Multicenter Validation of a Unified Evidence-Based Treatment Protocol Focusing on Clazosentan for Managing Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Comparison Between PrF and PoC
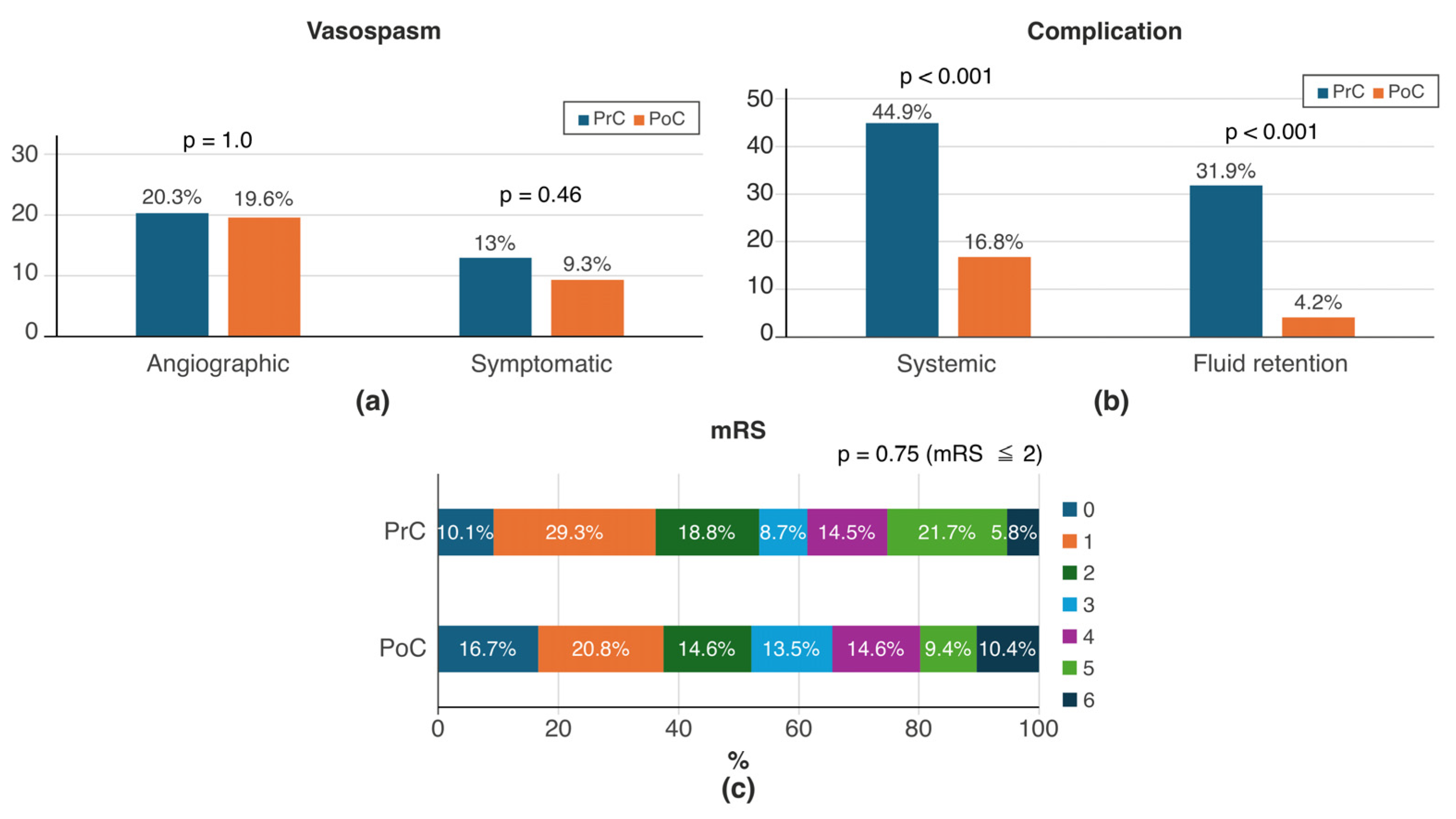
3.2. Comparison Between PrC and PoC
3.3. Comparison Between PoF and PoC
3.4. Comparison Between PrF and PoC in Elderly Patients (76 Years and Older)
3.5. Comparison Between PrF and PoC in Patients with WFNS Grade V
4. Discussion
4.1. PrF vs. PoC
4.2. PrC vs. PoC
4.3. PoF vs. PoC
4.4. PrF vs. PoC in Elderly Patients (76 Years and Older)
4.5. PrF vs. PoC in WFNS Grade V
4.6. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aSAH | aneurysmal subarachnoid hemorrhage |
| DCI | delayed cerebral ischemia |
| ET | endothelin |
| ETA | endothelin A receptor |
| ETB | endothelin B receptor |
| mRS | modified Rankin Scale |
| Na–K ATPase | sodium–potassium ATPase |
| PoC | postprotocol clazosentan |
| PoF | postprotocol fasudil |
| PrC | preprotocol clazosentan |
| PrF | preprotocol fasudil |
| WFNS | World Federation of Neurosurgical Societies |
References
- Endo, H.; Hagihara, Y.; Kimura, N.; Takizawa, K.; Niizuma, K.; Togo, O.; Tominaga, T. Effects of clazosentan on cerebral vasospasm-related morbidity and all-cause mortality after aneurysmal subarachnoid hemorrhage: Two randomized phase 3 trials in Japanese patients. J. Neurosurg. 2022, 137, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Asai, T.; Fukui, T.; Ota, S.; Shimato, S.; Koketsu, N.; Nishizawa, T.; Araki, Y.; Saito, R. Real-world data of clazosentan in combination therapy for aneurysmal subarachnoid hemorrhage: A multicenter retrospective cohort study. Neurosurg. Rev. 2023, 46, 195. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Okawara, M.; Osakabe, M.; Yamaguchi, H.; Maeda, T.; Kurita, H. Initial real-world experience of clazosentan for subarachnoid hemorrhage in Japan. World Neurosurg. X 2024, 21, 100253. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Ryu, B.; Shima, S.; Kamijyo, E.; Ito, K.; Ando, T.; Kushi, K.; Sato, S.; Inoue, T.; Kawashima, A.; et al. Comparison of efficacy between clazosentan and fasudil hydrochloride-based management of vasospasm after subarachnoid hemorrhage focusing on older and WFNS grade V patients: A single-center experience in Japan. Neurosurg. Rev. 2024, 47, 113. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Kanoke, A.; Uchida, H.; Haryu, S.; Omodaka, S.; Kimura, N.; Yoshida, M.; Niizuma, K.; Tominaga, T.; Endo, H. Prophylactic management of cerebral vasospasm with clazosentan in real clinical practice: A single-center retrospective cohort study. Front. Neurol. 2024, 15, 1413632. