Bleeding Complications of Anticoagulation Therapy Used in the Treatment of Acute Coronary Syndromes—Review of the Literature
Abstract
1. Introduction
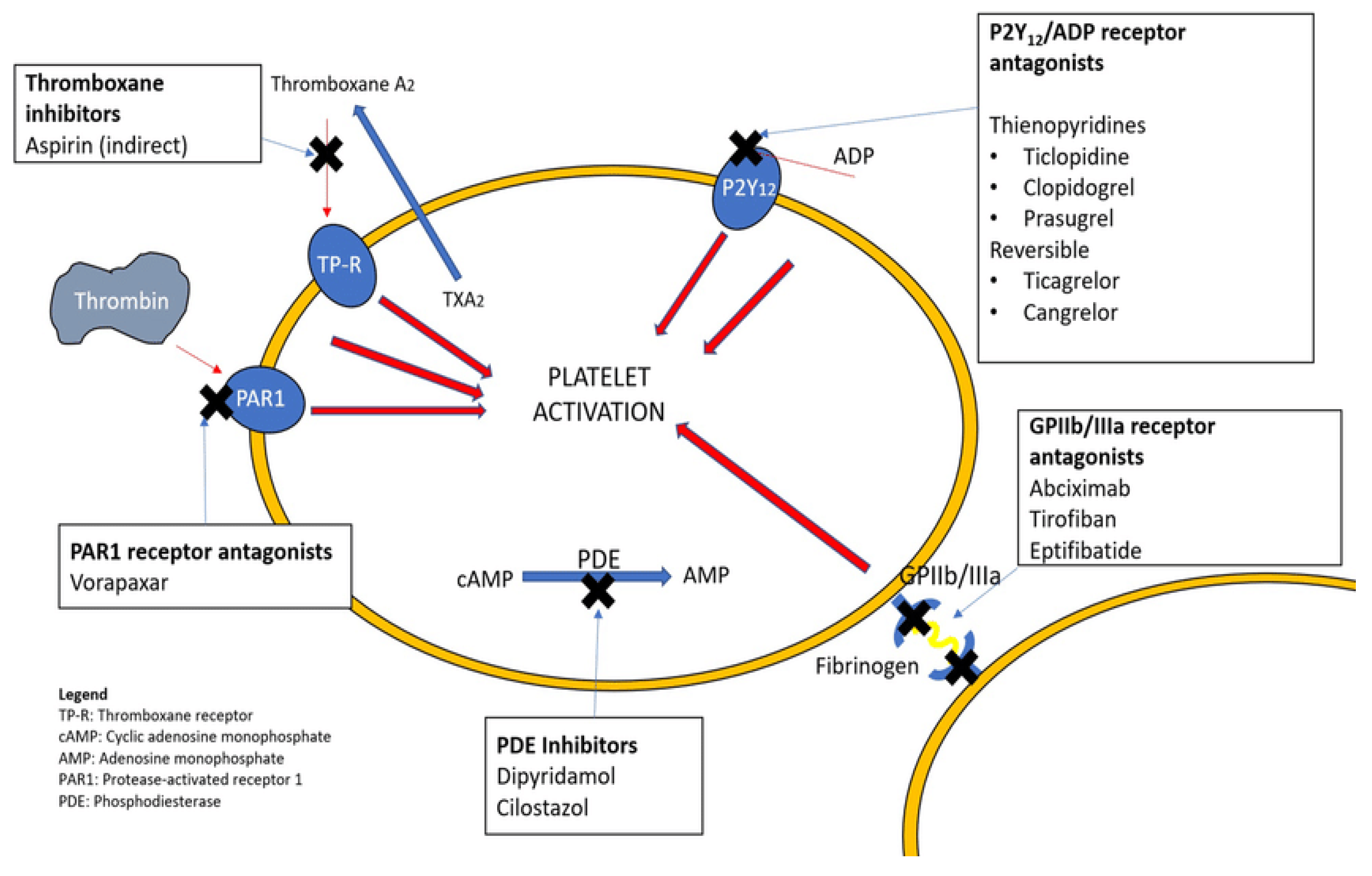
2. Antiplatelet Drugs
2.1. Mechanism of Action
- Platelet aggregation inhibitors:
- ○
- Aspirin and related cyclooxygenase inhibitors
- ○
- Oral thienopyridines, which are P2Y12 inhibitors such as clopidogrel, ticagrelor, ticlopidine, and prasugrel
- Glycoprotein platelet inhibitors (e.g., abciximab, eptifibatide, tirofiban)
- Protease-activated receptor-1 antagonists (e.g., vorapaxar)
- Miscellaneous (e.g., dipyridamole—a nucleoside transport inhibitor and phosphodiesterase type 3 [PDE3] inhibitor, cilostazol—a PDE3 inhibitor)
2.2. Risk of Bleeding
2.2.1. Oral Antiplatelet Drugs
2.2.2. Intravenous Antiplatelet Drugs—Review of Clinical Trials
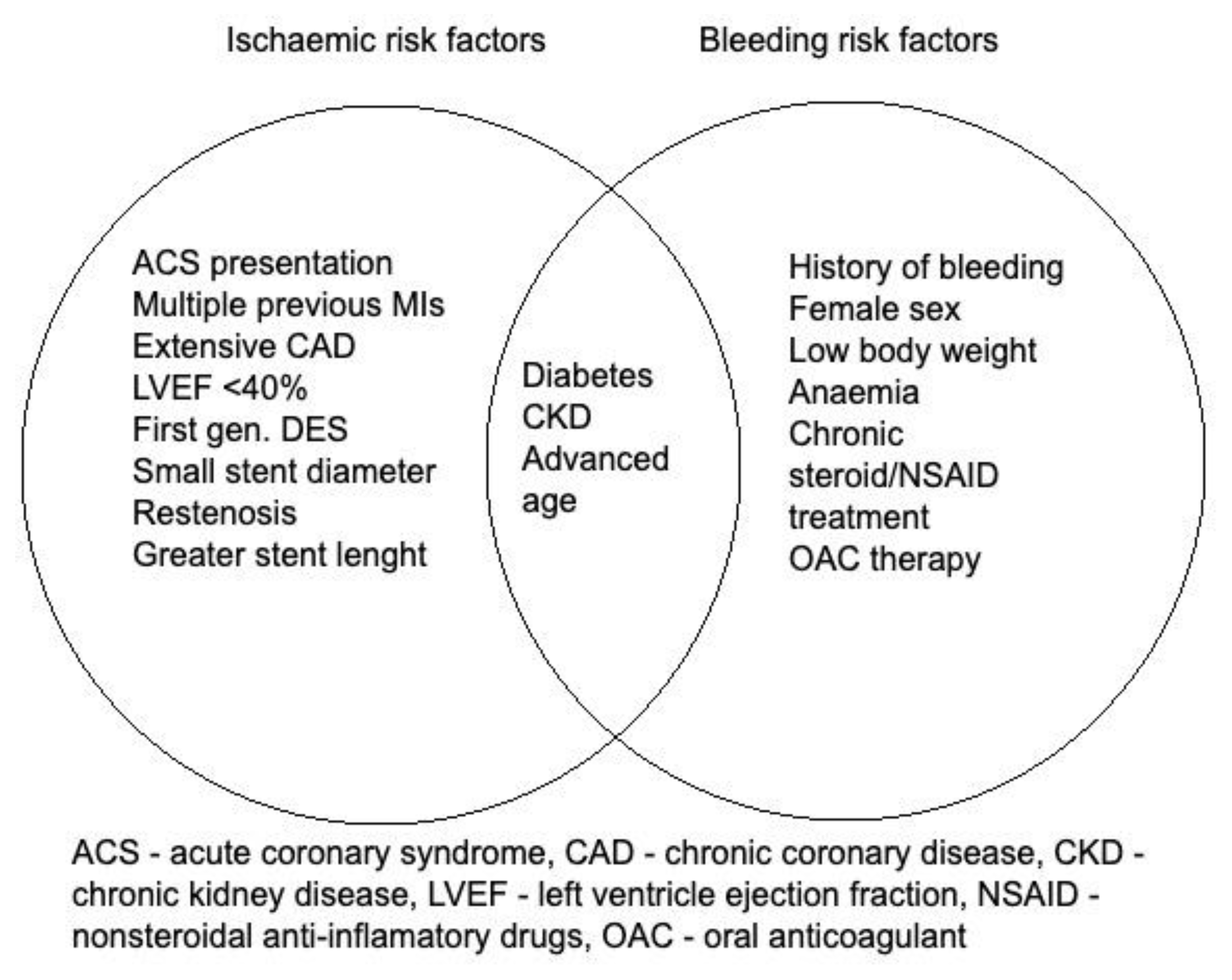
2.3. Special Group of Patients with High Bleeding Risk
3. Fibrinolytic Drugs
3.1. Mechanism of Action
3.2. Epidemiology of Bleeding Adverse Events After Using Fibrinolytics
3.3. Methods of Reducing the Risk of Bleeding in Patients Undergoing Fibrinolysis
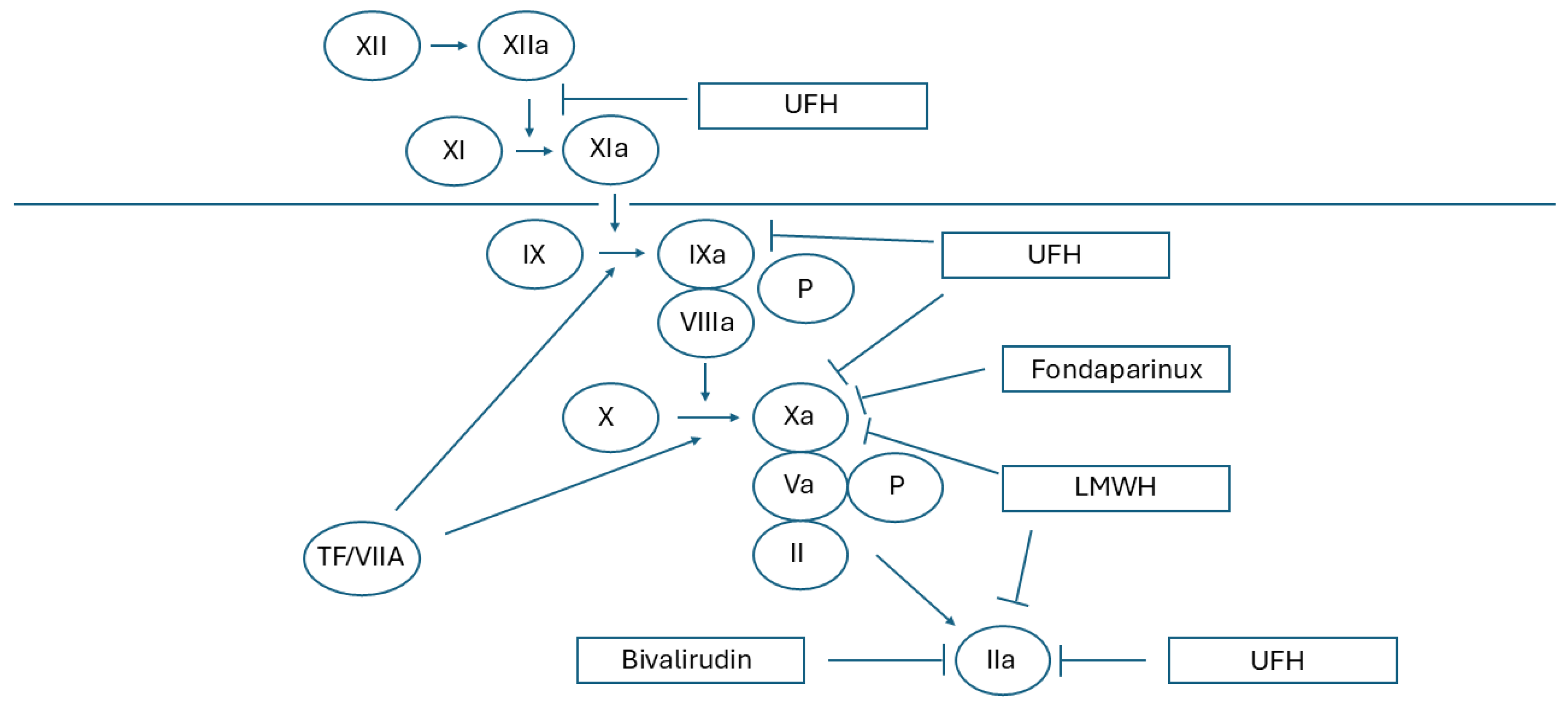
4. Heparins
4.1. Mechanism of Action
4.2. Risk of Bleeding
5. Prevention of Bleeding Complications After Anticoagulation Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vranckx, P.; White, H.D.; Huang, Z.; Mahaffey, K.W.; Armstrong, P.W.; Van de Werf, F.; Moliterno, D.J.; Wallentin, L.; Held, C.; Aylward, P.E.; et al. Validation of BARC bleeding criteria in patients with acute coronary syndromes: The TRACER trial. J. Am. Coll. Cardiol. 2016, 67, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Berger, P.B.; Mehilli, J.; Seyfarth, M.; Neumann, F.-J.; Schömig, A.; Kastrati, A. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: Appropriateness of including bleeding as a component of a quadruple end point. J. Am. Coll. Cardiol. 2008, 51, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Maingard, J.T.; Li, K.; Kok, H.K.; Barras, C.D.; Russell, J.H.; Hirsch, J.A.; Chandra, R.V.; Jhamb, A.; Thijs, V.; et al. Antiplatelet Drugs for Neurointerventions: Part 1 Clinical Pharmacology. Clin. Neuroradiol. 2020, 30, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, F.; Gragnano, F.; Pasceri, V.; Cesaro, A.; Zimarino, M.; Calabrò, P. Risk Scores of Bleeding Complications in Patients on Dual Antiplatelet Therapy: How to Optimize Identification of Patients at Risk of Bleeding after Percutaneous Coronary Intervention. J. Clin. Med. 2022, 11, 3574. [Google Scholar] [CrossRef]
- Costa, F.; van Klaveren, D.; James, S.; Heg, D.; Räber, L.; Feres, F.; Pilgrim, T.; Hong, M.K.; Kim, H.S.; Colombo, A.; et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: A pooled analysis of individual-patient datasets from clinical trials. Lancet 2017, 11, 1025–1034. [Google Scholar] [CrossRef]
- Natsuaki, M.; Morimoto, T.; Yamaji, K.; Watanabe, H.; Yoshikawa, Y.; Shiomi, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; et al. CREDO-Kyoto PCI/CABG Registry Cohort 2, RESET, and NEXT trial investigators. Prediction of Thrombotic and Bleeding Events After Percutaneous Coronary Intervention: CREDO-Kyoto Thrombotic and Bleeding Risk Scores. J. Am. Heart. Assoc. 2018, 7, e008708. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Abu-Assi, E.; Raposeiras-Roubín, S.; Henriques, J.P.; Saucedo, J.; González-Juanatey, J.R.; Wilton, S.B.; Kikkert, W.J.; Nuñez-Gil, I.; Ariza-Sole, A.; et al. BleeMACS: Rationale and design of the study. J. Cardiovasc. Med. 2016, 17, 744–749. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Tang, X.; Xian, Y.; Jiang, L.; Chen, J.; Gao, L.; Gao, Z.; Qiao, S.; Yang, Y.; et al. Prognostic Value of the PARIS Thrombotic Risk Score for 2-Year Mortality After Percutaneous Coronary Intervention. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029619853638. [Google Scholar] [CrossRef]
- García Rodríguez, L.A.; Martín-Pérez, M.; Hennekens, C.H.; Rothwell, P.M.; Lanas, A. Bleeding Risk with Long-Term Low-Dose Aspirin: A Systematic Review of Observational Studies. PLoS ONE 2016, 11, e0160046. [Google Scholar] [CrossRef]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar] [CrossRef]
