Prognostic Accuracy of Nutritional Assessment Tools in Critically-Ill COVID-19 Patients
Abstract
1. Introduction
2. Methods
2.1. Patients Selection
2.2. Data Collection
2.2.1. Nutritional Risk Assessment Tools and Outcomes
mNUTRIC, NRS 2002, MUST
2.2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Characteristics of Patients
3.2. Analysis of Nutrition Assessment Tools
4. Independent Variables for Predicting ICU Mortality
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total (n = 397) | Survivors (n = 273) | Non-Survivors (n = 124) | p Value | |
|---|---|---|---|---|
| Age *, years | 65 [55–76] | 62 [54–73] | 70 [60–82] | <0.01 |
| Male sex, n (%) | 254 (64.0) | 171 (62.6) | 83 (66.9) | 0.24 |
| Comorbidities, n (%) | ||||
| Hypertension | 209 (52.6) | 134 (49.1) | 75 (60.1) | 0.02 |
| Diabetes | 136 (34.3) | 93 (34.1) | 43 (34.7) | 0.50 |
| Cardiac disease | 134 (33.8) | 81 (29.7) | 53 (42.7) | <0.01 |
| Malignancy | 77 (19.4) | 33 (12.1) | 44 (35.5) | <0.01 |
| Chronic lung disease | 66 (16.6) | 45 (16.5) | 21 (16.9) | 0.51 |
| Chronic kidney disease | 35 (8.8) | 21 (7.7) | 14(11.3) | 0.16 |
| ECOG * | 1 [0–2] | 1 [0–2] | 2 [1–3] | <0.01 |
| CFS * | 3 [2–6] | 3 [1–4] | 6 [4–7] | <0.01 |
| APACHE II score * | 15 [11–19] | 13 [10–17] | 20 [16–25] | <0.01 |
| Admission SOFA score * | 4 [2–5] | 3 [2–4] | 6 [4–8] | <0.01 |
| PaO2/FiO2 on admission * | 150 [113–226] | 165 [120–244] | 134 [105–183] | <0.01 |
| IMV on admission, n (%) | 162 (40.8) | 48 (17.6) | 114 (91.9) | <0.01 |
| Duration of IMV, days * | 12 [5–22] | 9 [5–22] | 13 [5–22] | 0.92 |
| AKI on admission, n (%) | 112 (28.2) | 51 (18.7) | 61 (49.2) | <0.01 |
| Septic shock on admission, n (%) | 54 (13.6) | 18 (6.6) | 36 (29.0) | <0.01 |
| BMI *, kg/m2 | 26.1 [24.0–29.4] | 26.5 [24.2–29.4] | 25.7 [23.4–29.3] | 0.09 |
| BMI classification, n (%) | ||||
| Underweight | 9 (2.3) | 4 (1.5) | 5 (4.0) | 0.11 |
| Normal | 141 (35.5) | 91 (33.3) | 50 (40.3) | 0.10 |
| Overweight | 162 (40.8) | 114 (41.8) | 48 (38.8) | 0.32 |
| Obese | 85 (21.4) | 64 (23.4) | 21 (16.9) | 0.09 |
| BMI < 25 kg/m2, n (%) | 164 (41.3) | 97 (35.5) | 67 (46.0) | 0.03 |
| mNUTRIC score * | 3 [2–5] | 3 [1–4] | 5 [4–6] | <0.01 |
| NRS 2002 * | 3 [3–4] | 3 [3–4] | 4 [3–4] | <0.01 |
| MUST score * | 2 [2–2] | 2 [2–2] | 2 [2–2] | <0.01 |
| ICU LOS *, days | 11 [6–19] | 9 [5–15] | 15 [8–25] | <0.01 |
| Hospital LOS *, days | 19 [12–31] | 13 [7–17] | 19 [12–28] | <0.01 |
| ICU mortality, n (%) | 124 (31.2) | |||
| 28 days mortality, n (%) | 96 (24.2) | |||
| Hospital mortality, n (%) | 133 (33.5) |
| Total (n = 397) | Survivors (n = 273) | Non-Survivors (n = 124) | p Value | |
|---|---|---|---|---|
| Haemoglobin, g/dL | 12.7 [10.5–14.0] (n = 397) | 12.9 [10.8–14.1] (n = 273) | 11.5 [9.7–13.6] (n = 124) | <0.01 |
| Platelet, (×103) | 189 [142–270] (n = 397) | 197 [154–275] (n = 273) | 171 [114–252] (n = 124) | 0.03 |
| Lymphocyte (×103) | 0.69 [0.45–1.06] (n = 397) | 0.73 [0.50–1.07] (n = 273) | 0.59 [0.35–0.98] (n = 124) | 0.01 |
| NLR | 8.8 [4.2–17.0] (n = 397) | 8.3 [4.0–13.7] (n = 273) | 12.3 [4.4–23.4] (n = 124) | 0.04 |
| Prealbumin, mg/dL | 11.6 [8.6–16.9] (n = 292) | 12.0 [9.0–18.0] (n = 190) | 11.0 [8.0–16.0] (n = 102) | <0.01 |
| Albumin, g/dL | 3.20 [2.80–3.54] (n = 397) | 3.36 [3.00–3.84] (n = 273) | 3.01 [2.59–3.44] (n = 124) | <0.01 |
| Total protein, g/dL | 6.20 [5.69–6.74] (n = 397) | 6.3 [5.8–9.8] (n = 273) | 6.0 [5.5–6.6] (n = 124) | 0.04 |
