Biofeedback Training in Inpatient Mental Health Facilities: A Scoping Review
Abstract
1. Introduction
Objectives
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Charting
3. Results
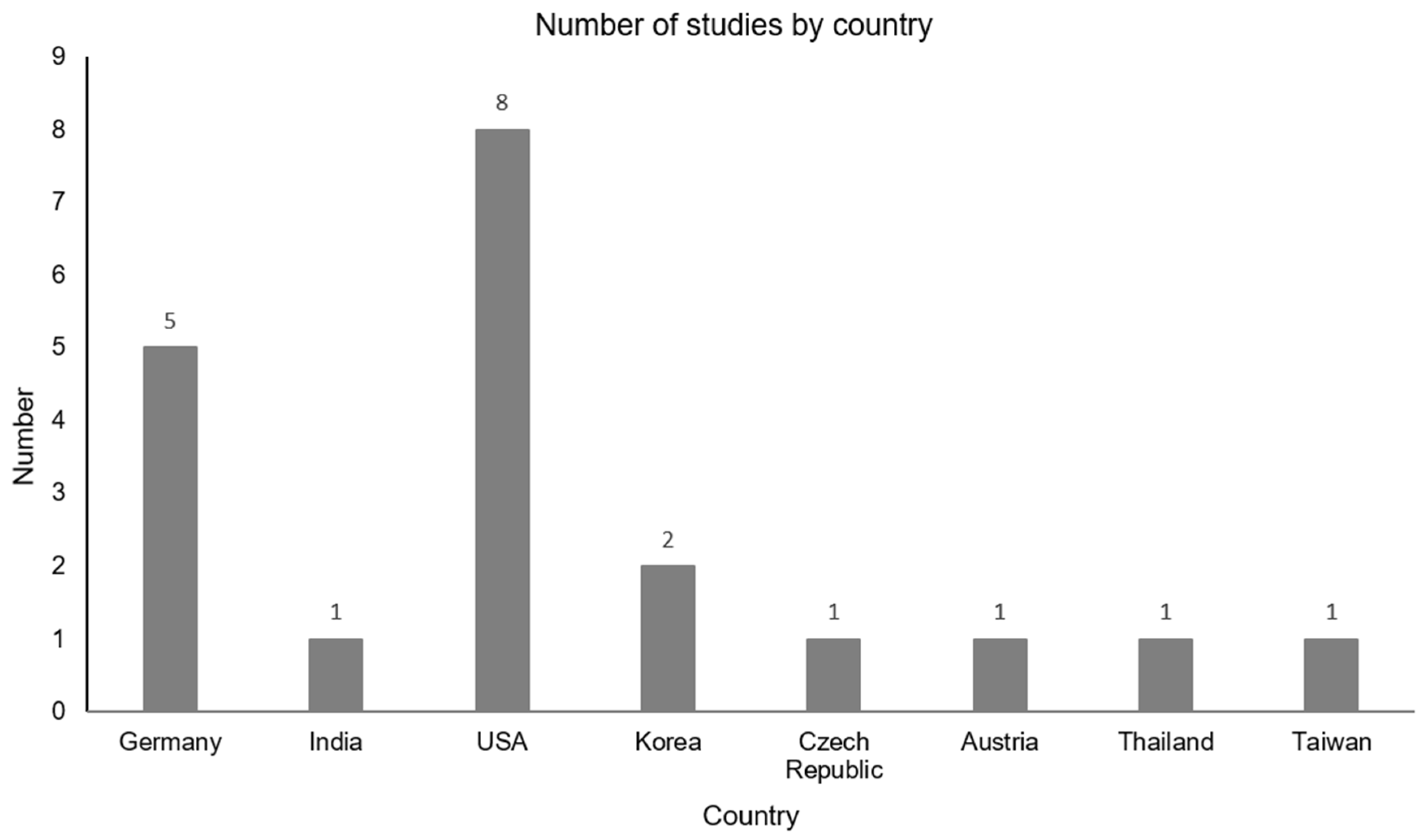
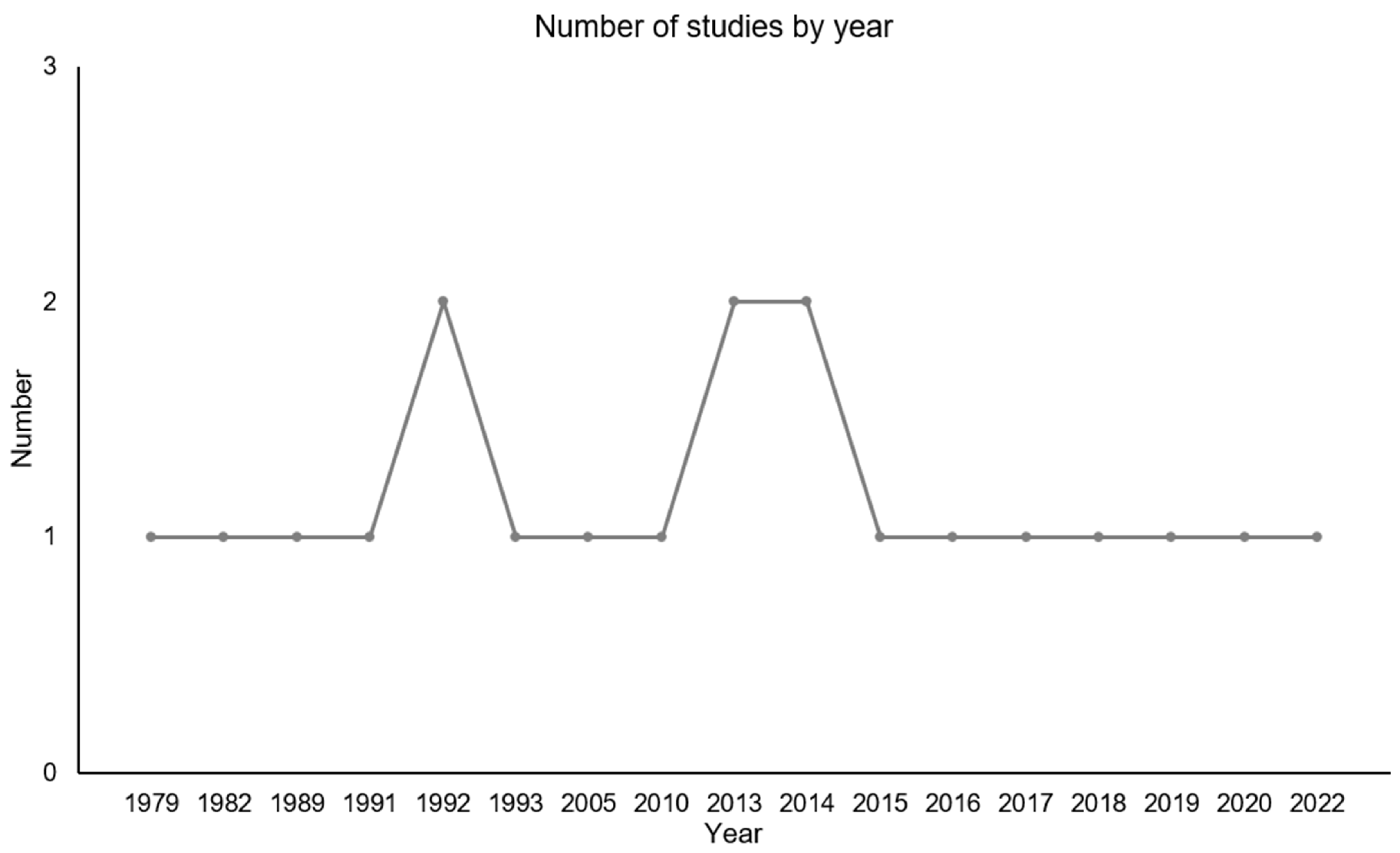
3.1. Overview
3.2. Classification
3.2.1. EEG Biofeedback
Structural Features of EEG BFB
Effectiveness of EEG BFB
Participants of EEG BFB
3.2.2. HRV Biofeedback
Structural Features of HRV BFB
Effectiveness of HRV BFB
Participants of HRV BFB
3.2.3. EMG Biofeedback
Structural Features of EMG BFB
Effectiveness of EMG BFB
Participants of EMG BFB
3.3. Subgroup Analyses
3.4. Reported Limitations
3.5. Future Directions
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BFB | Biofeedback |
| HRV | Heart rate variability |
| EEG | Electroencephalography |
| SCP | Slow cortical potentials |
| SMR | Sensory motor rhythm |
| PTSD | Post-traumatic stress disorder |
| BED | Binge eating disorder |
References
- Köllner, V.; Broda, M. Grundlagen und Denkmodelle der Verhaltensmedizin. In Praktische Verhaltensmedizin, 1st ed.; Thieme: New York, NY, USA, 2005; pp. 3–16. [Google Scholar]
- Windthorst, P.; Veit, R.; Enck, P.; Smolka, R.; Zipfel, S.; Teufel, M. Biofeedback und Neurofeedback: Anwendungsmöglichkeiten in Psychosomatik and Psychotherapie. Psychother. Psychosom. Med. Psychol. 2015, 65, 146–158. [Google Scholar] [CrossRef]
- Moss, D. Heart rate variability (HRV) biofeedback. Psychophysiol. Today 2004, 1, 4–11. [Google Scholar]
- Wang, R.; Zhang, S.; Zhang, J.; Tong, Q.; Ye, X.; Wang, K.; Li, J. Electromyographic biofeedback therapy for improving limb function after stroke: A systemativ review and meta-analysis. PLoS ONE 2024, 19, e0289572. [Google Scholar] [CrossRef]
- Cinnera, A.M.; Morone, G.; Bisirri, A.; Lucenti, T.; Rotundo, M.; Monaci, S.; Verton, C.; Paoluzzi, M.; Iosa, M.; Ciancarelli, I. Headaches treatment with EMG biofeedback: A focused systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2023, 59, 697–705. [Google Scholar] [CrossRef]
- Luctar-Flude, M.; Groll, D. A Systematic Review of the Safety and Effect of Neurofeedback on Fatigue and Cognition. Integr. Cancer Ther. 2015, 14, 318–340. [Google Scholar] [CrossRef] [PubMed]
- Rief, W.; Birbaumer, N. Grundsätzliches zu Biofeedback. In Biofeedback: Grundlagen, Indikationen, Kommunikation, Vorgehen, 3rd ed.; Schattauer GmbH: Stuttgart, Germany, 2011. [Google Scholar]
- Patil, A.U.; Lin, C.; Lee, S.H.; Huang, H.W.; Wu, S.C.; Madathil, D.; Huang, C.M. Review of EEG based neurofeedback as as therapeutic intervention to treat depression. Psychiatry Res. Neuroimaging 2023, 329, 111591. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, W.B.; Rice, K.M. Biofeedback as a placebo: Anxiety reduction facilitated by training in either suppression or enhancement of alpha brainwaves. J. Consult. Clin. Psychol. 1981, 49, 590–596. [Google Scholar] [CrossRef]