- Gwon, H.C.; Hahn, J.Y.; Park, K.W.; Song, Y.B.; Chae, I.H.; Lim, D.S.; Han, K.R.; Choi, J.H.; Choi, S.H.; Kang, H.J.; et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: The efficacy of Xience/Promus versus Cypher to reduce late loss after stenting (excellent) randomized, multicenter study. Circulation 2012, 125, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Campo, G.; Monti, M.; Vranckx, P.; Percoco, G.; Tumscitz, C.; Castriota, F.; Colombo, F.; Tebaldi, M.; Fucà, G.; et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: A randomized multicenter trial. Circulation 2012, 125, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Kerneis, M.; Gibson, C.M.; Chi, G.; Mehran, R.; AlKhalfan, F.; Talib, U.; Pahlavani, S.; Mir, M.; Bode, C.; Halperin, J.L.; et al. Effect of procedure and coronary lesion characteristics on clinical outcomes among atrial fibrillation patients undergoing percutaneous coronary intervention: Insights from the PIONEER AF-PCI trial. JACC Cardiovasc. Interv. 2018, 11, 626–634. [Google Scholar] [CrossRef]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.H.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Eng. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N. Eng. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef]
- Kubica, J.; Adamski, P.; Dobrzycki, S.; Gajda, R.; Gąsior, M.; Gierlotka, M.; Jaguszewski, M.; Legutko, J.; Lesiak, M.; Navarese, E.P.; et al. CangrelorExpanding therapeutic options in patients with acute coronary syndrome. Cardiol. J. 2024, 31, 133–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatt, D.L.; Lincoff, A.M.; Gibson, C.M.; Stone, G.W.; McNulty, S.; Montalescot, G.; Kleiman, N.S.; Goodman, S.G.; White, H.D.; Mahaffey, K.W.; et al. Intravenous platelet blockade with cangrelor during PCI. N. Eng. J. Med. 2009, 361, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.A.; Stone, G.W.; McNulty, S.; White, H.D.; Lincoff, A.M.; Gibson, C.M.; Pollack, C.V., Jr.; Montalescot, G.; Mahaffey, K.W.; Kleiman, N.S.; et al. Platelet inhibition with cangrelor in patients undergoing PCI. N. Eng. J. Med. 2009, 361, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.; Stone, G.; Mahaffey, K.; Gibson, C.M.; Steg, P.G.; Hamm, C.W.; Price, M.J.; Leonardi, S.; Gallup, D.; Bramucci, E.; et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N. Eng. J. Med. 2013, 368, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Boersma, E.; Akkerhuis, K.M.; Théroux, P.; Califf, R.M.; Topol, E.J.; Simoons, M.L. Platelet glycoprotein IIb/IIIa receptor inhibition in non-ST-elevation acute coronary syndromes: Early benefit during medical treatment only, with additional protection during percutaneous coronary intervention. Circulation 1999, 100, 2045–2048. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jimenez, J.; Tricoci, P. Safety and efficacy of abciximab as an adjunct to percutaneous coronary intervention. Vasc. Health Risk Manag. 2010, 6, 39–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Califf, R.M.; Lincoff, A.M.; Tcheng, J.E.; Topol, E.J. An overview of the results of the EPIC trial. Eur. Heart J. 1995, 16, 43–49. [Google Scholar] [CrossRef]
- EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N. Eng. J. Med. 1997, 336, 1689–1696. [Google Scholar] [CrossRef]
- EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. The EPISTENT investigators. Evaluation of platelet IIb/IIIa inhibitor for stenting. Lancet 1998, 352, 87–92. [Google Scholar] [CrossRef]
- Capture Investigators. Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: The CAPTURE Study. Lancet 1997, 349, 1429–1435. [Google Scholar] [CrossRef]
- The PURSUIT Investigators. Stroke in patients with acute coronary syndromes. Incidence and outcomes in platelet glycoprotein IIb/IIIa in unstable angina: Receptor suppresion using integrilin therapy (PURSUIT) Trial. Circulation 1999, 99, 2371–2377. [Google Scholar] [CrossRef]
- The PRISM Study Investigators: A comparison of aspirin plus tyrofibane with aspirin plus heparin for unstable angina. N. Eng. J. Med. 1998, 338, 1498–1505. [CrossRef]
- The ESPRIT Investigators. Novel dosing regimen of eptifibatide in planned coronary stent implantation (ESPRIT): A randomised, placebo-controlled trial. Lancet 2000, 356, 2037–2044. [Google Scholar] [CrossRef]