| Triglycerides, mg/dL | 134 [101–193] (n = 146) | 126 [100–193] (n = 114) | 146 [111–199] (n = 32) | 0.37 |
| BUN, mg/dL | 21.0 [14.7–31.8] (n = 397) | 18.7 [14.0–25.3] (n = 273) | 27.6 [18.0–53.7] (n = 124) | <0.01 |
| Creatinine, mg/dL | 0.86 [0.66–1.22] (n = 397) | 0.80 [0.64–1.06] (n = 273) | 1.03 [0.77–1.55] (n = 124) | <0.01 |
| CRP, mg/L | 8.0 [2.7–14.8] (n = 397) | 8.3 [2.2–14.9] (n = 273) | 7.8 [3.9–14.3] (n = 124) | 0.62 |
| IL-6, pg/mL | 42.0 [15.9–112.5] (n = 109) | 36 [13–94] (n = 79) | 65 [31–121] (n = 30) | 0.01 |
| Ferritin, mL/ng | 441 [194–915] (n = 397) | 421 [189–809] (n = 273) | 597 [213–1300] (n = 124) | <0.01 |
| Procalcitonin, ng/mL | 0.24 [0.09–0.81] (n = 397) | 0.19 [0.08–0.57] (n = 273) | 0.33 [0.14–1.79] (n = 124) | <0.01 |
References
- Correia, M.I.T.D.; Waitzberg, D.L. The Impact of Malnutrition on Morbidity, Mortality, Length of Hospital Stay and Costs Evaluated through a Multivariate Model Analysis. Clin. Nutr. 2003, 22, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Su Lim, L.; Ong, K.C.B.; Chan, Y.H.; Loke, W.C.; Ferguson, M.; Daniels, L. Malnutrition and Its Impact on Cost of Hospitalization, Length of Stay, Readmission and 3-Year Mortality. Clin. Nutr. 2012, 31, 345–350. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review. J. Parenter. Enter. Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults with COVID-19 in New York City: A Prospective Cohort Study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Halaçli, B.; Kaya, A.; Topeli, A. Critically Ill COVID-19 Patient. Turk. J. Med. Sci. 2020, 50, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK Patients in Hospital with Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet. Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Rahman, S.; Montero, M.T.V.; Rowe, K.; Kirton, R.; Kunik, F. Epidemiology, Pathogenesis, Clinical Presentations, Diagnosis and Treatment of COVID-19: A Review of Current Evidence. Expert. Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef]
- Bakouny, Z.; Hawley, J.E.; Choueiri, T.K.; Peters, S.; Rini, B.I.; Warner, J.L.; Painter, C.A. COVID-19 and Cancer: Current Challenges and Perspectives. Cancer. Cell 2020, 38, 629–646. [Google Scholar] [CrossRef]
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and Its Impact on 1590 Patients with COVID-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Singh, K.; Dhingra, S.; Charan, J.; Sharma, P.; Islam, S.; Jahan, D.; Iskandar, K.; Samad, N.; Haque, M. The Double Burden of the COVID-19 Pandemic and Polypharmacy on Geriatric Population—Public Health Implications. Ther. Clin. Risk. Manag. 2020, 16, 1007–1022. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Simbaña-Rivera, K.; Gómez- Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D.; et al. Clinical, Molecular, and Epidemiological Characterization of the SARS-CoV-2 Virus and the Coronavirus Disease 2019 (COVID-19), a Comprehensive Literature Review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef]
- Short, K.R.; Kedzierska, K.; van de Sandt, C.E. Back to the Future: Lessons Learned From the 1918 Influenza Pandemic. Front. Cell. Infect. Microbiol. 2018, 8, 343. [Google Scholar] [CrossRef]
- Czapla, M.; Juárez-Vela, R.; Gea-Caballero, V.; Zieliński, S.; Zielińska, M. The Association between Nutritional Status and In-Hospital Mortality of COVID-19 in Critically-Ill Patients in the ICU. Nutrients 2021, 13, 3302. [Google Scholar] [CrossRef]