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke 2012, 43, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.A.; Bruder, N.; Citerio, G.; Defreyne, L.; Dubois, C.; Gupta, R.; Higashida, R.; Marr, A.; Nguyen, T.N.; Roux, S.; et al. On Behalf of the REACT Investigators. REACT: A randomized trial to assess the efficacy and safety of clazosentan for preventing clinical deterioration due to delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2024, 142, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, S.; Kawamoto, T.; Hasegawa, Y.; Nakamura, Y.; Sakata, K.; Kikuchi, J.; Hirohata, M.; Morioka, M. Impact of clazosentan on vasospasm reduction and functioal recovery after aneurysmal subarachnoid hemorrage. Neuro. Med. Chir. 2025, 65, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.; Leclère, B.; Morisson, L.; Eude, Y.; Gayat, E.; Mebazaa, A.; Cinotti, R. A network meta-analysis of therapeutic and prophylactic management of vasospasm on aneurysmal subarachnoid hemorrhage outcomes. Front. Neurol. 2023, 14, 1217719. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Trensz, F.; Pasquali, A.; Cattaneo, C.; Strasser, D.S.; Hess, P.; Iglarz, M.; Clozel, M. Endothelin ETA receptor blockade, by activating ETB receptors, increases vascular permeability and induces exaggerated fluid retention. J. Pharmacol. Exp. Ther. 2017, 361, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Comellas, A.P.; Briva, A.; Dada, L.A.; Butti, M.L.; Trejo, H.E.; Yshii, C.; Azzam, Z.S.; Litvan, J.; Chen, J.; Lecuona, E.; et al. Endothelin-1 impairs alveolar epithelial function via endothelial ETB receptor. Am. J. Respir. Crit. Care Med. 2009, 179, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, Y.; Chida, K.; Miyoshi, K.; Kojima, D.; Nomura, J.-I.; Setta, K.; Chiba, T.; Koji, T.; Fujiwara, S.; Kashimura, H.; et al. Fluid balance management with loop diuretics in patients with aneurysmal subarachnoid hemorrhage treated with clazosentan: A case series. Brain Hemorrhages 2024, 5, 74–78. [Google Scholar] [CrossRef]
- Mutoh, T.; Aono, H.; Seto, W.; Kimoto, T.; Tochinai, R.; Moroi, J.; Ishikawa, T. Cardiopulmonary events of the elderly (≥75 years) during clazosentan therapy after subarachnoid hemorrhage: A retrospective study from a tertiary stroke center in Japan. Medicina 2024, 60, 185. [Google Scholar] [CrossRef] [PubMed]


| Initial response | Stabilize respiration and circulation (consider endotracheal intubation) Ensure adequate sedation and analgesia Lower blood pressure (sBP 100–140 mmHg) |
| Initial examination | Diagnosis of SAH with CT, MRI (FLAIR). Detection of aneurysm with CTA, or angiography if necessary Contrast-enhanced MRI to identify the rupture site |
