Effects of Treatment on Structural and Functional Parameters of the Left Heart in Naïve Acromegaly Patients: Prospective Single-Centre Study: 12-Month Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
- The inclusion criteria for APs were as follows:
- -
- Diagnosis of acromegaly: GH > 1 µg/l during 75 g oral glucose tolerance test before receiving any treatments, increased IGF-1 levels, and positive pituitary magnetic resonance imaging findings [20]
- -
- No history of acromegaly treatment.
- The control group comprised 34 healthy subjects (19 females and 15 males) matched for age, sex, and BMI, and without the presence of acromegaly (normal IGF-1 levels).
- The exclusion criteria for both groups were as follows:
- -
- History of coronary artery disease, myocardial infarction or stroke, Grade II to IV valve disease severity, history of pulmonary embolism, LVEF < 50%, peripheral arterial disease, chronic renal failure, or chronic obstructive pulmonary disease.
- Due to exclusion criteria, we excluded 4 APs.
2.2. Clinical Examination
2.3. Laboratory Examinations
2.4. Echocardiography
2.5. Dual-Energy X-Ray Absorptiometry (DXA)
2.6. Statistical Analyses
3. Results
3.1. Characterization of the Study Population at the Time of Diagnosis
3.2. Comparison Between APs and CON
3.3. Comparison Between Male and Female APs
3.4. Changes in Biochemical, Body Composition, and Echocardiography Parameters (12 Months After the Beginning of Acromegaly Treatment)
3.5. Relationships Between Activity of Acromegaly, Body Composition Parameters, and Echocardiography Parameters in APs at Baseline
3.6. Predictors of Baseline LVMI and Predictors of Reverse Remodelling of LVMI
3.7. Association Between LVM and Body Composition Scaling Parameters
4. Discussion
5. Conclusions
Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleseriu, M.; Langlois, F.; Lim, D.S.T.; Varlamov, E.V.; Melmed, S. Acromegaly: Pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022, 10, 804–826. [Google Scholar] [CrossRef] [PubMed]
- Alhawyan, F.S. Mortality in Acromegalic Patients: Etiology, Trends, and Risk Factors. Cureus 2021 13, e14265. [CrossRef]
- Colao, A.; Grasso, L.F.; Giustina, A.; Melmed, S.; Chanson, P.; Pereira, A.M.; Pivonello, R. Acromegaly. Nat. Rev. Dis. Primers 2019, 5, 20. [Google Scholar] [CrossRef]
- Iglesias, P. Acromegaly and Cardiovascular Disease: Associated Cardiovascular Risk Factors, Cardiovascular Prognosis, and Therapeutic Impact. J. Clin. Med. 2025, 14, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ságová, I.; Dragula, M.; Mokáň, M.; Vaňuga, P. Filling the gap between the heart and the body in acromegaly: A case-control study. Endocrine 2023, 79, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Jin, J.; Zhang, P.; Yan, K.; Zhang, H.; Chen, X.; He, W.; Guan, H.; Liao, Z.; Xiao, H.; et al. Use of speckle tracking echocardiography in evaluating cardiac dysfunction in patients with acromegaly: An update. Front. Endocrinol. 2023, 14, 1260842. [Google Scholar] [CrossRef]