- Martinuzzi, A.L.N.; Manzanares, W.; Quesada, E.; Reberendo, M.J.; Baccaro, F.; Aversa, I.; Kecskes, C.E.; Magnífico, L.; González, V.; Bolzico, D.; et al. Nutritional Risk and Clinical Outcomes in Critically Ill Adult Patients with COVID-19. Nutr. Hosp. 2021, 38, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Alikiaii, B.; Heidari, Z.; Fazeli, A.; Rahimi Varposhti, M.; Moradi Farsani, D.; Fattahpour, S.; Rafiee, S.; Bagherniya, M. Evaluation of the Effectiveness of the Nutritional Risk Screening System 2002 (NRS-2002) in COVID-19 Patients Admitted to the Intensive Care Unit. Int. J. Clin. Pract. 2021, 75, e14934. [Google Scholar] [CrossRef] [PubMed]
- Jia, H. Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and Inflammatory Lung Disease. Shock 2016, 46, 239–248. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Rahman, A.; Hasan, R.M.; Agarwala, R.; Martin, C.; Day, A.G.; Heyland, D.K. Identifying Critically-Ill Patients Who Will Benefit Most from Nutritional Therapy: Further Validation of the “Modified NUTRIC” Nutritional Risk Assessment Tool. Clin. Nutr. 2016, 35, 158–162. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z.; Ad Hoc ESPEN Working Group. Nutritional Risk Screening (NRS 2002): A New Method Based on an Analysis of Controlled Clinical Trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in Hospital Outpatients and Inpatients: Prevalence, Concurrent Validity and Ease of Use of the “Malnutrition Universal Screening Tool” (‘MUST’) for Adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Dhaliwal, R.; Jiang, X.; Day, A.G. Identifying Critically Ill Patients Who Benefit the Most from Nutrition Therapy: The Development and Initial Validation of a Novel Risk Assessment Tool. Crit. Care 2011, 15, R268. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Henry, J.; Ong, V.; Leong, C.S.-F.; Teh, A.L.; van Dam, R.M.; Kowitlawakul, Y. Association of Modified NUTRIC Score with 28-Day Mortality in Critically Ill Patients. Clin. Nutr. 2017, 36, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maciel, L.R.M.d.A.; Franzosi, O.S.; Nunes, D.S.L.; Loss, S.H.; Dos Reis, A.M.; Rubin, B.d.A.; Vieira, S.R.R. Nutritional Risk Screening 2002 Cut-Off to Identify High-Risk Is a Good Predictor of ICU Mortality in Critically Ill Patients. Nutr. Clin. Pract. 2019, 34, 137–141. [Google Scholar] [CrossRef]
- Vong, T.; Yanek, L.R.; Wang, L.; Yu, H.; Fan, C.; Zhou, E.; Oh, S.J.; Szvarca, D.; Kim, A.; Potter, J.J.; et al. Malnutrition Increases Hospital Length of Stay and Mortality among Adult Inpatients with COVID-19. Nutrients 2022, 14, 1310. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, S.; Mao, Z.; Wang, W.; Hu, H. Clinical Significance of Nutritional Risk Screening for Older Adult Patients with COVID-19. Eur. J. Clin. Nutr. 2020, 74, 876–883. [Google Scholar] [CrossRef]
- de Vries, M.C.; Koekkoek, W.K.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional Assessment of Critically Ill Patients: Validation of the Modified NUTRIC Score. Eur. J. Clin. Nutr. 2018, 72, 428–435. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P.; Endorsed by the ESPEN Council. ESPEN Expert Statements and Practical Guidance for Nutritional Management of Individuals with SARS-CoV-2 Infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines for Acute Kidney Injury. 2012. Available online: https://kdigo.org/guidelines/acute-kidney-injury/ (accessed on 16 April 2022).