- Adhia, D.B.; Mani, R.; Mathew, J.; O’Leary, F.; Smith, M.; Vanneste, S.; De Ridder, D. Exploring electronencephalographic infraslow neurofeedback treatment for chronic low back pain: A double-blinded safety and feasibility randomizes placebo-controlled trial. Sci. Rep. 2023, 13, 1177. [Google Scholar] [CrossRef]
- Schmidt, K.; Barac-Dammeyer, D.; Kowalski, A.; Teigelack, P.; Pfeiffer, C.; Robitzsch, A.; Dörrie, N.; Skoda, E.-M.; Bäuerle, A.; Fink, M.; et al. Implementing biofeedback treatment in a psychosomatic-psychotherapeutic inpatient unit: A mixed methods evaluation of acceptance, satisfaction, and feasibility. Front. Psychiatry 2023, 14, 1140880. [Google Scholar] [CrossRef]
- Fisher, S.F.; Lanius, R.A.; Frewen, P.A. EEG neurofeedback as adjunct to psychotherapy for complex developmental trauma-related disorders: Case study and treatment rationale. Traumatology 2016, 22, 255–260. [Google Scholar] [CrossRef]
- Blume, M.; Schmidt, R.; Schmidt, J.; Martin, A.; Hilbert, A. EEG Neurofeedback in the Treatment of Adults with Binge-Eating Disorder: A Randomized Controlled Pilot Study. Neurotherapeutics 2022, 19, 352–365. [Google Scholar] [CrossRef]
- Rahmani, M.; Mahvelati, A.; Farajinia, A.H.; Shahyad, S.; Khaksarian, M.; Nooripour, R.; Hassanvandi, S. Comparison of Vitamin D, Neurofeedback, and Neurofeedback Combined with Vitamin D Supplementation in Children with Attention-Deficit/Hyperactivity Disorder. Arch. Iran. Med. 2022, 25, 285–393. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Beckham, A.J.; Greene, T.B.; Meltzer-Brody, S. A pilot study of heart rate variability biofeedback therapy in the treatment of perinatal depression on a specialized perinatal psychiatry inpatient unit. Arch. Women’s Ment. Health 2012, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tatschl, J.M.; Hochfellner, S.M.; Schwerdtfeger, A.R. Implementing Mobile HRV Biofeedback as Adjunctive Therapy During Inpatient Psychiatric Rehabilitiation Facilitates Recovery of Depressive Symptoms and Enhances Autonomic Functioning Short-Term: A 1-Year Pre-Post-intervention Follow-Up Pilot Study. Front. Neurosci. 2020, 14, 738. [Google Scholar] [CrossRef]
- Bhat, P. Efficacy of Alfa EEG wave biofeedback in the management of anxiety. Ind. Psychiatry J. 2010, 19, 111–114. [Google Scholar] [CrossRef]
- Cheon, E.; Koo, B.; Choi, J. The Efficacy of Neurofeedback in Patients with Major Depressive Disorder: An Open Labeled Prospective Study. Appl. Psychol. Biofeedback 2015, 41, 103–110. [Google Scholar] [CrossRef]