- Gadelha, P.; Santos, E.C.L.; Castillo, J.; Vilar, L. Subclinical ventricular dysfunction in long-term acromegaly assessed by speckle-tracking echocardiography. Front. Endocrinol. 2022, 13, 812964. [Google Scholar] [CrossRef]
- Crisafulli, S.; Luxi, N.; Sultana, J.; Fontana, A.; Spagnolo, F.; Giuffrida, G.; Ferraù, F.; Gianfrilli, D.; Cozzolino, A.; Cristina De Martino, M.; et al. Global epidemiology of acromegaly: A systematic review and meta-analysis. Eur. J. Endocrinol. 2021, 185, 251–263. [Google Scholar] [CrossRef]
- Esposito, D.; Olsson, D.S.; Franzén, S.; Miftaraj, M.; Nåtman, J.; Gudbjörnsdottir, S.; Johannsson, G. Effect of diabetes on morbidity and mortality in patients with acromegaly. J. Clin. Endocrinol. Metab. 2022, 107, 2483–2492. [Google Scholar] [CrossRef]
- Melmed, S.; Bronstein, M.D.; Chanson, P.; Klibanski, A.; Casanueva, F.F.; Wass, J.A.; Strasburger, C.J.; Luger, A.; Clemmons, D.R.; Giustina, A. A Consensus Statement on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 2018, 14, 552–561. [Google Scholar] [CrossRef]
- Maione, L.; Brue, T.; Beckers, A.; Delemer, B.; Petrossians, P.; Borson-Chazot, F.; Chabre, O.; François, P.; Bertherat, J.; Cortet-Rudelli, C.; et al. Changes in the management and comorbidities of acromegaly over three decades: The French Acromegaly Registry. Eur. J. Endocrinol. 2017, 176, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Găloiu, S.; Toma, I.D.; Tănasie, D.I.; Bărbulescu, A.; Baciu, I.; Niculescu, D.A.; Trifănescu, R.A.; Căpăţînă, C.; Radian, Ş.; Poiană, C. High mortality risk among women with acromegaly persists. Front. Endocrinol. 2024, 15, 1348972. [Google Scholar] [CrossRef]
- Arosio, M.; Reimondo, G.; Malchiodi, E.; Berchialla, P.; Borraccino, A.; De Marinis, L.; Pivonello, R.; Grottoli, S.; Losa, M.; Cannavò, S.; et al. Predictors of morbidity and mortality in acromegaly: An Italian survey. Eur. J. Endocrinol. 2012, 167, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cao, Y.; Cao, J.; Li, X.; Liu, P.; Wang, Z.; Gao, L.; Bao, X.; Xing, B.; Wang, Y. Reversibility of Cardiac Involvement in Acromegaly Patients After Surgery: 12-Month Follow-up Using Cardiovascular Magnetic Resonance. Front. Endocrinol. 2020, 11, 598948. [Google Scholar] [CrossRef]
- Bogazzi, F.; Di Bello, V.; Palagi, C.; Donne, M.G.; Di Cori, A.; Gavioli, S.; Talini, E.; Cosci, C.; Sardella, C.; Brogioni, S.; et al. Improvement of intrinsic myocardial contractility and cardiac fibrosis degree in acromegalic patients treated with somatostatin analogues: A prospective study. Clin. Endocrinol. 2005, 62, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Maison, P.; Tropeano, A.I.; Macquin-Mavier, I.; Giustina, A.; Chanson, P. Impact of somatostatin analogs on the heart in acromegaly: A metaanalysis. J. Clin. Endocrinol. Metab. 2007, 92, 1743–1747. [Google Scholar] [CrossRef]