| Preoperative management | Avoid invasive procedures (e.g., urethral balloon, gastric tube after sedation) Maintain blood pressure (sBP 100–140 mmHg) Head elevated by 20° Cardiology evaluation for pulmonary edema, takotsubo cardiomyopathy, etc. |
| Surgical intervention | Direct or endovascular surgery (based on facility and patient) |
| Postoperative management | Postoperative BP: sBP 120–160 mmHg Prevention of DCI: First choice: Clazosentan (10 mg/h) Others: Fasudil (60 mg/day) or Ozagrel sodium (80 mg/day) The use of cilostazol and statins is facility-specific Fluid management: Normovolemia Basic infusion: extracellular fluid (1 mL/kg/h) Target water balance (0–500 mL); infusion volume was adjusted in case of overbalance. Diuretics: in case of fluid overload (over 1500 mL) Monitor for CSWS and SIADH (Na 130–150 mEq/L) Hb > 8 g/dL, Alb > 2.5 g/dL, Blood glucose < 180 mg/dL, Normothermia Administering antiulcer medications, prefer enteral feeding for oral intake difficulties |
| CSF management | Manage SD, VD, and CD (drainage volume: 150–250 mL/day) |
| Postoperative examination | CT (day after surgery and around day 4), CTA (1 week after clipping), chest CT (at the same time as head CT, if possible) MRI and MRA (day after surgery, around day 8, day 14, and any time of suspected vasospasm) Chest X-ray (as needed) Blood tests (every other day) |
| Treatment for DCI | Extracellular fluid loading Increase target blood pressure Consider endovascular treatment |
| Rehabilitation | Implemented to prevent contractures and maintain ADL |
| PrF (n = 128) | PoC (n = 97) | p-Value | |
|---|---|---|---|
| Age (year) | 65 ± 14.2 | 64 ± 14.6 | 0.78 |
| Sex (female) | 101 (78.9%) | 68 (70.1%) | 0.16 |
| Past history | |||
| Hypertension | 56 (43.8%) | 39 (40.2%) | 0.68 |
| Diabetes mellitus | 10 (7.8%) | 4 (4.1%) | 0.4 |
| Past stroke | 11 (8.6%) | 5 (5.2%) | 0.43 |
| WFNS grade | 0.2 | ||
| I | 39 (30.5%) | 21 (21.7%) | |
| II | 29 (22.7%) | 24 (24.7%) | |
| III | 12 (9.4%) | 15 (15.5%) | |
| IV | 21 (16.4%) | 10 (10.3%) | |
| V | 27 (21.1%) | 27 (27.8%) | |
| Fisher group | 0.12 | ||
| 1 | 6 (4.7%) | 2 (2.1%) | |
| 2 | 10 (7.8%) | 6 (6.2%) | |
| 3 | 102 (79.7%) | 72 (74.2%) | |
| 4 | 10 (7.8%) | 17 (17.5%) | |
| Treatment | 0.07 | ||
| IVR | 82 (65.6%) | 50 (51.6%) | |
| DS | 43 (34.4%) | 46 (47.4%) | |
| Combined | 0 (0) | 1 (1%) | |
| Spinal drainage | 75 (59.5%) | 61 (63.5%) | 0.58 |
| Cilostazol | 94 (74.0%) | 50 (51.6%) | <0.001 |
| Statin | 88 (68.8%) | 39 (40.2%) | <0.001 |
| Vasospasm (Yes) (n = 59/33) | Vasospasm (No) (n = 166/191) | Univariate Analysis p-Value | Multivariate Analysis p-Value (Adjusted OR [95% CI]) | ||