- Body Mass Index—BMI. Available online: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/body-mass-index (accessed on 16 April 2022).
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Jensen, G.L.; Compher, C.; Sullivan, D.H.; Mullin, G.E. Recognizing Malnutrition in Adults: Definitions and Characteristics, Screening, Assessment, and Team Approach. JPEN J. Parenter. Enteral. Nutr. 2013, 37, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, B.; Dang, Q.; Chen, Z.; Zhou, Q.; Luo, H.; Yuan, W.; Sun, Z. Pathogenesis and Mechanism of Gastrointestinal Infection With COVID-19. Front. Immunol. 2021, 12, 674074. [Google Scholar] [CrossRef] [PubMed]
- Wełna, M.; Adamik, B.; Kübler, A.; Goździk, W. The NUTRIC Score as a Tool to Predict Mortality and Increased Resource Utilization in Intensive Care Patients with Sepsis. Nutrients 2023, 15, 1648. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G.; A.S.P.E.N. Board of Directors; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enteral. Nutr. 2009, 33, 277–316. [Google Scholar] [CrossRef]
- Mendes, R.; Policarpo, S.; Fortuna, P.; Alves, M.; Virella, D.; Heyland, D.K.; Portuguese NUTRIC Study Group. Nutritional Risk Assessment and Cultural Validation of the Modified NUTRIC Score in Critically Ill Patients-A Multicenter Prospective Cohort Study. J. Crit. Care 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Wang, N.; Wang, M.-P.; Jiang, L.; Du, B.; Zhu, B.; Xi, X.-M. Association between the Modified Nutrition Risk in Critically Ill (mNUTRIC) Score and Clinical Outcomes in the Intensive Care Unit: A Secondary Analysis of a Large Prospective Observational Study. BMC Anesthesiol. 2021, 21, 220. [Google Scholar] [CrossRef]
- Jeong, D.H.; Hong, S.-B.; Lim, C.-M.; Koh, Y.; Seo, J.; Kim, Y.; Min, J.-Y.; Huh, J.W. Comparison of Accuracy of NUTRIC and Modified NUTRIC Scores in Predicting 28-Day Mortality in Patients with Sepsis: A Single Center Retrospective Study. Nutrients 2018, 10, E911. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Tu, C.-Y.; Chen, C.-H.; Wang, Y.-T.; Chen, W.-C.; Fu, P.-K.; Chen, C.-M.; Lai, C.-C.; Kuo, L.-K.; Ku, S.-C.; et al. Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients 2021, 14, 198. [Google Scholar] [CrossRef]