- Yang, H.; Tan, H.; Huang, H.; Li, J. Advances in Research on the Cardiovascular Complications of Acromegaly. Front. Oncol. 2021, 11, 640999. [Google Scholar] [CrossRef]
- Heidarpour, M.; Shafie, D.; Aminorroaya, A.; Sarrafzadegan, N.; Farajzadegan, Z.; Nouri, R.; Najimi, A.; Dimopolou, C.; Stalla, G. Effects of somatostatin analog treatment on cardiovascular parameters in patients with acromegaly: A systematic review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24, 29. [Google Scholar] [CrossRef]
- Ságová, I.; Kantárová, D.; Mokáň, M.; Dragula, M.; Vaňuga, P. Kardiovaskulárne komplikácie akromegálie. Cor Vasa 2022, 64, 46–52. [Google Scholar] [CrossRef]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A. Endocrine Society. Acromegaly: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 109. [Google Scholar] [CrossRef]
- Mancia, G.; De Backer, G.; Dominiczak, A.; Cifkova, R.; Fagard, R.; Germano, G.; Grassi, G.; Heagerty, A.M.; Kjeldsen, S.E.; Laurent, S.; et al. ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care 2022, 45, 17–38. [Google Scholar] [CrossRef]
- Chamberlain, J.J.; Rhinehart, A.S.; Shaefer, C.F., Jr.; Neuman, J. Diagnosis and management of diabetes: Synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann. Intern. Med. 2016, 164, 542–552. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Jafary, F.H. Devereux formula for left ventricular mass-be careful to use the right units of measurement. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2007, 20, 783. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Popielarz-Grygalewicz, A.; Gąsior, J.S.; Konwicka, A.; Grygalewicz, P.; Stelmachowska-Banaś, M.; Zgliczyński, W.; Dąbrowski, M. Heart in Acromegaly: The Echocardiographic Characteristics of Patients Diagnosed with Acromegaly in Various Stages of the Disease. Int. J. Endocrinol. 2018, 11, 6935054. [Google Scholar] [CrossRef] [PubMed]
- Lie, J.T. Pathology of the heart in acromegaly: Anatomic findings in 27 autopsied patients. Am. Heart J. 1980, 100, 41–52. [Google Scholar] [CrossRef]
- Ramos-Leví, A.M.; Marazuela, M. Bringing Cardiovascular Comorbidities in Acromegaly to an Update. How Should We Diagnose and Manage Them? Front. Endocrinol. 2019, 10, 120. [Google Scholar] [CrossRef]
- Uziȩbło-Życzkowska, B.; Jurek, A.; Witek, P.; Zieliński, G.; Gielerak, G.; Krzesiński, P. Left Heart Dysfunction in Acromegaly Revealed by Novel Echocardiographic Methods. Front. Endocrinol. 2020, 11, 418. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Pivonello, R.; Lombardi, G.; Colao, A. Cardiac abnormalities in acromegaly: Pathophysiology and implications for management. Treat. Endocrinol. 2004, 3, 309–318. [Google Scholar] [CrossRef]
- Sherin, R.P.V.; Vietor, N.O.; Usman, A.; Hoang, T.D.; Shakir, M.K.M. Cardiovascular Disorders Associated with Acromegaly: An Update. Endocr. Pract. 2024, 30, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, C.M.; Gottlieb, I.; Volschan, I.; Kasuki, L.; Warszawski, L.; Balarini Lima, G.A.; Xavier, S.S.; Pedrosa, R.C.; Neto, L.V.; Gadelha, M.R. Low Frequency of Cardiomyopathy Using Cardiac Magnetic Resonance Imaging in an Acromegaly Contemporary Cohort. J. Clin. Endocrinol. Metab. 2015, 100, 4447–4455. [Google Scholar] [CrossRef] [PubMed]