- IMPACT-II Investigators. Randomized placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Lancet 1997, 349, 1422–1428. [Google Scholar] [CrossRef]
- Topol, E.J.; Moliterno, D.J.; Herrmann, H.C.; Powers, E.R.; Grines, C.L.; Cohen, D.J.; Cohen, E.A.; Bertrand, M.; Neumann, F.J.; Stone, G.W.; et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N. Eng. J. Med. 2001, 344, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Hong, M.K.; Shin, D.H.; Nam, C.M.; Kim, J.S.; Ko, Y.G.; Choi, D.; Kang, T.S.; Park, B.E.; Kang, W.C.; et al. A new strategy for discontinuation of dual antiplatelet therapy: The RESET trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J. Am. Coll. Cardiol. 2012, 60, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Feres, F.; Costa, R.A.; Abizaid, A.; Leon, M.B.; Marin-Neto, J.A.; Botelho, R.V.; King, S.B., 3rd; Negoita, M.; Liu, M.; de Paula, J.E.; et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: The OPTIMIZE randomized trial. JAMA 2013, 310, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.Y.; Song, Y.B.; Oh, J.H.; Chun, W.J.; Park, Y.H.; Jang, W.J.; Im, E.S.; Jeong, J.O.; Cho, B.R.; Oh, S.K.; et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: The SMART-CHOICE randomized clinical trial. JAMA 2019, 321, 2428–2437. [Google Scholar] [CrossRef]
- Mehran, R.; Baber, U.; Sharma, S.K.; Cohen, D.J.; Angiolillo, D.J.; Briguori, C.; Cha, J.Y.; Collier, T.; Dangas, G.; Dudek, D.; et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N. Eng. J. Med. 2019, 381, 2032–2042. [Google Scholar] [CrossRef]
- Kim, B.K.; Hong, S.J.; Cho, Y.H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.Y.; Cho, S.; Jeon, D.W.; et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: The TICO randomized clinical trial. JAMA 2020, 323, 2407–2416. [Google Scholar] [CrossRef]
- Giacoppo, D.; Matsuda, Y.; Fovino, L.N.; D’Amico, G.; Gargiulo, G.; Byrne, R.A.; Capodanno, D.; Valgimigli, M.; Mehran, R.; Tarantini, G. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: A systematic review and meta-analysis of randomized clinical trials. Eur. Heart J. 2021, 42, 308–319. [Google Scholar]
- Valgimigli, M.; Frigoli, E.; Heg, D.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.C.; Chevalier, B.; Onuma, Y.; et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N. Eng. J. Med. 2021, 385, 1643–1655. [Google Scholar] [CrossRef]
- Mehran, R.; Cao, D.; Angiolillo, D.J.; Bangalore, S.; Bhatt, D.L.; Ge, J.; Hermiller, J.; Makkar, R.R.; Neumann, F.J.; Saito, S.; et al. 3- or 1-month DAPT in patients at high bleeding risk undergoing everolimus-eluting stent implantation. JACC Cardiovasc. Interv. 2021, 14, 1870–1883. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, J.S.; Hong, S.J.; Lim, D.S.; Lee, S.Y.; Yun, K.H.; Park, J.K.; Kang, W.C.; Kim, Y.H.; Yoon, H.J.; et al. 1-month dual-antiplatelet therapy followed by aspirin monotherapy after polymer-free drug-coated stent implantation: One-Month DAPT Trial. JACC Cardiovasc. Interv. 2021, 14, 1801–1811. [Google Scholar] [CrossRef]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: The STOPDAPT-2 randomized clinical trial. JAMA 2019, 321, 2414–2427. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Montalto, C.; Branca, M.; Hong, S.J.; Watanabe, H.; Franzone, A.; Vranckx, P.; Hahn, J.Y.; Gwon, H.C.; Feres, F.; et al. Dual antiplatelet therapy duration after percutaneous coronary intervention in high bleeding risk: A meta-analysis of randomized trials. Eur. Heart J. 2023, 44, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Agbaedeng, T.A.; Noubiap, J.J.; Roberts, K.A.; Chew, D.P.; Psaltis, P.J.; Amare, A.T. Sex-Based Outcomes of Dual-Antiplatelet Therapy After Percutaneous Coronary Intervention: A Pairwise and Network Meta-Analysis. Drugs 2024, 84, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Jüni, P.; Hamm, C.; Steg, P.G.; Heg, D.; van Es, G.A.; McFadden, E.P.; Onuma, Y.; van Meijeren, C.; et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: A multicentre, open-label, randomised superiority trial. Lancet 2018, 392, 940–949. [Google Scholar] [PubMed]