- Chen, M.; Wang, S.-A.; Yang, J.; Bai, J.; Gu, J.; Luo, H.; Zhang, X.; Han, Y.; Shao, J.; Xu, Y.; et al. Association of Systemic Immune-Inflammation Index with Malnutrition among Chinese Hospitalized Patients: A Nationwide, Multicenter, Cross-Sectional Study. Front. Nutr. 2024, 11, 1375053. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Aguilar, R.; Malih, N.; Abbate, M.; Fresneda, S.; Yañez, A.; Bennasar-Veny, M. Validity of Nutrition Screening Tools for Risk of Malnutrition among Hospitalized Adult Patients: A Systematic Review and Meta-Analysis. Clin. Nutr. 2024, 43, 1094–1116. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Verma, S.; Shrivastava, P.; Saran, K.; Kumari, A.; Raj, K.; Kumar, A.; Ray, H.N.; Bhattacharya, P.K. Modified NUTRIC Score as a Predictor of All-Cause Mortality in Critically Ill Patients: A Systematic Review and Meta-Analysis. Indian J. Crit. Care Med. 2024, 28, 495–503. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kalaiselvan, M.S.; Renuka, M.K.; Arunkumar, A.S. Use of Nutrition Risk in Critically Ill (NUTRIC) Score to Assess Nutritional Risk in Mechanically Ventilated Patients: A Prospective Observational Study. Indian J. Crit. Care Med. 2017, 21, 253–256. [Google Scholar] [CrossRef]
- Cattani, A.; Eckert, I.C.; Brito, J.E.; Tartari, R.F.; Silva, F.M. Nutritional Risk in Critically Ill Patients: How It Is Assessed, Its Prevalence and Prognostic Value: A Systematic Review. Nutr. Rev. 2020, 78, 1052–1068. [Google Scholar] [CrossRef]
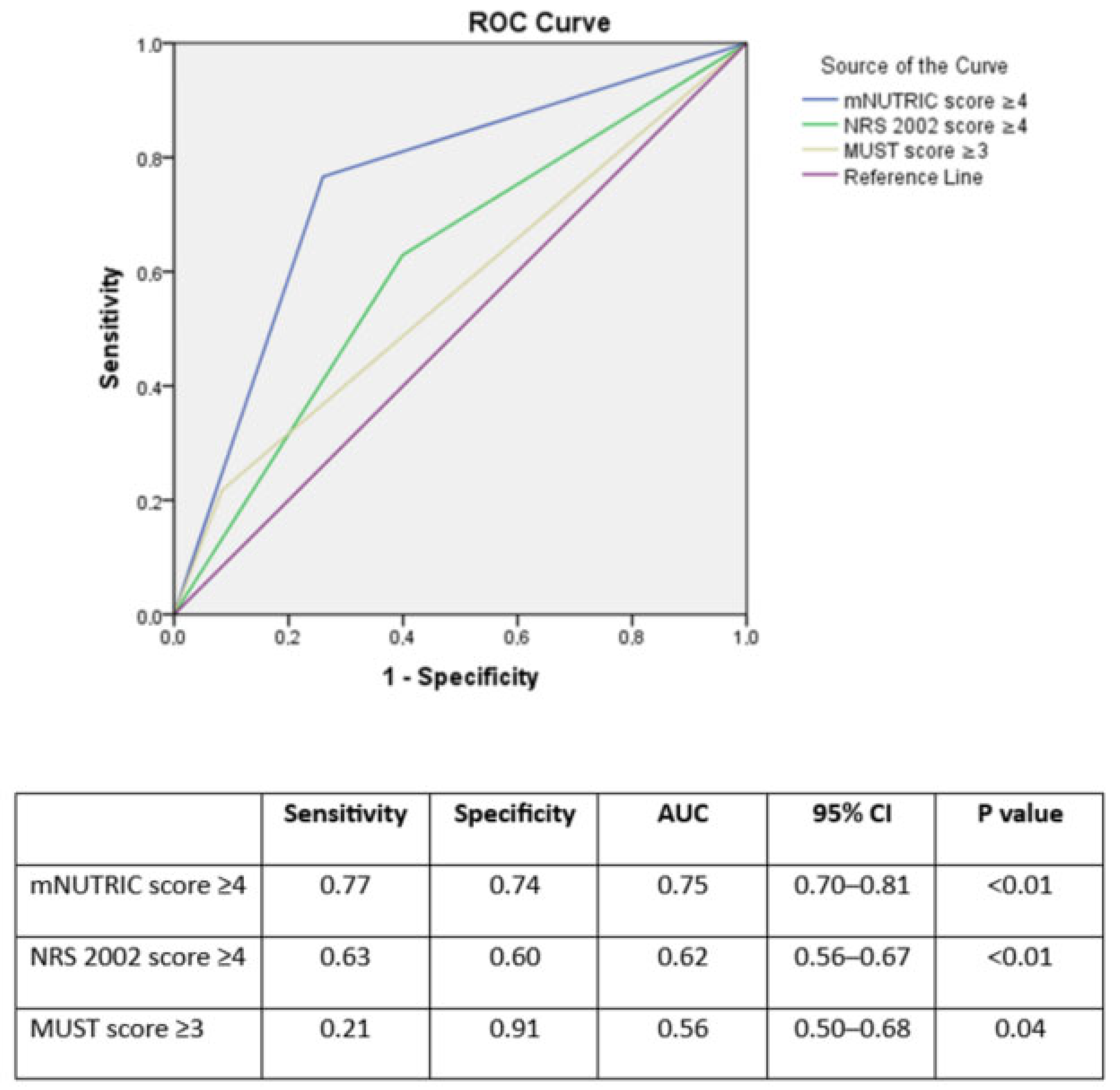
| Total (n = 397) | Patients at Malnutrition Risk According to mNUTRIC (mNUTRIC ≥ 5) (n = 103, 25.9%) | Patients at Malnutrition Risk According to NRS 2002 (NRS 2002 ≥ 4) (n = 187, 47.1%) | |
|---|---|---|---|
| Age *, years | 65 [55–76] | 77 [68–82] | 77 [71–82] |
| Male sex, n (%) | 254 (64.0) | 61 (59.2) | 117 (62.6) |
| Comorbidities, n (%) | |||
| Hypertension | 209 (52.6) | 75 (72.8) | 118 (63.1) |
| Diabetes | 136 (34.3) | 46 (44.7) | 72 (38.5) |
| Cardiac disease | 134 (33.8) | 59 (57.3) | 85 (45.5) |
| Malignancy | 77 (19.4) | 33 (32.0) | 48 (25.7) |
| Chronic lung disease | 66 (16.6) | 20 (19.4) | 35 (18.7) |
| Chronic kidney disease | 35 (8.8) | 19 (18.4) | 21 (11.2) |
| ECOG * | 1 [0–2] | 2 [2–3] | 2 [1–3] |