- Denney, M.R.; Baugh, J.L.; Hardt, H.D. Sobriety Outcome After Alcoholism Treatment with Biofeedback Participation: A Pilot Inpatient Study. Int. J. Addict. 1991, 26, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Penzlin, A.I.; Siepmann, T.; Illigens, B.M.; Weidner, K.; Siepmann, M. Heart rate variability biofeedback in patients with alcohol dependence: A randomized controlled study. Neuropsychiatr. Dis. Treat. 2015, 2015, 2619–2627. [Google Scholar] [CrossRef]
- Teeravisutkul, P.; Chumchua, V.; Saengcharnchai, P.; Leelahanaj, T. Stress and craving reduction under treatment with heart rate variability biofeedback and the Phramongkutklao model among patients with alcohol use disorder. Psychol. Res. Behav. Manag. 2019, 12, 619–627. [Google Scholar] [CrossRef]
- Scott, W.C.; Kaiser, D.; Othmer, S.; Sideroff, S.I. Effects of an EEG Biofeedback Protocol on a Mixed Substance Abusing Population. Am. J. Drug Alcohol Abus. 2005, 31, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Eddie, D.; Kim, C.; Lehrer, P.; Deneke, E.; Bates, M.E. A Pilot Study of Brief Heart Rate Variability Biofeedback to Reduce Craving in Young Adult Men Receiving Inpatient Treatment for Substance Use Disorders. Appl. Psychol. Biofeedback 2014, 39, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kopřivová, J.; Congedo, M.; Raszka, M.; Praško, J.; Brunovský, M.; Horáček, J. Prediction of Treatment Response and the Effect of Independent Component Neurofeedback in Obsessive-Compulsive Disorder: A Randomized, Sham-Controlled, Double-Blind Study. Neuropsychobiology 2013, 67, 210–223. [Google Scholar] [CrossRef]
- Pharr, O.M.; Coursey, R.D. The use and utility of EMG biofeedback with chronic schizophrenic patients. Biofeedback Self-Regul. 1989, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Hsu, W.; Shen, S.; Hsu, M.; Lin, M. Dose-Response Relationships of Multisensory Intervention on Hospitalized Patients with Chronic Schizophrenia. J. Nurs. Res. 2017, 25, 13–20. [Google Scholar] [CrossRef]
- Winkeler, A.; Winkeler, M.; Imgart, H. Infra-Low Frequency Neurofeedback in the Treatment of Patients with Chronic Eating Disorder and Comorbid Post-Traumatic Stress Disorder. Front. Hum. Neurosci. 2022, 16, 890682. [Google Scholar] [CrossRef]
- Ko, S.; Park, W. Effects of Quantitative Electroencephalography Based Neurofeedback Training on Autonomous Regulations in Patients with Alcohol Use Disorder. Asian Nurs. Res. 2018, 12, 136–144. [Google Scholar] [CrossRef]
- Schneider, F.; Heimann, H.; Mattes, R.; Lutzenberger, W.; Birbaumer, N. Self-regulation of slow cortical potentials in psychiatric patients: Depression. Biofeedback Self-Regul. 1992, 17, 203–214. [Google Scholar] [CrossRef]