- Gragnano, F.; Mehran, R.; Branca, M.; Franzone, A.; Baber, U.; Jang, Y.; Kimura, T.; Hahn, J.Y.; Zhao, Q.; Windecker, S.; et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after complex percutaneous coronary interventions. J. Am. Coll. Cardiol. 2023, 81, 537–552. [Google Scholar] [CrossRef]
- Verstraete, M.; Collen, D. Pharmacology of thrombolytic drugs. J. Am. Coll. Cardiol. 1986, 8, 33B–40B. [Google Scholar] [CrossRef]
- Tang, M.; Hu, C.; Lin, H.; Yan, H. Fibrinolytic drugs induced hemorrhage: Mechanisms and solutions. Blood Coagul. Fibrinol. 2023, 34, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Sabu, A. Fibrinolytic Enzymes for Thrombolytic Therapy. Adv. Exp. Med. Biol. 2019, 1148, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Marder, V.J.; Novokhatny, V. Direct fibrinolytic agents: Biochemical attributes, preclinical foundation and clinical potential. J. Thromb. Haemost. 2010, 8, 433–444. [Google Scholar] [CrossRef]
- Baharifar, H.; Khoobi, M.; Arbabi Bidgoli, S.; Amani, A. Preparation of PEG-grafted chitosan/streptokinase nanoparticles to improve biological half-life and reduce immunogenicity of the enzyme. Int. J. Biol. Macromol. 2020, 143, 181–189. [Google Scholar] [CrossRef]
- Zhang, N.; Li, C.; Zhou, D.; Ding, C.; Jin, Y.; Tian, Q.; Meng, X.; Pu, K.; Zhu, Y. Cyclic RGD functionalized liposomes encapsulating urokinase for thrombolysis. Acta Biomater. 2018, 70, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Hu, C.; Yu, W.; Hu, T. Conjugation with eight-arm PEG markedly improves the in vitro activity and prolongs the blood circulation of staphylokinase. Bioconjug. Chem. 2018, 29, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, A.; Takio, K.; Fujikawa, K. Localization of the binding site of tissue-type plasminogen activator to fibrin. J. Clin. Investig. 1986, 78, 163–169. [Google Scholar] [CrossRef] [PubMed]
- LeCouffe, N.E.; Kappelhof, M.; Treurniet, K.M.; Rinkel, L.A.; Bruggeman, A.E.; Berkhemer, O.A.; Wolff, L.; van Voorst, H.; Tolhuisen, M.L.; Dippel, D.W.J.; et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N. Eng. J. Med. 2021, 385, 1833–1844. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Liu, Y.; Xu, Y.; Lin, H.; Cheng, Y.; Li, J.; Meng, C.; Liang, M.; Yuan, C.; et al. Enhanced clot lysis by a single point mutation in a reteplase variant. Br. J. Haematol. 2022, 196, 1076–1085. [Google Scholar] [CrossRef]
- Warach, S.J.; Dula, A.N.; Milling, T.J. Tenecteplase thrombolysis for acute ischemic stroke. Stroke 2020, 51, 3440–3451. [Google Scholar] [CrossRef]
- Mori, E.; Yoneda, Y.; Tabuchi, M.; Yoshida, T.; Ohkawa, S.; Ohsumi, Y.; Kitano, K.; Tsutsumi, A.; Yamadori, A. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992, 42, 976–1976. [Google Scholar] [CrossRef]
- Ding, J.; Pan, L.; Hu, Y.; Rajah, G.B.; Zhou, D.; Bai, C.; Ya, J.Y.; Wang, Z.A.; Jin, K.X.; Guan, J.W.; et al. Batroxobin in combination with anticoagulation may promote venous sinus recanalization in cerebral venous thrombosis: A real-world experience. CNS Neurosci. Ther. 2019, 25, 638–646. [Google Scholar] [CrossRef]
- Bourgain, R.H.; Six, F. The effect of defibrase on arterial thrombus formation. Thromb. Res. 1975, 6, 195–200. [Google Scholar] [CrossRef]
- Gasmi, A.; Chabchoub, A.; Guermazi, S.; Karoui, H.; Elayeb, M.; Dellagi, K. Further characterization and thrombolytic activity in a rat model of a fibrinogenase from vipera lebetina venom. Thromb. Res. 1997, 86, 233–242. [Google Scholar] [CrossRef]
- Tillett, W.S.; Garner, R.L. The Fibrinolytic Activity of Hemolytic Streptococci. J. Exp. Med. 1933, 58, 485–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, A.J.; Tillett, W.S. The lysis in rabbits of intravascular blood clots by the streptococcal fibrinolytic system (streptokinase). J. Exp. Med. 1952, 95, 449–464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sherry, S. The fibrinolytic activity of streptokinase activated human plasmin. J. Clin. Investing 1954, 33, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fletcher, A.P.; Alkjaersig, N.; Smyrniotis, F.E.; Sherry, S. The treatment of patients suffering from early myocardial infarction with massive and prolonged streptokinase therapy. Trans. Assoc. Am. Physicians 1958, 71, 287. [Google Scholar] [PubMed]
