14(10):3513. https://doi.org/10.3390/jcm14103513
Chicago/Turabian StyleBeyazal Polat, Hatice, Mehmet Beyazal, Medeni Arpa, Bayram Kızılkaya, Teslime Ayaz, Ömer Lütfi Gündoğdu, Kamil Konur, Zehra Polat, Fatma Beyazal Çeliker, and Halil Atasoy. 2025. "Exploring the Prevalence and Risk Factors of MASLD in Patients with Newly Diagnosed Diabetes Mellitus: A Comprehensive Investigation" Journal of Clinical Medicine 14, no. 10: 3513. https://doi.org/10.3390/jcm14103513
APA StyleBeyazal Polat, H., Beyazal, M., Arpa, M., Kızılkaya, B., Ayaz, T., Gündoğdu, Ö. L., Konur, K., Polat, Z., Beyazal Çeliker, F., & Atasoy, H. (2025). Exploring the Prevalence and Risk Factors of MASLD in Patients with Newly Diagnosed Diabetes Mellitus: A Comprehensive Investigation. Journal of Clinical Medicine, 14(10), 3513. https://doi.org/10.3390/jcm14103513