| CFS * | 3 [2–6] | 6 [4–7] | 5 [3–7] |
| APACHE II score * | 15 [11–19] | 22 [18–27] | 17 [14–21] |
| Admission SOFA score * | 4 [2–5] | 4 [3–6] | 4 [3–6] |
| PaO2/FiO2 on admission * | 150 [113–226] | 134 [101–187] | 154 [118–230] |
| IMV on admission, n (%) | 162 (40.8) | 77 (74.8) | 90 (48.1) |
| Duration of IMV, days * | 12 [5–22] | 12 [5–21] | 13 [7–22] |
| AKI on admission, n (%) | 112 (28.2) | 54 (52.4) | 64 (34.2) |
| Septic shock on admission, n (%) | 54 (13.6) | 28 (27.2) | 32 (17.1) |
| BMI *, kg/m2 | 26.1 [24.0–29.4] | 25.7 [23.1–29.3] | 25.5 [23.1–29.3] |
| BMI classification, n (%) | |||
| Underweight | 9 (2.3) | 5 (4.9) | 9 (4.8) |
| Normal | 141 (35.5) | 38 (36.9) | 79 (42.2) |
| Overweight | 162 (40.8) | 40 (38.8) | 65 (34.8) |
| Obese | 85 (21.4) | 20 (19.4) | 34 (18.2) |
| BMI < 25 kg/m2, n (%) | 164 (41.3) | 46 (44.7) | 90 (48.1) |
| ICU LOS *, days | 11 [6–19] | 14 [8–23] | 12 [6–20] |
| Hospital LOS *, days | 19 [12–31] | 23 [15–33] | 22 [13–32] |
| ICU mortality, n (%) | 124 (31.2) | 65 (63.1) | 78 (41.7) |
| 28 days mortality, n (%) | 96 (24.2) | 51 (49.5) | 62 (33.2) |
| Hospital mortality, n (%) | 133 (33.5) | 68 (66.0) | 84 (44.9) |
| mNUTRIC score * | 3 [2–5] | ||
| NRS 2002 * | 3 [3–4] | ||
| MUST score * | 4 [4–4] |
| Sensitivity | Specificity | p Value | |
|---|---|---|---|
| mNUTRIC ≥ 1 | 1.00 | 0.06 | <0.01 |
| mNUTRIC ≥ 2 | 0.99 | 0.26 | |
| mNUTRIC ≥ 3 | 0.90 | 0.49 | |
| mNUTRIC ≥ 4 | 0.77 | 0.74 | |
| mNUTRIC ≥ 5 | 0.52 | 0.86 | |
| mNUTRIC ≥ 6 | 0.35 | 0.60 | |
| mNUTRIC ≥ 7 | 0.19 | 0.99 | |
| mNUTRIC ≥ 8 | 0.08 | 0.99 | |
| mNUTRIC ≥ 9 | 0.04 | 1.00 | |
| mNUTRIC = 10 | 0.01 | 1.00 | |
| NRS 2002 ≥ 2 | 1.00 | 0.01 | <0.01 |
| NRS 2002 ≥ 3 | 0.88 | 0.21 | |
| NRS 2002 ≥ 4 | 0.63 | 0.60 | |
| NRS 2002 ≥ 5 | 0.17 | 0.45 | |
| NRS 2002 = 6 | 0.07 | 0.98 | |
| MUST ≥ 2 | 1.00 | 0.00 | 0.04 |
| MUST ≥ 3 | 0.21 | 0.91 | |
| MUST ≥ 4 | 0.11 | 0.95 | |
| MUST ≥ 5 | 0.08 | 0.95 | |
| MUST = 6 | 0.04 | 0.99 |
| mNUTRIC < 4 (n = 231) | mNUTRIC ≥ 4 (n = 166) | p Value | NRS 2002 < 4 (n = 210) | NRS 2002 ≥ 4 (n = 187) | p Value | MUST < 3 (n = 347) | MUST ≥ 3 (n = 50) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age *, years | 59 [51–67] | 75 [66–82] | <0.01 | 57 [51–63] | 77 [71–82] | <0.01 | 65 [55–76] | 66 [53–74] | 0.77 |