- Ruegsegger, P.; Nydick, I.; Hutter, R.C.; Freiman, A.H.; Bang, N.U.; Cliffton, E.E.; Ladue, J.S. Fibrinolytic (plasmin) therapy of experimental coronary thrombi with alteration of the evolution of myocardial infarction. Circulation 1959, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. GISSI-2: A factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Lancet 1990, 336, 65. [Google Scholar] [PubMed]
- Armstrong, P.W.; Gershlick, A.H.; Goldstein, P.; Wilcox, R.; Danays, T.; Lambert, Y.; Sulimov, V.; Rosell Ortiz, F.; Ostojic, M.; Welsh, R.C.; et al. Fibrinolysis or primary PCI in ST-segment elevation myocardial infarction. N. Eng. J. Med. 2013, 368, 1379–1387. [Google Scholar] [CrossRef]
- Roule, V.; Ardouin, P.; Blanchart, K.; Lemaitre, A.; Wain-Hobson, J.; Legallois, D.; Alexandre, J.; Sabatier, R.; Milliez, P.; Beygui, F. Prehospital fibrinolysis versus primary percutaneous coronary intervention in ST-elevation myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Pan, Y.; Zhou, L.; Wang, Y. Low-dose rt-PA may not decrease the incidence of symptomatic intracranial haemorrhage in patients with high risk of symptomatic intracranial haemorrhage. Neurol. Res. 2019, 41, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.; Schulman, S.; Witt, D.M.; Vandvik, P.O.; Fish, J.; Kovacs, M.J.; Svensson, P.J.; Veenstra, D.L.; Crowther, M.; Guyatt, G.H. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e152S–e184S. [Google Scholar] [CrossRef] [PubMed]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [PubMed]
- Warnock, L.B.; Huang, D. Heparin; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Solari, F.; Varacallo, M. Low-Molecular-Weight Heparin (LMWH). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001, 21, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Walenga, J.M.; Fareed, J.; Jeske, W.P.; Bıck, R.L.; Samama, M.M. Development of a Synthetic Heparin Pentasaccharide: Fondaparinux. Turk. J. Haematol. 2002, 19, 137. [Google Scholar] [PubMed]
- Kumar, A.; Talwar, A.; Farley, J.F.; Muzumdar, J.; Schommer, J.C.; Balkrishnan, R.; Wu, W. Fondaparinux Sodium Compared with Low-Molecular-Weight Heparins for Perioperative Surgical Thromboprophylaxis: A Systematic Review and Meta-analysis. J. Am. Heart. Assoc. 2019, 8, e012184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omerovic, E.; James, S.; Råmundal, T.; Fröbert, O.; Linder, R.; Danielewicz, M.; Hamid, M.; Pagonis, C.; Henareh, L.; Wagner, H.; et al. Bivalirudin versus heparin in ST and non-ST-segment elevation myocardial infarction-Outcomes at two years. Cardiovasc. Revasc. Med. 2024, 26, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Guo, J.; Wang, X.; Wang, G.; Fan, Z.; Yin, D.; Wang, Z.; Zhang, F.; Tian, C.; Gong, W.; et al. RIGHT Investigators. Postprocedural Anticoagulation After Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction: A Multicenter, Randomized, Double-Blind Trial. Circulation 2024, 149, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xie, T.; Chen, Y.; Zhou, Y.; Han, X. The Effects of Bivalirudin and Ordinary Heparin on the Incidence of Bleeding Events and the Level of Inflammation after Interventional Therapy for Acute Myocardial Infarction. Altern. Ther. Health Med. 2024, 24, AT10782. [Google Scholar] [PubMed]
- Alturkmani, H.; Uretsky, B.; Patel, S.; Albadaineh, M.N.; Alqaisi, O.; Alaiwah, M.; Cross, M.; Abbasi, D.; Rollefson, W. Safety and Efficacy of Enoxaparin During Low-Risk Elective Percutaneous Coronary Intervention. Am. J. Cardiol. 2024, 218, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Shaik, M.; Yuan, J. Choosing between enoxaparin and fondaparinux for the management of patients with acute coronary syndrome: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 116. [Google Scholar]