- Schneider, F.; Rockstroh, B.; Heimann, H.; Lutzenberger, W.; Mattes, R.; Elbert, T.; Birbaumer, N.; Bartels, M. Self-regulation of slow cortical potentials in psychiatric patients: Schizophrenia. Biofeedback Self-Regul. 1992, 17, 277–292. [Google Scholar] [CrossRef]
- Schneider, F.; Elbert, T.; Heimann, H.; Welker, A.; Stetter, F.; Mattes, R.; Birbaumer, N.; Mann, K. Self-regulation of low cortical potentials in psychiatric patients: Alcohol dependency. Biofeedback Self-Regul. 1993, 18, 23–32. [Google Scholar] [CrossRef]
- Scolnick, B.; Mostofsky, D.I.; Keane, R.J. Pilot study employing heart rate variability biofeedback training to decrease anxiety in patients with eating disorders. J. Eat. Disord. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Blue, L.A.; Blue, F.R. Effects of Biofeedback on Muscular Tension in Selected Personality States. J. Psychol. Interdiscip. Appl. 1979, 101, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Ford, M.R.; Stroebel, C.F.; Strong, P.; Szarek, B.L. Quieting response training: Treatment of psychiatric impatients. Biofeedback Self-Regul. 1982, 7, 331–339. [Google Scholar] [CrossRef]
- Schönberg, P.L.A.; David, A.S. Biofeedback for Psychiatric Disorders: A Systematic Review. Appl. Psychol. Biofeedback 2014, 29, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Appl. Psychophysiol. Biofeedback 2000, 25, 177–191. [Google Scholar] [CrossRef]
- Walker, J.E.; Lawson, R. FP02 Beta Training for Drug-Resistant Depression-A new protocol that usually reduces depression and keeps it reduced. J. Neurother. 2013, 17, 198–200. [Google Scholar] [CrossRef]
- Yu, S.; Tseng, C.; Lin, W. A neurofeedback Protocol for Executive Function to Reduce Depression and Rumination: A Controlled Study. Clin. Psychopharmacol. Neurosci. 2020, 18, 375–385. [Google Scholar] [CrossRef]
- Spetter, M.S.; Malekshahi, R.; Birbaumer, N.; Lührs, M.; Van Der Veer, A.H.; Scheffler, K.; Spuckti, S.; Preissl, H.; Veit, R.; Hallschmid, M. Volitional regulation of brain responses to food stimuli in overweight and obese subjects: A real-time fMRI feedback study. Appetite 2017, 112, 188–195. [Google Scholar] [CrossRef]
- Fattahi, S.; Naderi, F.; Asgari, P.; Ahadi, H. Neuro-Feedback training for overweight women: Improvement of food craving and mental health. NeuroQuantology 2017, 15, 232–238. [Google Scholar] [CrossRef]
- Orendáčová, M.; Kvašňák, E.; Vránová, J. Effect of neurofeedback therapy on neurological post-COVID-19 complications (A pilot study). PLoS ONE 2022, 17, e0271350. [Google Scholar] [CrossRef]
- Ros, T.; Enriquez-Geppert, S.; Zotev, V.; Young, K.D.; Wood, G.; Whitfield-Gabrieli, S.; Wan, F.; Vuilleumier, P.; Vialatte, F.; Van de Ville, D.; et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain J. Neurol. 2020, 143, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Anil, K.; Demain, S.; Burridge, J.; Simpson, D.; Taylor, J.; Cotter, I.; Vuckovic, A. The importance of self-efficacy and negative affect for neurofeedback success for central neuropathic pain after a spinal cord injury. Sci. Rep. 2022, 12, 10949. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomized Trials. Ann. Intern. Med. 2010, 152, 726–732. [Google Scholar] [CrossRef]