|---|---|---|---|---|---|
| Angiographic vasospasm | Age | 66.0 ± 13.6 | 63.9 ± 14.5 | 0.33 | 0.21 (1.01 [0.99–1.04]) |
| Sex (female) | 39 (66.1%) | 129(78.2%) | 0.08 | 0.025 (0.45 [0.22–0.90]) | |
| WFNS grade IV,V | 28 (47.4%) | 56 (33.9%) | 0.08 | 0.07 (1.8 [0.96–3.40]) | |
| Operation DS | 22 (37.3%) | 67 (41.4%) | 0.64 | 0.94 (1.8 [0.51–1.86]) | |
| Postprotocol | 19 (32.2%) | 78 (47.3%) | 0.048 | 0.02 (0.48 [0.24–0.90]) | |
| Symptomatic vasospasm | Age | 65.4 ± 12.8 | 64.3 ± 14.5 | 0.67 | 0.51 (1.97 [0.23–17.6]) |
| Sex (female) | 21 (63.6%) | 147 (77%) | 0.13 | 0.053 (0.43 [0.19–1.01]) | |
| WFNS grade IV,V | 16 (48.5%) | 67 (25.6%) | 0.18 | 0.17 (1.73 [0.79–3.78]) | |
| Operation DS | 11 (33.3%) | 78 (41.5%) | 0.44 | 0.64 (0.82 [0.35–1.84]) | |
| Postprotocol | 9 (27.3%) | 103 (53.9%) | 0.057 | 0.03 (0.4 [0.16–0.91]) |
| PrC (n = 69) | PoC (n = 97) | p-Value | |
|---|---|---|---|
| Age (year) | 69 ± 16.3 | 64 ± 14.6 | 0.50 |
| Sex (female) | 45 (65.2%) | 68 (70.1%) | 0.61 |
| Past history | |||
| Hypertension | 35 (50.7%) | 39 (40.2%) | 0.21 |
| Diabetes mellitus | 3 (4.4%) | 4 (4.1%) | 1.0 |
| Past stroke | 7 (10.1%) | 5 (5.2%) | 0.24 |
| WFNS grade | 0.1 | ||
| I | 17 (25%) | 21 (21.7%) | |
| II | 15 (22.1%) | 24 (24.7%) | |
| III | 3 (4.4%) | 15 (15.5%) | |
| IV | 14 (20.6%) | 10 (10.3%) | |
| V | 19 (27.9%) | 27 (27.8%) | |
| Fisher group | 0.22 | ||
| 1 | 1 (1.5%) | 2 (2.1%) | |
| 2 | 5 (7.3%) | 6 (6.2%) | |
| 3 | 42 (60.9%) | 72 (74.2%) | |
| 4 | 21 (30.4%) | 17 (17.5%) | |
| Treatment | 0.09 | ||
| IVR | 44 (66.7%) | 50 (51.6%) | |
| DS | 22 (33.3%) | 46 (47.4%) | |
| Combined | 0 (0%) | 1 (1%) | |
| Spinal drainage | 41 (60.3%) | 61 (63.5%) | 0.74 |
| Cilostazol | 29 (42%) | 50 (51.6%) | 0.27 |
| Statin | 31 (44.9%) | 39 (40.2%) | 0.63 |
| Complication (Yes) (n = 47/26) | Complication (No) (n = 117/139) | Univariate Analysis p-Value | Multivariate Analysis p-Value (Adjusted OR [95% CI]) | ||
|---|---|---|---|---|---|
| Systemic complication | Age | 68.2 ± 15.1 | 62.9 ± 15.0 | 0.046 | 0.02 (1.03 [1.01–1.06]) |
| Sex (female) | 31 (66.0%) | 81 (72.3%) | 0.71 | 0.22 (0.57 [0.23–1.4]) | |
| WFNS grade IV,V | 19 (40.4%) | 51 (44%) | 0.73 | 0.2 (0.6 [0.27–1.3]) | |
| Operation DS | 13 (28.3%) | 53 (46.1%) | 0.05 | 0.09 (0.5 [0.22–1.1]) | |
| Postprotocol | 16 (34.0%) | 79 (67.5%) | <0.001 | <0.001 (0.24 [0.11–0.50]) | |
| Fluid retention | Age | 68.6 ± 15.3 | 63.8 ± 15.2 | 0.14 | 0.15 (1.03 [0.99–1.07]) |
| Sex (female) | 19 (73.1%) | 94 (67.6%) | 0.65 | 0.87 (0.91 [0.27–3.2]) | |
| WFNS grade IV,V | 11 (42.3%) | 59 (42.8%) | 1.0 | 0.39 (0.65 [0.24–1.7]) | |