- Qiao, J.; Zhang, X.; Zhang, J.; Li, P.; Xu, B.; Wang, S.; Jiang, H.; Shen, Y.; Wang, K. Comparison between fondaparinux and low-molecular-weight heparin in patients with acute coronary syndrome: A meta-analysis. Cardiology 2016, 133, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Imberti, J.F.; Mei, D.A.; Vitolo, M.; Bonini, N.; Proietti, M.; Potpara, T.; Lip, G.Y.H.; Boriani, G. Comparing atrial fibrillation guidelines: Focus on stroke prevention, bleeding risk assessment and oral anticoagulant recommendations. Eur. J. Intern. Med. 2022, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Choi, S.Y.; Kim, M.H.; Serebruany, V. CRUSADE Score is Superior to Platelet Function Testing for Prediction of Bleeding in Patients Following Coronary Interventions. eBioMedicine 2017, 21, 213–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorog, D.A.; Gue, Y.X.; Chao, T.F.; Fauchier, L.; Ferreiro, J.L.; Huber, K.; Konstantinidis, S.V.; Lane, D.A.; Marin, F.; Oldgren, J.; et al. Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: A Position Paper from the ESC Working Group on Thrombosis, in collaboration with the European Heart Rhythm Association, the Association for Acute CardioVascular Care and the Asia-Pacific Heart Rhythm Society. Europace 2022, 24, 1844–1871. [Google Scholar] [CrossRef]
- Lip, G.Y.; Proietti, M.; Potpara, T.; Mansour, M.; Savelieva, I.; Tse, H.F.; Goette, A.; Camm, A.J.; Blomstrom-Lundqvist, C.; Gupta, D.; et al. Atrial fibrillation and stroke prevention: 25 years of research at EP Europace journal. Europace 2023, 25, euad226. [Google Scholar] [CrossRef]
- Shin, D.G.; Kim, S.; Kim, Y.R. Bleeding risk in patients with atrial fibrillation treated with combined anti-platelet and non-vitamin K antagonist oral anticoagulant therapy. Rev. Cardiovasc. Med. 2022, 23, 2. [Google Scholar] [CrossRef]
- Jiménez Díaz, V.A.; Hovasse, T.; Íñiguez, A.; Copt, S.; Byrne, J.; Brunel, P.; Morice, M.C.; Abizaid, A.; Tespilli, M.; Walters, D.; et al. Impact of vascular access on outcome after PCI in patients at high bleeding risk: A pre-specified sub-analysis of the LEADERS FREE trial. Rev. Esp. Cardiol. 2020, 73, 536. [Google Scholar] [CrossRef] [PubMed]
- Borlich, M.; Zeymer, U.; Wienbergen, H.; Hobbach, H.P.; Cuneo, A.; Bekeredjian, R.; Ritter, O.; Hailer, B.; Hertting, K.; Hennersdorf, M.; et al. Impact of Access Site on Periprocedural Bleeding and Cerebral and Coronary Events in High-Bleeding-Risk Percutaneous Coronary Intervention: Findings from the RIVA-PCI Trial. Cardiol. Ther. 2024, 13, 89–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piran, S.; Schulman, S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood 2019, 133, 425–435. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Cryer, B.L.; Contant, C.F.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; Lapuerta, P.; Goldsmith, M.A.; Laine, L.; et al. Clopidogrel with or without omeprazole in coronary artery disease. N. Eng. J. Med. 2010, 363, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Cannon, C.P.; Cryer, B.L.; Liu, Y.; Hsieh, W.H.; Doros, G.; Cohen, M.; Lanas, A.; Schnitzer, T.J.; Shook, T.L.; et al. Efficacy and safety of proton-pump inhibitors in high-risk cardiovascular subsets of the COGENT trial. Am. J. Med. 2016, 129, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Y.; Siddiqui, W.J.; Alvarez, C.; Aggarwal, S.; Hasni, S.F.; Ahmad, A.; Eisen, H. Reduction in postpercutaneous coronary intervention angina in addition to gastrointestinal events in patients on combined proton pump inhibitors and dual antiplatelet therapy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 847–853. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Asad, Z.U.; Rahman, H.; Khan, M.S.; Saleem, M.A.; Arshad, A.; Nawaz, N.; Sattur, S.; Kaluski, E. Meta-analysis of efficacy and safety of proton pump inhibitors with dual antiplatelet therapy for coronary artery disease. Cardiovasc. Revasc. Med. 2019, 20, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Sehested, T.S.; Carlson, N.; Hansen, P.W.; Gerds, T.A.; Charlot, M.G.; Torp-Pedersen, C.; Køber, L.; Gislason, G.H.; Hlatky, M.A.; Fosbøl, E.L. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur. Heart J. 2019, 40, 1963–1970. [Google Scholar] [CrossRef]
- Spadafora, L.; Betti, M.; D’Ascenzo, F.; De Ferrari, G.; De Filippo, O.; Gaudio, C.; Collet, C.; Sabouret, P.; Agostoni, P.; Zivelonghi, C.; et al. Impact of In-Hospital Bleeding on Post-Discharge Therapies and Prognosis in Acute Coronary Syndromes. J. Cardiovasc. Pharmacol. 2022, 13, 10–97. [Google Scholar] [PubMed]
| Name | Mechanism of Action | Route of Administration | Recommended Dosage |
|---|---|---|---|