- Schmidt, K.; Kowalski, A.; Schweda, A.; Dörrie, N.; Skoda, E.-M.; Bäuerle, A.; Teufel, M. Evaluation of a manualised neurofeedback training in psychosomatic-psychotherapeutic outpatient treatment (Neuro-pp-out): Study protocol for a clinical mixed-methods pilot study. BMJ Open 2024, 14, e079098. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.; Göke, H.; Kowalski, A.; Dörrie, N.; Skoda, E.-M.; Bäuerle, A.; Teufel, M.; Schmidt, K. Implementing a Manual for Neurofeedback Training in a Psychosomatic-Psychotherapeutic Outpatient Unit: A Mixed Methods Evaluation of Acceptance, Satisfaction, and Feasibility in Patients and Practitioners. Psychiatry Int. 2025, 6, 28. [Google Scholar] [CrossRef]

| Author (Year) | Patient Condition | Number of Subjects | Control Group | BFB Type | Type of Intervention | Manualized Training | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Winkeler et al. (2022) [28] | Eating disorder and post-traumatic stress disorder | n = 18 | n = 18 | Infra-low frequency EEG | 12 sessions 30–40 min 2×/week | No | EG significantly improved in restrained eating, more weight gain, reduced avoidance behavior, and less complications in course of treatment. | Lack of homogeneity in diagnoses; small sample size |
| Bhat (2010) [18] | Anxiety and depression | n = 50 | n = 50 | Alpha EEG | 40 sessions 5×/week | No | Improvement of anxiety symptoms: medication showed higher improvement than BF; in EG, women showed higher improvement with BF than men; | Lack of a follow-up to assess continued benefit |
| Scott et al. (2005) [23] | Substance use disorder | n = 60 | n = 61 | Beta EEG and SMR | 40–50 sessions 45 min 4–5×/week 2×/day | No | significantly more dropout in CG, EG significantly longer abstinent, and significant improvement in TOVA Questionnaire and in 5/10 MMPI Scales. | Relied on self-report for abstinence check |
| Cheon et al. (2016) [19] | Depression | n = 20 | n = 0 | Beta and alpha/theta EEG | 16–24 sessions 60 min 2–3×/week | No | Significant improvement in depressive symptoms, anxiety, and clinical illness; increased remission and response rates. | Small sample size, lack of control group, non-blinding subjects; patients received medication |