| Male sex, n (%) | 157 (68.0) | 97 (58.4) | 0.06 | 137 (65.2) | 117 (62.6) | 0.32 | 226 (65.1) | 28 (56.0) | 0.21 |
| ECOG * | 1 [0–2] | 2 [1–3] | <0.01 | 1 [0–2] | 2 [1–3] | <0.01 | 1 [0–2] | 2 [1–3] | <0.01 |
| CFS * | 2 [1–4] | 6 [4–7] | <0.01 | 2 [1–4] | 5 [3–7] | <0.01 | 3 [2–6] | 6 [3–7] | <0.01 |
| APACHE II score * | 12 [10–14] | 20 [17–24] | <0.01 | 13 [10–16] | 17 [14–21] | <0.01 | 14 [11–18] | 17 [14–21] | <0.01 |
| Admission SOFA score * | 3 [2–4] | 5 [4–8] | <0.01 | 3 [2–4] | 4 [3–6] | <0.01 | 4 [2–5] | 4 [3–7] | 0.02 |
| PaO2/FiO2 on admission * | 164 [120–240] | 138 [108–194] | <0.01 | 148 [111–225] | 154 [118–230] | 0.66 | 149 [112–225] | 156 [125–232] | 0.56 |
| BMI *, kg/m2 | 26.1 [24.2–29.4] | 26.0 [23.4–29.4] | 0.21 | 26.9 [24.6–29.5] | 25.5 [23.1–29.3] | <0.01 | 26.5 [24.3–29.5] | 21.2 [19.0–25.2] | <0.01 |
| BMI classification, n (%) | |||||||||
| Underweight | 3 (1.3) | 6 (3.6) | 0.49 | 0 (0) | 9 (4.8) | <0.01 | 0 (0) | 9 (18.0) | <0.01 |
| Normal | 82 (35.5) | 59 (35.5) | 0.42 | 62 (29.5) | 79 (42.2) | <0.01 | 113 (32.6) | 28 (56.0) | <0.01 |
| Overweight | 95 (41.1) | 67 (40.4) | 0.33 | 97 (46.2) | 65 (34.8) | <0.01 | 153 (44.1) | 9 (18.0) | <0.01 |
| Obese | 51 (22.1) | 34 (20.5) | 0.35 | 51 (24.3) | 34 (18.2) | <0.01 | 81 (23.3) | 4 (8.0) | 0.01 |
| BMI <25 kg/m2, n (%) | 86 (37.2) | 68 (41.0) | 0.25 | 64 (30.5) | 90 (48.1) | <0.01 | 117 (33.7) | 37 (74.0) | <0.01 |
| AKI on admission, n (%) | 36 (15.6) | 76 (45.8) | <0.01 | 48 (22.9) | 64 (34.2) | <0.01 | 96 (27.7) | 16 (32.0) | 0.52 |
| Septic shock on admission, n (%) | 19 (8.2) | 35 (21.1) | <0.01 | 22 (10.5) | 32 (17.1) | 0.03 | 38 (11.0) | 16 (32.0) | <0.01 |
| IMV on admission, n (%) | 54 (23.4) | 108 (65.1) | <0.01 | 72 (34.3) | 90 (48.1) | <0.01 | 134 (38.6) | 28 (56.0) | 0.02 |
| Duration of IMV, days * | 12 [5–22] | 12 [5–21] | 0.68 | 10 [4–21] | 13 [7–22] | <0.01 | 12 [6–21] | 11 [2–26] | 0.41 |
| ICU mortality, n (%) | 29 (12.6) | 95 (57.2) | <0.01 | 46 (21.9) | 78 (41.7) | <0.01 | 97 (28.0) | 27 (54.0) | <0.01 |
| 28 days mortality, n (%) | 21 (9.1) | 75 (45.2) | <0.01 | 34 (16.2) | 62 (33.2) | <0.01 | 76 (21.9) | 20 (40.0) | <0.01 |
| Hospital mortality, n (%) | 34 (14.7) | 99 (59.6) | <0.01 | 49 (23.3) | 84 (44.9) | <0.01 | 103 (29.7) | 30 (60.0) | <0.01 |