| Aspirin | antiplatelet drug COX (TXA2 inhibitor) | orally or intravenously | LD 150–300 mg orally or 75–250 mg i.v, followed by an oral MD of 75–100 mg * |
| Clopidogrel | antiplatelet drug, P2Y12 receptor inhibitor | Orally | LD of 300–600 mg orally, followed by an MD of 75 mg o.d. * |
| Prasugrel | antiplatelet drug, P2Y12 receptor inhibitor | Orally | LD of 60 mg orally, followed by an MD of 10 mg o.d. * |
| Ticagrelor | antiplatelet drug, P2Y12 receptor inhibitor | Orally | LD of 180 mg orally, followed by an MD of 90 mg b.i.d. * |
| Cangrelor | antiplatelet drug, P2Y12 receptor inhibitor | Intravenously | Bolus of 30 mcg/kg i.v. followed by 4 mcg/kg/min infusion for at least 2 h or the duration of the procedure * |
| Eptifibatide | antiplatelet drug, GP IIb/IIIa receptor inhibitor | Intravenously | Double bolus of 180 mcg/kg i.v. (given at a 10-min interval) followed by an infusion of 2.0 mcg/kg/min for up to 18 h. For CrCl 30–50 mL/min: first LD, 180 mcg/kg i.v. bolus (max 22.6 mg); maintenance infusion, 1 mcg/kg/min (max 7.5 mg/h) |
| Tirofiban | antiplatelet drug, GP IIb/IIIa receptor inhibitor | intravenously | Bolus of 25 mcg/kg i.v. over 3 min, followed by an infusion of 0.15 mcg/kg/min for up to 18 h. For CrCl ≤60 mL/min: LD, 25 mcg/kg i.v. over 5 min followed by a maintenance infusion of 0.075 mcg/kg/min continued for up to 18 h |
| UFH | anticoagulant drug | Intravenously | Initial treatment: i.v. bolus 70–100 U/kg followed by i.v. infusion titrated to achieve the aPTT of 60–80 s * |
| Enoxaparin | anticoagulant drug | Subcutaneously | Initial treatment: for treatment of ACS 1 mg/kg b.i.d. subcutaneously for a minimum of 2 days and continued until clinical stabilization. For CrCl below 30 mL per minute (by Cockcroft–Gault equation), the dosage should be reduced to 1 mg per kg o.d. |
| Bivalirudin | anticoagulant drug | Intravenously | During PPCI: 0.75 mg/kg i.v. bolus followed by i.v. infusion of 1.75 mg/kg/h for 4 h after the procedure. For CrCl below 30 mL/min (by Cockcroft–Gault equation), maintenance infusion should be reduced to 1 mg/kg/h. |
| Fondaparinux | Anticoagulant drug | Subcutaneously | Initial treatment: 2.5 mg/d subcutaneously. During PCI: A single bolus of UFH is recommended. Avoid if CrCl < 20 mL/min. |
| Study Name | Group Size | Drug | Conclusions | Complications |
|---|---|---|---|---|
| CHAMPION PLATFORM | 5362 | Cangrelor | Lower rate of stent thrombosis and all-cause mortality in cangrelor vs. placebo group | No statistical differences in the incidence of bleeding between the cangrelor vs. placebo group |
| CHAMPION PCI | 8877 | Cangrelor | No statistical differences in the mortality and myocardial infarction between the cangrelor vs. placebo group | Minor but not major bleeding occurred more often in the cangrelor vs. placebo group |
| CHAMPION PHOENIX | 10,942 | Cangrelor | Lower rate of all-cause death, myocardial infarction, ischemia-induced revascularization, or stent thrombosis within 48 h in cangrelor vs. placebo group | Higher risk of major bleeding or transfusion in cangrelor vs. placebo group |
| EPIC | 2099 | Abciximab | Reduction of death, nonfatal myocardial infarction, repeat revascularization in abciximab group | Significant increase in both major bleeding and transfusion events in the abciximab group |
| EPILOG | 2792 | Abciximab + LMWH | Reduction of death, nonfatal myocardial infarction, repeat revascularization in abciximab + LMWH group | Lower rate of major bleeding in abciximab + low-dose heparin group vs. standard-dose heparin groups |
| EPISTENT | 2399 | Abciximab | Reduction of death, nonfatal myocardial infarction, repeat revascularization in abciximab groups | No statistical differences in the incidence of bleeding |
| CAPTURE | 1265 | Abciximab | Reduction of death, nonfatal myocardial infarction, repeat revascularization in abciximab group in patients with unstable angina | No statistical differences in the incidence of bleeding |
| PURSUIT | 10,948 | Eptifibatide | Decrease of mortality and incidence of myocardial infarction in the group of patients undergoing coronary artery bypass grafting | No statistical differences in the incidence of bleeding |