| Ko & Park (2018) [29] | Alcohol use disorder | n = 17 | n = 19 | Alpha EEG and high-beta training | 10 sessions 40 min 2–3×/week | No | Significant increase in basic psychological need satisfaction, alcohol abstinence self-efficacy, and self-regulation in EG, with no significant increase in alpha waves and decrease in high-beta waves; significant increase in high beta of CG. | Lack of comparability; difficult to extrapolate the results to patients with alcohol use disorder |
| Kopřivová et al. (2013) [25] | Obsessive–compulsive disorder | n = 10 | n = 10 | EEG and sham feedback | 18 sessions 25–30 min 3×/week | No | NFB group showed significantly higher percentage-based reduction in compulsions. | Limited spatial specificity; results limited to specific phenotype |
| Schneider et al. (1992a) [30] | Depression | n = 8 | n = 8 | SCP | 20 sessions 5×/week | No | SCP self-regulation impairment specific for schizophrenic patients, with no comparable deficits found for patients with depression. | No significant self-regulation of SCP in the control group, contradicting other study findings; small sample size |
| Schneider et al. (1992b) [31] | Schizophrenia | n = 12 | n = 12 | SCP | 20 sessions on 20 consecutive days | No | After direct feedback, schizophrenic patients were able to regulate SCP systematically compared to patients with alcohol dependence. The schizophrenia EG was unable to achieve a transfer performance of self-regulation for SCP; a correlation was found between the inability to regulate SCP and the duration of the illness. | Observed group effect may be more related to the use of medication than to schizophrenia |
| Schneider et al. (1993) [32] | Alcohol use disorder | n = 10 | n = 0 | SCP | 4 sessions on 4 consecutive days | No | The greatest increase in SCP differentiation is expected in the transfer condition with increasing abstinence; learning self-regulation of SCP with feedback takes more time compared to the four sessions on four consecutive days. | The information processing of the feedback stimulus may have prevented the modification of the SCP |
| Author (Year) | Patient Condition | Number of Subjects | Control Group | BFB Type | Type of Intervention | Manualized Training | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Beckham et al. (2013) [16] | Depression and anxiety | n = 15 | n = 0 | HRV | 8 sessions 30–60 min 2×/week | No | STAI, WEMWBS, and LASA showed significant improvements at time points A and B compared to baseline. | Lack of control group; results only on short-term effects; small sample size; follow-up survey after discharge: social desirability bias |
| Eddie et al. (2014) [24] | Substance use disorder | n = 21 | n = 20 | HRV | 3 sessions 60–75 min 1×/week | No | Treatment + BFB: greater effect on craving reduction than CG but not significant; negative correlation of HRV and stress interaction: HRV at beginning of treatment only predicts change in craving in CG; high HRV is connected to higher reduction in craving between begin and end of treatment. | Lack of a significant overall effect of HRV BFB despite a mean effect size reduction in abstinence due to the short duration of the training |