| Operation DS | 4 (15.4%) | 63 (46.3%) | 0.004 | 0.009 (0.24 [0.06–0.71]) | |
| Postprotocol | 4 (15.4%) | 92 (66.2%) | <0.001 | <0.001 (0.085 [0.02–0.25]) |
| PoF (n = 28) | PoC (n = 97) | p-Value | |
|---|---|---|---|
| Age (year) | 74.5 ± 13.4 | 64 ± 14.6 | <0.001 |
| Sex (female) | 21 (75%) | 68 (70.1%) | 0.81 |
| Past history | |||
| Hypertension | 15 (53.6%) | 39 (40.2%) | 0.28 |
| Diabetes mellitus | 1 (3.6%) | 4 (4.1%) | 1.0 |
| Past stroke | 3 (10.7%) | 5 (5.2%) | 0.38 |
| WFNS grade | 0.54 | ||
| I | 7 (25%) | 21 (21.7%) | |
| II | 9 (32.1%) | 24 (24.7%) | |
| III | 1 (3.6%) | 15 (15.5%) | |
| IV | 3 (10.7%) | 10 (10.3%) | |
| V | 8 (28.6%) | 27 (27.8%) | |
| Fisher group | 0.3 | ||
| 1 | 2 (2.1%) | 2 (2.1%) | |
| 2 | 2 (2.1%) | 6 (6.2%) | |
| 3 | 17 (60.7%) | 72 (74.2%) | |
| 4 | 7 (25%) | 17 (17.5%) | |
| Treatment | 0.006 | ||
| IVR | 20 (74.1%) | 50 (51.6%) | |
| DS | 5 (18.5%) | 46 (47.4%) | |
| Combined | 2 (7.4%) | 1 (1%) | |
| Spinal drainage | 16 (57.1%) | 61 (63.5%) | 0.66 |
| Cilostazol | 16 (57.1%) | 50 (51.6%) | 0.67 |
| Statin | 16 (57.1%) | 39 (40.2%) | 0.13 |
| Vasospasm | |||
| Angiographic | 7 (28.0%) | 19 (19.6%) | 0.41 |
| Symptomatic | 6 (24.0%) | 9 (9.3%) | 0.08 |
| Complication | |||
| Systemic | 4 (14.3%) | 16 (16.8%) | 1.0 |
| Fluid retention | 1 (3.6%) | 4 (4.2%) | 1.0 |
| mRS 0–2 at discharge | 11 (39.3%) | 50 (52.1%) | 0.29 |
| Elderly (>75) | PrF (n = 33) | PoC (n = 23) | p-Value |
|---|---|---|---|
| Age (year) | 83.3 ± 5.7 | 82.3 ± 5.3 | 0.56 |
| Sex (female) | 32 (97.0%) | 20 (87.0%) | 0.29 |
| Past history | |||
| Hypertension | 18 (54.6%) | 14 (60.9%) | 0.78 |
| Diabetes mellitus | 3 (9.1%) | 0 (0%) | 0.26 |
| Past stroke | 3 (9.1%) | 2 (8.7%) | 1.0 |
| WFNS grade | 0.41 | ||
| I | 8 (24.2%) | 6 (26.1%) | |
| II | 3 (9.1%) | 4 (17.4%) | |
| III | 3 (9.1%) | 4 (17.4%) | |
| IV | 9 (27.3%) | 2 (8.7%) | |
| V | 10 (30.3%) | 7 (30.4%) | |
| Fisher group | 0.86 | ||
| 1 | 1 (3.0%) | 0 (0%) | |
| 2 | 1 (3.0%) | 2 (8.7%) | |
| 3 | 27 (81.8%) | 18 (78.3%) | |
| 4 | 4 (12.1%) | 3 (13.0%) | |
| Treatment | 0.55 | ||
| IVR | 22 (68.8%) | 14 (60.9%) | |
| DS | 10 (31.3%) | 8 (34.8%) | |
| Combined | 0 (0%) | 1 (4.4%) | |
| Spinal drainage | 20 (62.5%) | 17 (73.9%) | 0.4 |
| Cilostazol | 29 (87.9%) | 10 (43.5%) | <0.001 |
| Statin | 20 (60.6%) | 7 (30.4%) | 0.03 |
| Vasospasm | |||
| Angiographic | 13 (40.6%) | 5 (21.7%) | 0.16 |
| Symptomatic | 8 (25.0%) | 2 (8.7%) | 0.17 |
| Complication | |||
| Systemic | 13 (40.6%) | 5 (22.7%) | 0.24 |
| Fluid retention | 3 (9.4%) | 3 (13.0%) | 0.69 |
| mRS 0–2 at discharge | 6 (20.7%) | 5 (21.7%) | 1.0 |