- Warszawski, L.; Kasuki, L.; Sá, R.; dos Santos Silva, C.M.; Volschan, I.; Gottlieb, I.; Pedrosa, R.C.; Gadelha, M.R. Low frequency of cardniac arrhythmias and lack of structural heart disease in medically-naïve acromegaly patients: A prospective study at baseline and after 1 year of somatostatin analogs treatment. Pituitary 2016, 19, 582–589. [Google Scholar] [CrossRef]
- Bredella, M.A.; Schorr, M.; Dichtel, L.E.; Gerweck, A.V.; Young, B.J.; Woodmansee, W.W.; Swearingen, B.; Miller, K.K. Body composition and ectopic lipid changes with biochemical control of acromegaly. J. Clin. Endocrinol. Metab. 2017, 102, 4218–4225. [Google Scholar] [CrossRef][Green Version]
- Lee, M.J.; Kim, J. The pathophysiology of visceral adipose tissues in cardiometabolic diseases. Biochem. Pharmacol. 2024, 222, 116116. [Google Scholar] [CrossRef]
- Guo, X.; Gao, L.; Shi, X.; Li, H.; Wang, Q.; Wang, Z.; Chen, W.; Xing, B. Pre-and postoperative body composition and metabolic characteristics in patients with acromegaly: A prospective study. Int. J. Endocrinol. 2018, 4125013. [Google Scholar] [CrossRef]
- Reyes-Vidal, C.M.; Mojahed, H.; Shen, W.; Jin, Z.; Arias-Mendoza, F.; Fernandez, J.C.; Gallagher, D.; Bruce, J.N.; Post, K.D.; Freda, P.U. Adipose tissue redistribution and ectopic lipid deposition in active acromegaly and effects of surgical treatment. J. Clin. Endocrinol. Metab. 2015, 100, 2946–2955. [Google Scholar] [CrossRef]
- Ságová, I.; Kantárová, D.; Mokáň, M.; Vaňuga, P. Changes in Cross-Sectional Area of the Median Nerve and Body Composition Parameters after Treatment of Acromegaly: 1 year Follow-Up. Int. J. Endocrinol. 2022, 2022, 8766046. [Google Scholar] [CrossRef]
- Füchtbauer, L.; Olsson, D.S.; Bengtsson, B.Å.; Norrman, L.L.; Sunnerhagen, K.S.; Johannsson, G. Muscle strength in patients with acromegaly at diagnosis and during long-term follow-up. Eur. J. Endocrinol. 2017, 177, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Popielarz-Grygalewicz, A.; Stelmachowska-Banaś, M.; Raczkiewicz, D.; Czajka-Oraniec, I.; Zieliński, G.; Kochman, W.; Dąbrowski, M.; Zgliczyński, W. Effects of acromegaly treatment on left ventricular systolic function assessed by speckle tracking echocardiography in relation to sex differences: Results from a prospective single center study. Front. Endocrinol. 2023, 14, 1154615. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Pivonello, R.; Galderisi, M.; Cappabianca, P.; Auriemma, R.S.; Galdiero, M.; Cavallo, L.M.; Esposito, F.; Lombardi, G. Impact of treating acromegaly first with surgery or somatostatin analogs on cardiomyopathy. J. Clin. Endocrinol. Metab. 2008, 93, 2639–2646. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Marzullo, P.; Cuocolo, A.; Spinelli, L.; Pivonello, R.; Bonaduce, D.; Salvatore, M.; Lombardi, G. Reversal of acromegalic cardiomyopathy in young but not in middle-aged patients after 12 months of treatment with the depot long acting somatostatin analogue octreotide. Clin. Endocrinol. 2003, 58, 169–176. [Google Scholar] [CrossRef]