| Penzlin et al. (2015) [21] | Alcohol use disorder | n = 24 | n = 24 | HRV | 6 sessions 20 min 3×/weeks | No | BFB group: perceived reduction in craving sooner than control; decrease in anxiety (vs. control); improved cardiac autonomic function; improved vasomotor function after completion. | Late-stage ethyl-toxic damage to cardiac autonomic fibers may have reduced HRV BFB responsivity; small sample size; Laser Doppler flowmetry insensitive to individual vasomotor dysfunction |
| Scolnick et al. (2014) [33] | Eating disorder | n = 24 | n = 0 | HRV | 12 sessions 10 min 5–7×/week | No | HRV BFB training is safe in this population; can be used alongside yoga and meditation. | Lack of control group |
| Tatschl et al. (2020) [17] | Depression | n = 34 | n = 34 | HRV | 5 sessions 35 min 1×/week | No | Larger recovery in depressive symptoms than CG (but decreased in follow-up), as well as increases in resting low-frequency HRV and cardiorespiratory coherence. | No assessment of symptoms between post-assessment and follow-up (12 months); no assessment of slow breathing training between post-intervention and follow-up; potential placebo effect; CG did not get additional control intervention |
| Teeravisutkul et al. (2019) [22] | Alcohol use disorder | n = 17 | n = 18 | HRV | 16 sessions 30 min 4×/week | No | EG: decreased stress and craving after training and 1-month follow-up, CG only immediately after training; higher difference in craving and stress scores at baseline and post-intervention than CG. | Follow-up performed on outpatients, so no proper control for factors that could affect follow-up results |
| Cheng et al. (2017) [27] | Schizophrenia | n = 30 | n = 30 | HRV, EMG, GSR, and RR | 6 sessions 2×/week | No | Significant improvement in anxiety (EG); significant decrease in HR and RR (EG); HADS score and anxiety subscores decreased significantly as number of interventions increased. | Highly functioning chronic schizophrenic patients only |
| Author (Year) | Patient Condition | Number of Subjects | Control Group | BFB Type | Type of Intervention | Manualized Training | Results | Limitations |
|---|---|---|---|---|---|---|---|---|
| Blue & Blue (1979) [34] | Depression | n = 30 | n = 10 | EMG | 14 sessions 30–40 min on 14 consecutive days | No | (In individual sessions 6, 7, and12) muscle tension lower in manic and agitated group (vs. depressed and comparative group). | No limitations mentioned |