| WFNS Grade V | PrF (n = 27) | PoC (n = 27) | p-Value |
|---|---|---|---|
| Age (year) | 66.9 ± 15.4 | 66.4 ± 13.2 | 0.94 |
| Sex (female) | 21 (77.8%) | 17 (63.0%) | 0.37 |
| Past history | |||
| Hypertension | 16 (59.3%) | 9 (33.3%) | 0.10 |
| Diabetes mellitus | 2 (7.4%) | 1 (3.7%) | 1.0 |
| Past stroke | 2 (7.4%) | 2 (7.4%) | 1.0 |
| Fisher group | 0.25 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 0 (0%) | 0 (0%) | |
| 3 | 20 (74.1%) | 15 (55.6%) | |
| 4 | 7 (25.9%) | 12 (44.4%) | |
| Treatment | 0.03 | ||
| IVR | 21 (80.8%) | 13 (48.2%) | |
| DS | 5 (19.2%) | 13 (48.2%) | |
| Combined | 0 (0%) | 1 (3.7%) | |
| Spinal drainage | 17 (63%) | 15 (57.7%) | 0.78 |
| Cilostazol | 20 (74.1%) | 11 (40.7%) | 0.027 |
| Statin | 17 (63.0%) | 12 (44.4%) | 0.27 |
| Vasospasm | |||
| Angiographic | 10 (37.0%) | 7 (25.9%) | 0.56 |
| Symptomatic | 6 (22.2%) | 3 (11.1%) | 0.47 |
| Complication | |||
| Systemic | 11 (40.7%) | 3 (11.1%) | 0.028 |
| Fluid retention | 5 (18.5%) | 0 (0%) | 0.051 |
| mRS 0–2 at discharge | 7 (29.2%) | 8 (30.8%) | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, H.; Ishii, D.; Kuwabara, M.; Hara, T.; Kurisu, K.; Sumida, M.; Ikawa, F.; Ohba, S.; Tominaga, A.; Obayashi, N.; et al. Multicenter Validation of a Unified Evidence-Based Treatment Protocol Focusing on Clazosentan for Managing Subarachnoid Hemorrhage. J. Clin. Med. 2025, 14, 3423. https://doi.org/10.3390/jcm14103423
Kondo H, Ishii D, Kuwabara M, Hara T, Kurisu K, Sumida M, Ikawa F, Ohba S, Tominaga A, Obayashi N, et al. Multicenter Validation of a Unified Evidence-Based Treatment Protocol Focusing on Clazosentan for Managing Subarachnoid Hemorrhage. Journal of Clinical Medicine. 2025; 14(10):3423. https://doi.org/10.3390/jcm14103423
Chicago/Turabian StyleKondo, Hiroshi, Daizo Ishii, Masashi Kuwabara, Takeshi Hara, Kaoru Kurisu, Masayuki Sumida, Fusao Ikawa, Shinji Ohba, Atsushi Tominaga, Naohiko Obayashi, and et al. 2025. "Multicenter Validation of a Unified Evidence-Based Treatment Protocol Focusing on Clazosentan for Managing Subarachnoid Hemorrhage" Journal of Clinical Medicine 14, no. 10: 3423. https://doi.org/10.3390/jcm14103423
APA StyleKondo, H., Ishii, D., Kuwabara, M., Hara, T., Kurisu, K., Sumida, M., Ikawa, F., Ohba, S., Tominaga, A., Obayashi, N., Kuroki, K., Sadatomo, T., Hamasaki, O., Sakamoto, S., Matsushige, T., Watanabe, Y., Araki, H., Abiko, M., Ichinose, N., ... Horie, N. (2025). Multicenter Validation of a Unified Evidence-Based Treatment Protocol Focusing on Clazosentan for Managing Subarachnoid Hemorrhage. Journal of Clinical Medicine, 14(10), 3423. https://doi.org/10.3390/jcm14103423