- Colao, A. Improvement of cardiac parameters in patients with acromegaly treated with medical therapies. Pituitary 2012, 15, 50–58. [Google Scholar] [CrossRef]
- Lombardi, G.; Colao, A.; Marzullo, P.; Biondi, B.; Palmieri, E.; Fazio, S. Multicenter italian study group on lanreotide: Improvement of left ventricular hypertrophy and arrhythmias after lanreotide-induced GH and IGF-I decrease in acromegaly. A prospective multi-Center study. J. Endocrinol. Invest. 2002, 25, 971–976. [Google Scholar] [CrossRef]
- Colao, A.; Cappabianca, P.; Caron, P.; De Menis, E.; Farrall, A.J.; Gadelha, M.R.; Hmissi, A.; Rees, A.; Reincke, M.; Safari, M.; et al. Octreotide LAR vs. surgery in newly diagnosed patients with acromegaly: A randomized, open-label, multicentre study. Clin. Endocrinol. 2009, 70, 757–768. [Google Scholar] [CrossRef]

| Characteristics | Patients with Acromegaly (APs) (n = 34) | Control Group (CON) (n = 34) | Comparison APs vs. CON p-Value | Male APs (n = 15) | Female APs (n = 19) | Comparison Male vs. Female APs p-Value |
|---|---|---|---|---|---|---|
| Sex (M/F) | 15/19 | 15/19 | - | - | - | - |
| Age at the time of study (year) | 52 [41.5–59] | 53 [40.8–61] | NS | 49.7 [37–53.8] | 53.5 [46–59.3] | 0.043 |
| Smoking history (%) | 19% | 17% | NS | 23% | 13% | NS |
| Arterial hypertension (%) | 46.7% | 18% | <0.001 | 40.7% | 47.6% | NS |
| Systolic BP (mmHg) | 123.6 [115.7–140] | 122.0 [110–137] | NS | 122.7 [114–139] | 123.9 [116.5–141] | NS |
| Diastolic BP (mmHg) | 78.3 [66.5–89.2] | 77.1 [64.7–87.9] | NS | 80.2 [73–92.1] | 78.6 [66–90.7] | NS |
| Diabetes (%) | 3.3% | 0% | NS | 0% | 5.6% | NS |
| Prediabetes (%) | 32% | 7% | <0.001 | 29% | 35% | NS |
| Dyslipidaemia (%) | 66.7% | 33% | <0.001 | 60.3% | 72.2% | NS |
| Laboratory examinations | ||||||
| Baseline GH (ng/mL) | 6.5 [5.2–6.1] | 0.2 [0.1–0.3] | <0.001 | 10.5 [6.7–12.6] | 4.4 [2.9–7] | 0.002 |
| IGF-1 (ng/mL) | 526 [359–710] | 139 [109–165] | <0.001 | 654 [525–942] | 417 [323–605] | 0.029 |
| IGF1/ULN | 2.3 [1.7–3.4] | 0.6 [0.5–0.7] | <0.001 | 3 [2.1–4.6] | 2 [1.5–3] | 0.023 |
| Creatinine (µmol/l) | 65.9 [2.8–11.1] | 73.5 [61.8–87] | 0.033 | 71.8 [65.7–89.9] | 57.2 [51.3–73] | 0.007 |
| FPG (mmol/L) | 5.8 [5.2–6.1] | 5.4 [4.7–5.6] | 0.012 | 5.5 [5.2–5.9] | 5.7 [5.1–6.2] | NS |
| HOMA IR | 3.1 [2.2–4.5] | 1.7 [1.1–3.3] | 0.002 | 3.4 [2.2–4.6] | 2.9 [2.2–4.2] | NS |
| Glycated haemoglobin (%) | 5.6 [5.3–5.8] | 5.2 [5.0–5.5] | 0.004 | 5.7 [5.4–5.9] | 5.4 [5.3–5.8] | 0.032 |
| Total cholesterol (mmol/L) | 5.2 [4.7–5.6] | 5.1 [4.2–5.6] | NS | 5.3 [4.4–5.7] | 4.9 [4–5.5] | NS |
| LDL cholesterol (mmol/L) | 3.5 [2.9–4.4] | 3 [2.2–3.4] | 0.008 | 3.6 [2.2–4.5] | 2.9 [2.2–3.2] | 0.035 |