| Pharr & Coursey (1989) [26] | Schizophrenia | n = 10 | n = 20 | EMG | 7 sessions 30 min 3×/week | No | Significant lower muscle tension in EG; no increase in psychopathy in EG patient. | Possible practice effect in Finger-Tapping Test; no balancing of negative symptoms of chronic schizophrenia |
| Denney et al. (1991) [20] | Alcohol use disorder | n = 20 | n = 0 | EMG and thermal BFB | 0–8 sessions | No | 0–5 group: no significant difference to no training; 6–7 group and 8+: significantly better than no training and 0–5 group; strongest effect in 8+ group at 3-month mark; abstinence decreases after 6 months post-discharge but slower in the BFB group. | Retrospective pilot study: not all variables that should be considered in a formal research format were examined |
| Ford et al. (1982) [35] | Psychophysio-logical disorders | n = 37 | n = 0 | EMG and thermal BFB | 8 sessions 60 min 1×/week | No | Lower MMPI scores = the lower the initial burden of mental illness of the inpatients; younger patients (17–24 years of age) were unsuccessful in therapy (due to lack of adherence); patients older than 30 years achieved no effect in only 21% of subjects with lower levels of distress. | Patients learn and implement BFB at different speeds; the familiar, quiet environment and the support provided by the clinic staff in the inpatient setting was not guaranteed during the homework exercise |
| EEG BFB (N) | HRV BFB (N) | EMG BFB (N) | |
|---|---|---|---|
| Depression and anxiety | 3 (33.3%) | 2 (28.6%) | 1 (25%) |
| Substance use disorder | 3 (33.3%) | 3 (42.9%) | 1 (25%) |
| Eating disorder | 1 (11.1%) | 1 (14.3%) | 0 (0%) |
| Schizophrenia | 1 (11.1%) | 1 (14.3%) | 1 (25%) |
| Obsessive–compulsive disorder | 1 (11.1%) | 0 (0%) | 0 (0%) |
| Other | 0 (0%) | 0 (0%) | 1 (25%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, K.; Schlicht, M.; Deutschendorf, L.; Smets, L.; Bäuerle, A.; Teufel, M. Biofeedback Training in Inpatient Mental Health Facilities: A Scoping Review. J. Clin. Med. 2025, 14, 3491. https://doi.org/10.3390/jcm14103491
Schmidt K, Schlicht M, Deutschendorf L, Smets L, Bäuerle A, Teufel M. Biofeedback Training in Inpatient Mental Health Facilities: A Scoping Review. Journal of Clinical Medicine. 2025; 14(10):3491. https://doi.org/10.3390/jcm14103491
Chicago/Turabian StyleSchmidt, Kira, Maike Schlicht, Lina Deutschendorf, Lena Smets, Alexander Bäuerle, and Martin Teufel. 2025. "Biofeedback Training in Inpatient Mental Health Facilities: A Scoping Review" Journal of Clinical Medicine 14, no. 10: 3491. https://doi.org/10.3390/jcm14103491
APA StyleSchmidt, K., Schlicht, M., Deutschendorf, L., Smets, L., Bäuerle, A., & Teufel, M. (2025). Biofeedback Training in Inpatient Mental Health Facilities: A Scoping Review. Journal of Clinical Medicine, 14(10), 3491. https://doi.org/10.3390/jcm14103491