| ESPRIT | 2064 | Eptifibatide | Reduction of death, nonfatal myocardial infarction, repeat revascularization in eptifibatide group | No statistical differences in the incidence of bleeding |
| IMPACT II | 4010 | Eptifibatide | Reduction of death, nonfatal myocardial infarction, and repeat revascularization in the eptifibatide group, regardless of the dose | No statistical differences in the incidence of bleeding |
| PRISM | 3232 | Tirofiban vs. heparin | Reduction of death, myocardial infarction or recurrent ischemia in the tirofiban group | No statistical differences in the incidence of bleeding |
| TARGET | 5308 | Abciximab vs. Tirofiban | Abciximab is superior than tirofiban | No statistical differences in the incidence of bleeding |
| Name | Types | Plasminogen Activation | Half-Life Time (Min) | References |
|---|---|---|---|---|
| Streptokinase | serine proteinase (plasminogen activator) | Indirect | 15–30 | [50] |
| Urokinase | serine proteinase (plasminogen activator) | Indirect | 15 | [51] |
| Staphylokinase | serine proteinase (plasminogen activator) | Indirect | 6 | [52] |
| Tissue-type plasminogen activator | serine proteinase (plasminogen activator) | Direct | 4–6 | [53] |
| Alteplase | serine proteinase (plasminogen activator) | Direct | 16 | [54] |
| Reteplase | serine proteinase (plasminogen activator) | Direct | 15–18 | [55] |
| Tenecteplase | serine proteinase (plasminogen activator) | Direct | 24 | [56] |
| Duteplase | serine proteinase (plasminogen activator) | Direct | 14–16 | [57] |
| Batroxobin | Metalloproteinase (plasmin) | No | 360 | [58] |
| Defibrase | Metalloproteinase (plasmin) | No | 180–360 | [59] |
| Fibrinogenase for Injection | Metalloproteinase (plasmin) | No | 150–250 | [60] |
| Absolute |
|---|
|
| Relavite |
|
| Risk Score | Risk Factors |
|---|---|
| HAS-BLED | systolic BP > 160 mm Hg; severe renal or hepatic disease; stroke; previous bleeding; labile INR; age > 65; use of antiplatelets or NSAIDs; alcohol excess |
| CRUSADE | heart rate; systolic BP; Hct; creatinine clearance; sex; signs of CHF at presentation; diabetes mellitus; history of vascular disease |
| ABC | age; biomarkers (Hb, hs-cTnT, GDF-15 or cystatin C); previous bleeding |
| ATRIA | anemia; severe renal disease; age ≥ 75; previous bleeding; hypertension |
| Alfalfa-MB | age > 65; previous bleeding; anemia; vascular disease; no PPI; use of antiplatelets or NSAIDs; use of rivaroxaban |
| HEMORRHAGES | hepatic/renal disease; ethanol abuse; malignancy; age > 75; low platelets; re-bleeding risk; hypertension; anemia; genetic factors; increased falls risk; stroke |
| ORBIT | age ≥ 75; reduced Hb/Hct/anemia; previous bleeding; reduced renal function; use of antiplatelets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowski, M.; Kocjan, M.; Mazurkiewicz, M.; Gamrot-Wrzoł, M.; Ryl, S.; Nowakowski, K.; Kawecki, J.; Kukulski, T.; Kawecki, D.; Morawiec-Migas, B. Bleeding Complications of Anticoagulation Therapy Used in the Treatment of Acute Coronary Syndromes—Review of the Literature. J. Clin. Med. 2025, 14, 3391. https://doi.org/10.3390/jcm14103391
Kosowski M, Kocjan M, Mazurkiewicz M, Gamrot-Wrzoł M, Ryl S, Nowakowski K, Kawecki J, Kukulski T, Kawecki D, Morawiec-Migas B. Bleeding Complications of Anticoagulation Therapy Used in the Treatment of Acute Coronary Syndromes—Review of the Literature. Journal of Clinical Medicine. 2025; 14(10):3391. https://doi.org/10.3390/jcm14103391
Chicago/Turabian StyleKosowski, Michał, Maciej Kocjan, Michalina Mazurkiewicz, Marta Gamrot-Wrzoł, Sabina Ryl, Krzysztof Nowakowski, Jakub Kawecki, Tomasz Kukulski, Damian Kawecki, and Beata Morawiec-Migas. 2025. "Bleeding Complications of Anticoagulation Therapy Used in the Treatment of Acute Coronary Syndromes—Review of the Literature" Journal of Clinical Medicine 14, no. 10: 3391. https://doi.org/10.3390/jcm14103391
APA StyleKosowski, M., Kocjan, M., Mazurkiewicz, M., Gamrot-Wrzoł, M., Ryl, S., Nowakowski, K., Kawecki, J., Kukulski, T., Kawecki, D., & Morawiec-Migas, B. (2025). Bleeding Complications of Anticoagulation Therapy Used in the Treatment of Acute Coronary Syndromes—Review of the Literature. Journal of Clinical Medicine, 14(10), 3391. https://doi.org/10.3390/jcm14103391