| HDL cholesterol (mmol/L) | 1.3 [1.1–1.8] | 1.4 [1.2–1.7] | NS | 1.4 [1.1–1.9] | 1.3 [1.1–1.8] | NS |
| Triglycerides (mmol/L) | 1.2 [1.0–1.6] | 1.35 [1.2–1.9] | NS | 1.2 [1.1–1.5] | 1.2 [1.0–1.8] | NS |
| Anthropometric measurements | ||||||
| Height (cm) | 173 [166–181] | 170 [164–175] | NS | 180 [175–189] | 165 [161–176] | <0.001 |
| Weight (kg) | 90 [73–110] | 88 [69–106] | NS | 109 [85–118] | 78 [69–94] | <0.001 |
| Waist circumference (cm) | 100 [89–116] | 102 [83–106] | NS | 107 [95–119] | 94 [86–109] | 0.003 |
| BMI (kg/m2) | 31 [25.0–34.1] | 30.5 [24.1–33.9] | NS | 33 [29–36] | 29 [24–34] | <0.001 |
| BSA (m2) | 2 [1.81–2.23] | 1.99 [1.75–2.18] | NS | 2.28 [2.04–2.46] | 2 [1.78–2.01] | <0.001 |
| DXA measurements | ||||||
| Fat mass total (kg) | 30.1 [22.8–37.5] | 31.3 [25.1–37.8] | NS | 29 [21.9–36.8] | 32.3 [23.8–37.8] | 0.019 |
| Fat mass trunk (kg) | 15 [10.1–17.2] | 15.2 [11.8–19,6] | NS | 14.6 [9.7–17.7] | 15.9 [10.1–17.2] | 0.048 |
| Fat mass limb (kg) | 14.8 [11.6–17.5] | 14.1 [11.6–18.5] | NS | 13.2 [9.1–16.2] | 15.1 [12.6–19.2] | 0.026 |
| Lean mass total (kg) | 60.4 [46.8–75] | 49.1 [42.5–66.7] | 0.033 | 77.8 [64.1–81.8] | 50.5 [45–65.3] | <0.001 |
| Lean mass trunk (kg) | 30.9 [24.6–38.1] | 26.8 [22.8–34] | 0.048 | 40 [31.8–44.8] | 26.9 [24.2–33.4] | <0.001 |
| Lean mass limb (kg) | 27.7 [20.4–35.6] | 21.5 [18.9–31.2] | 0.041 | 36.2 [31.7–42] | 23 [19.5–28.1] | <0.001 |
| Characteristics | Patients with Acromegaly (APs) (n = 34) | Control Group (CON) (n = 34) | Comparison APs vs. CON p-Value | Male APs | Female APs | Comparison Male vs. Female APs p-Value * |
|---|---|---|---|---|---|---|
| Ao. Asc. (mm) | 34 [32–35] | 33 [31–34] | NS | 34 [32.3–35] | 33 [31.8–35] | NS |
| LAVI (mL/m2) | 38.4 [35–41] | 28.7 [24–31] | <0.001 | 38.6 [35–42] | 36.9 [33.9–38] | 0.048 |
| LVEDd (mm) | 50 [46.8–56] | 49 [46–50] | 0.079 | 53 [50–61] | 48 [44.8–55.3] | <0.001 |
| RVEDd (mm) | 32 [29.6–35] | 31 [29–33.7] | NS | 33.5 [30.4–36] | 30 [29.1–33.7] | 0.036 |
| IVSDd (mm) | 13 [11–15] | 10 [9–11] | <0.001 | 14 [13–16] | 12 [9.8–13.3] | <0.001 |
| PWDd (mm) | 12 [11–15] | 10 [9–10] | <0.001 | 13 [12–15] | 12 [10–13] | 0.027 |
| LVM (g) | 248 [180–375] | 167 [142–196] | <0.001 | 348 [249–438] | 215 [158–315] | <0.001 |
| LVMI (g/m2) | 124 [97–173] | 86 [74–100] | <0.001 | 153 [107–190] | 116 [90–160] | <0.001 |
| LVH (%) | 70% | 23% | <0.001 | 72% | 67% | NS |
| RWT > 0.42 (%) | 86.7% | 13.3% | <0.001 | 91.7% | 83.3% | NS |
| LVEF (%) | 60 [55–60] | 56 [55–60] | NS | 58.5 [55–60] | 60 [55.8–60.5] | NS |
| E/É | 11.0 [9–12] | 9.3 [8.6–10] | 0.033 | 11.2 [9–12.2] | 10.6 [8.8–11] | NS |
| E/A | 0.9 [0.6–1.2] | 1.16 [0.7–1.6] | 0.027 | 0.87 [0.7–1.1] | 0.9 [0.75–1.2] | NS |
| TAPSE | 23 (21; 24) | 23 (23; 24) | NS | 23 [20.5–23.8] | 23.5 [20–24.5] | NS |
| Characteristics | At Diagnosis (All APs) | 1-Year Follow-Up (All APs) | p-Value (All APs) | 1-Year Follow-Up Change (Female APs) | p-Value (Female) | 1-Year Follow-Up Change (Male APs) | p-Value (Male) |
|---|---|---|---|---|---|---|---|
| IGF-1 (ng/mL) | 526 [359–710] | 212 [167–269] | <0.001 | −211 (−378; −130) | <0.001 | −332 (−592; −317) | 0.002 |
| IGF-1/ULN | 2.3 [1.7–3.4] | 0.99 [0.71–1.25] | <0.001 | −1.05 (−1.87; −0.61) | <0.001 | −2.02 (−2.94; −1.2) | 0.003 |
| BMI (kg/m2) | 31.8 [25.0–34.1] | 30.7 [26.0–34.0] | NS | 0 (−1; 1) | NS | 0 (−2; 1) | NS |
| BSA (m2) | 2.00 [1.81–2.23] | 1.99 [1.83–2.24] | NS | −0.01 (−0.04; 0.02) | NS | 0 (−0.05; 0.02) | NS |
| Fat mass (kg) | 30.1 [22.8–37.5] | 31.2 [24.6–39.4] | 0.035 | 1.0 (0.2; 2.8) | 0.048 | 2.1 (0.3; 5.1) | 0.030 |
| Fat mass trunk (kg) | 15 [10.1–17.2] | 15.9 [10.5–17.4] | 0.040 | 0.4 (0; 1.8) | 0.057 | 0.8 (0.2; 2.2) | 0.036 |
| Fat mass limb (kg) | 14.8 [11.6–17.5] | 15 [11.7–17.6] | NS | 0 (0; 0.5) | NS | 0 (0.1; 0.9) | NS |
| Lean mass (kg) | 60.4 [46.8–75] | 56.1 [45.9–72] | 0.010 | −1.5 (−3.2; −0.4) | 0.010 | −3.5 (−7.4; −1.2) | 0.018 |
| Lean mass trunk (kg) | 30.9 [24.6–38.1] | 28.8 [23.1–36.9] | 0.006 | −1.2 (−2.7; −0.2) | 0.008 | −2.4 (−5.3; −1) | 0.012 |
| Lean mass limb (kg) | 27.7 [20.4–35.6] | 26.4 [19.3–35] | 0.031 | −0.6 (−1.2; −0.1) | 0.025 | −1.5 (−3.2; −0.7) | 0.039 |
| LAVI (ml/m2) | 38.4 [35–41] | 36.2 [34.1–39.7] | 0.027 | −0.7 (−1; 0) | 0.026 | −1.2 (−1.6; 0) | 0.032 |
| LVEDd (mm) | 50 [46.8–56] | 49 [46.5–56] | NS | −0.4 (−0.8; 0) | NS | −0.5 (−1.8; 0) | NS |
| IVSd (mm) | 13 [11–15] | 11 [10–14] | <0.001 | −1 (−1; −0,8) | <0.001 | −1 (−2; −1) | 0.004 |
| PWDd (mm) | 12 [11–15] | 12 [10–14.5] | <0.001 | −0.2 (−1; 0) | 0.017 | −1 (−1; 0) | 0.008 |
| LVM (g) | 248 [180–375] | 217 [163–328] | <0.001 | −21 (−30; −11) | <0.001 | −44 (−66; −18) | 0.003 |
| LVMI (g/m2) | 124 [97–173] | 111 [83–154] | <0.001 | −11 (−14; −6) | <0.001 | −20 (−24; −6) | 0.004 |
| LVEF (%) | 60 [55–60] | 59 [55–60] | NS | 0 (0; 1) | NS | −0.5 (0; 2) | 0.053 |
| E/É | 11.0 [9–12] | 10.7 [8.8–11.6] | 0.037 | −0.2 (−0.4; 0.3) | 0.031 | 0 (0; 0) | NS |
| E/A | 0.9 [0.6–1.2] | 0.9 [0.4–1.3] | 0.047 | 0.15 (−0.15; 0.1) | 0.039 | 0 (−0.02; −0.05) | NS |
| Characteristics | IGF-1 (ng/mL) | IGF1/ULN | BMI (kg/m2) | BSA (m2) | Lean Mass (kg) | Lean Mass Trunk (kg) | Lean Mass Limb (kg) | Fat Mass (kg) | Glycated Haemoglobin (%) |
|---|---|---|---|---|---|---|---|---|---|
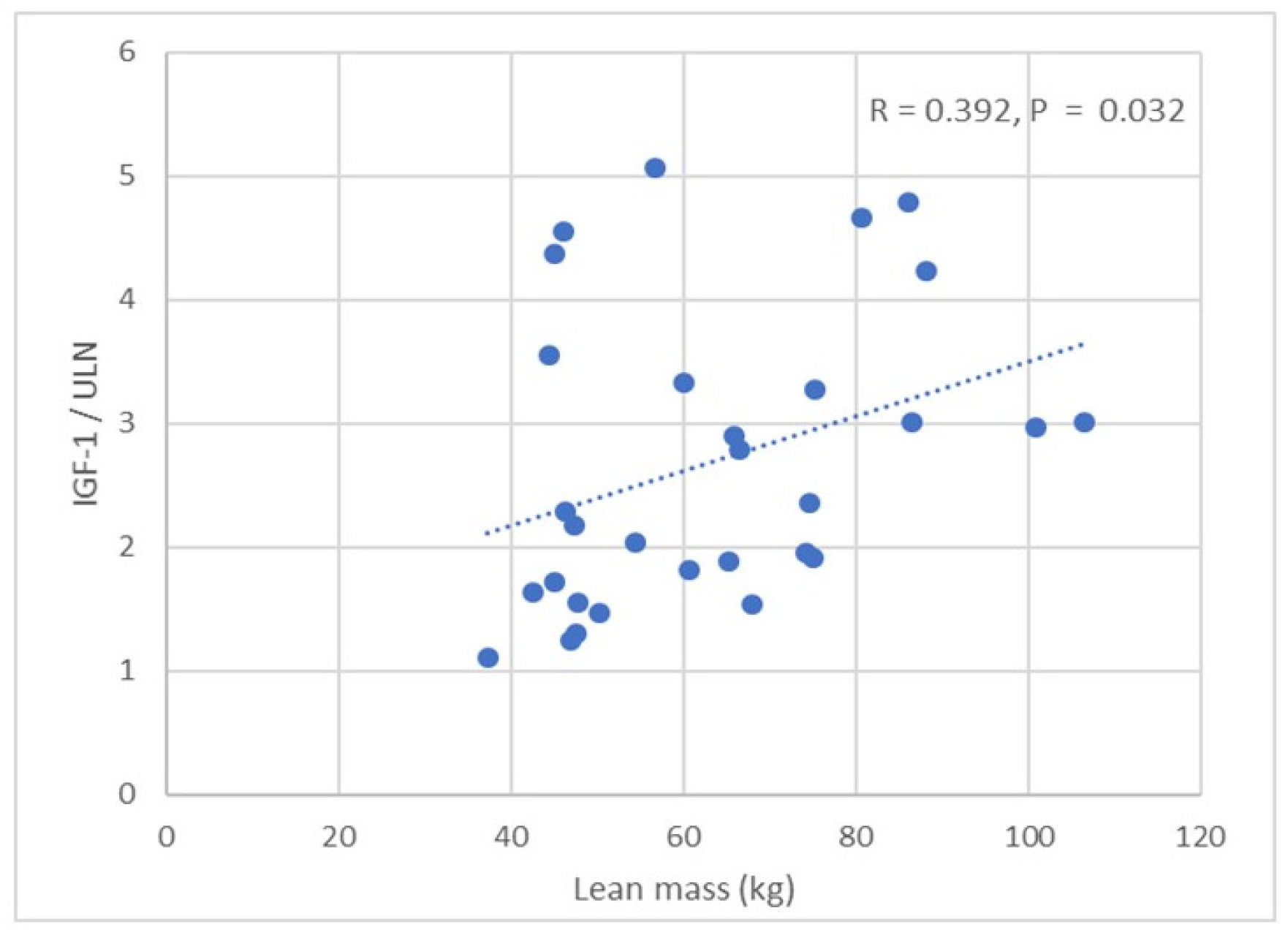
| LAVI (mL/m2) | R = 0.313 p = 0.045 | R = 0.385 p = 0.034 | R = 0.432 p = 0.025 | R = 0.621 p < 0.001 | R = 0.424 p = 0.027 | R = 0.432 p = 0.017 | R = 0.4382 p = 0.037 | NS | NS |
| LVEDd (mm) | NS | NS | R = 0.549 p < 0.001 | R = 0.599 p < 0.001 | R = 0.624 p < 0.001 | R = 0.701 p < 0.001 | R = 0.553 p = 0.002 | NS | R = 0.369 p = 0.045 |
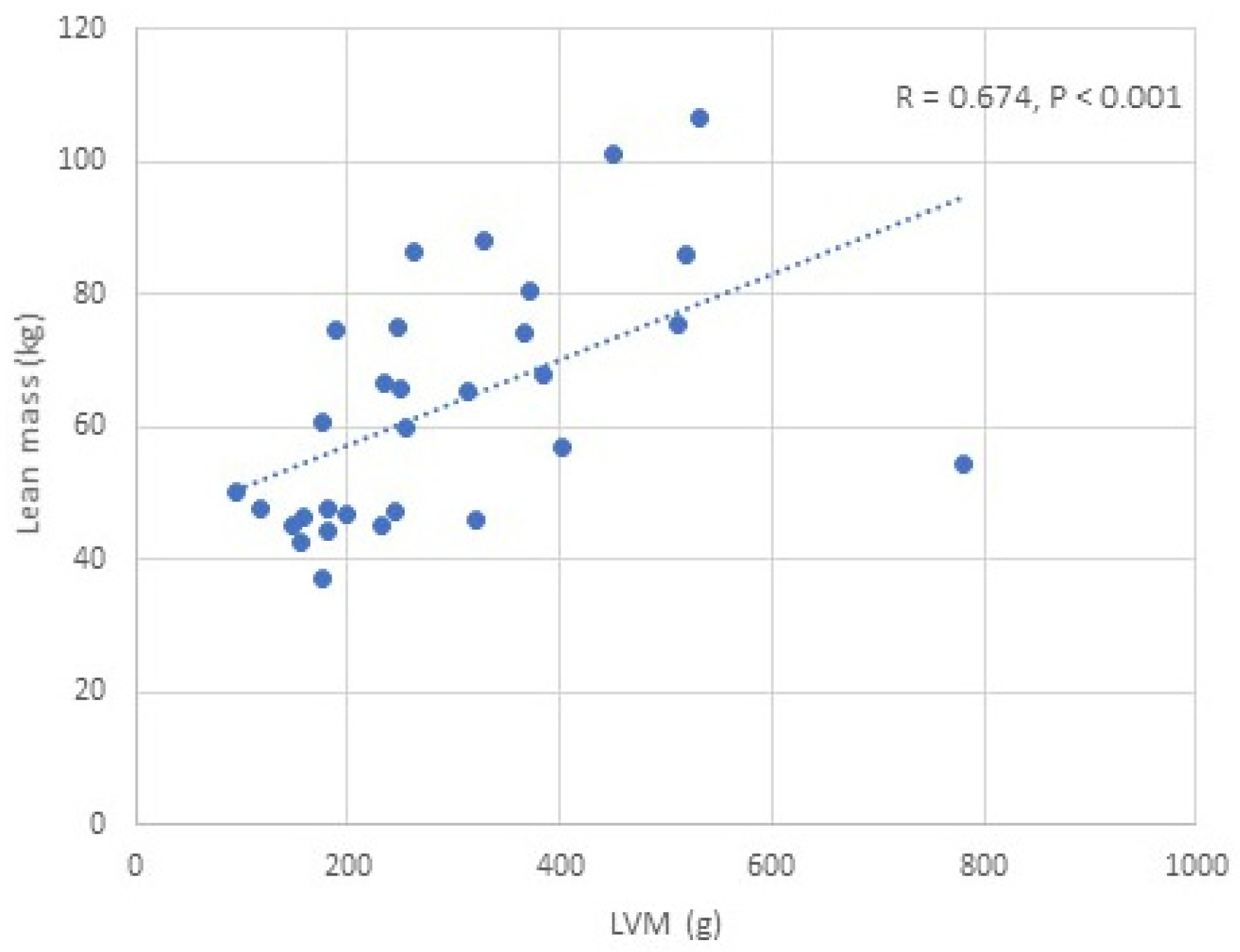
| LVM (g) | R = 0.463 p = 0.016 | R = 0.511 p = 0.004 | R = 0.532 p = 0.002 | R = 0.591 p = 0.001 | R = 0.674 p < 0.001 | R = 0.689 p < 0.001 | R = 0.593 p < 0.001 | R = 0.323 p = 0.082 | R = −0.473 p = 0.008 |
| LVMI (g/m2) | R = 0.394 p = 0.031 | R = 0.489 p = 0.006 | R = 0.395 p = 0.031 | R = 0.399 p = 0.029 | R = 0.451 p = 0.012 | R = 0.422 p = 0.020 | R = 0.328 p = 0.080 | NS | R = −0.371 p = 0.043 |
| LVEF (%) | NS | NS | R = 0.394 p = 0.032 | R = 0.421 p = 0.020 | R = 0.459 p = 0.011 | R = 0.464 p = 0.010 | R = 0.457 p = 0.011 | R = 0.364 p = 0.048 | NS |
| E/É | NS | NS | NS | NS | R = −0.374 p = 0.055 | NS | NS | NS | NS |
| E/A | NS | NS | NS | NS | NS | R = 0.397 p = 0.033 | NS | NS | NS |
| Baseline Variable | Univariate Analysis | Multiple Analysis | |||
|---|---|---|---|---|---|
| ρ | p-Value | B | β | p-Value | |
| IGF-1 | 0.394 | 0.031 | 11.194 | 0.200 | 0.525 |
| HBA1c (%) | 0.371 | 0.043 | 27.846 | 0.118 | 0.258 |
| BMI (kg/m2) | 0.395 | 0.031 | −2.747 | −0.240 | 0.551 |
| BSA (m2) | 0.381 | 0.038 | −52.865 | −0.229 | 0.697 |
| Lean mass (kg) | 0.438 | 0.016 | −1.385 | −0.385 | <0.001 |
| LVEDd (mm) | 0.875 | <0.001 | 9.850 | 1.11 | <0.001 |
| Univariate Analysis | Multiple Analysis | ||||
|---|---|---|---|---|---|
| Variable | ρ | p-Value | B | β | p-Value |
| Δ IGF-1 | 0.218 | 0.342 | |||
| Δ HBA1c | 0.122 | 0.598 | |||
| Δ BMI | 0.392 | 0.072 | |||
| Δ BSA | 0.453 | 0.026 | −127.313 | −0.444 | <0.001 |
| Δ Lean mass | 0.484 | 0.018 | −3.081 | −0.298 | 0.027 |
| Δ LVEDd | 0.469 | 0.032 | |||
| Δ IVSD | 0.436 | 0.046 | |||
| Δ PWDd | 0.335 | 0.138 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ságová, I.; Bolek, T.; Dragula, M.; Péč, M.J.; Benko, J.; Jurica, J.; Tonhajzerová, I.; Kantárová, D.; Mokáň, M.; Vaňuga, P.; et al. Effects of Treatment on Structural and Functional Parameters of the Left Heart in Naïve Acromegaly Patients: Prospective Single-Centre Study: 12-Month Follow-Up. J. Clin. Med. 2025, 14, 3397. https://doi.org/10.3390/jcm14103397
Ságová I, Bolek T, Dragula M, Péč MJ, Benko J, Jurica J, Tonhajzerová I, Kantárová D, Mokáň M, Vaňuga P, et al. Effects of Treatment on Structural and Functional Parameters of the Left Heart in Naïve Acromegaly Patients: Prospective Single-Centre Study: 12-Month Follow-Up. Journal of Clinical Medicine. 2025; 14(10):3397. https://doi.org/10.3390/jcm14103397
Chicago/Turabian StyleSágová, Ivana, Tomáš Bolek, Milan Dragula, Martin Jozef Péč, Jakub Benko, Jakub Jurica, Ingrid Tonhajzerová, Daniela Kantárová, Marián Mokáň, Peter Vaňuga, and et al. 2025. "Effects of Treatment on Structural and Functional Parameters of the Left Heart in Naïve Acromegaly Patients: Prospective Single-Centre Study: 12-Month Follow-Up" Journal of Clinical Medicine 14, no. 10: 3397. https://doi.org/10.3390/jcm14103397
APA StyleSágová, I., Bolek, T., Dragula, M., Péč, M. J., Benko, J., Jurica, J., Tonhajzerová, I., Kantárová, D., Mokáň, M., Vaňuga, P., & Samoš, M. (2025). Effects of Treatment on Structural and Functional Parameters of the Left Heart in Naïve Acromegaly Patients: Prospective Single-Centre Study: 12-Month Follow-Up. Journal of Clinical Medicine, 14(10), 3397. https://doi.org/10.3390/jcm14103397