| ICU LOS *, days | 9 [5–15] | 14 [7–23] | <0.01 | 10 [6–18] | 12 [6–20] | 0.06 | 11 [6–19] | 10 [5–19] | 0.71 |
| Hospital LOS *, days | 17 [11–29] | 23 [15–35] | <0.01 | 17 [11–29] | 22 [13–32] | <0.01 | 19 [12–31] | 21 [12–39] | 0.28 |
| mNUTRIC < 4 (n = 231) | mNUTRIC ≥ 4 (n = 166) | p Value | NRS 2002 < 4 (n = 210) | NRS 2002 ≥ 4 (n = 187) | p Value | MUST < 3 (n = 347) | MUST ≥ 3 (n = 50) | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| Haemoglobin, g/dL | 13.1 [11.1–14.1] | 11.5 [9.8–13.4] | <0.01 | 13.0 [10.7–14.1] | 12.1 [10.1–13.7] | <0.01 | 12.9 [10.8–17.0] | 12.7 [10.5–14.0] | <0.01 |
| Platelet, (×103) | 190 [149–276] | 185 (129–256) | 0.05 | 191 [146–272] | 187 [137–265] | 0.48 | 192 [144–270] | 189 [142–270] | 0.06 |
| Lymphocyte (×103) | 0.7 [0.5–1.1] | 0.6 [0.4–1.0] | 0.08 | 0.7 [0.5–1.1] | 0.6 [0.4–1.0] | 0.11 | 0.7 [0.5–1.0] | 0.7 [0.5–1.1] | 0.55 |
| NLR | 8.5 [4.0–14.3] | 10.1 [4.4–20.0] | 0.07 | 8.1 [4.0–14.7] | 10.1 [4.5–19.5] | 0.06 | 8.8 [4.1–17.0] | 8.8 [4.1–17.0] | 0.97 |
| Prealbumin, mg/dL | 13 [9–18] (n = 157) | 11 [8–15] (n = 135) | <0.01 | 13 [10–18] (n = 141) | 11 [8–15] (n = 151) | <0.01 | 12 [9–17] (n = 124) | 11 [9–17] (n = 168) | 0.08 |
| Albumin, g/dL | 3.3 [3.0–3.6] | 3.0 [2.7–3.4] | <0.01 | 3.3 [3.0–3.6] | 3.1 [2.7–3.5] | <0.01 | 3.3 [2.9–3.6] | 3.2 [2.8–3.5] | 0.01 |
| Total protein, g/dL | 6.3 [5.8–6.8] | 6.1 [5.5–6.6] | <0.01 | 6.2 [5.7–6.7] | 6.2 [5.6–6.7] | 0.30 | 6.3 [5.8–6.8] | 6.2 [5.7–6.7] | 0.04 |
| BUN, mg/dL | 17.7 [13.8–23.1] | 28.7 [19.2–48.3] | <0.01 | 17.5 [13.5–24.7] | 24.6 [18.0–40.4] | <0.01 | 21.0 [14.6–31.6] | 21.0 [14.7–31.8] | 0.50 |
| Creatinine, mg/dL | 0.8 [0.6–1.0] | 1.1 [0.8–1.6] | 0.25 | 0.8 [0.7–1.0] | 1.0 [0.7–1.3] | <0.01 | 0.9 [0.7–1.2] | 0.9 [0.7–1.2] | 0.76 |
| CRP, mg/L | 7.7 [2.0–14.1] | 8.5 [3.9–15.8] | 0.05 | 7.8 [2.1–14.4] | 8.3 [3.3–15.0] | 0.51 | 8.2 [2.7–15.0] | 8.0 [2.7–14.8] | 0.65 |
| IL-6, pg/mL | 34 [12–90] (n = 65) | 56 [29–125] (n = 44) | 0.02 | 35 [15–114] (n = 56) | 55 [24–96] (n = 53) | 0.68 | 40 [16–100] (n = 63) | 42 [16–113] (n = 46) | 0.53 |
| Procalcitonin, ng/mL | 0.21 [0.10–0.45] | 0.47 [0.14–1.91] | <0.01 | 0.25 [0.10–0.67] | 0.32 [0.11–1.22] | <0.01 | 0.22 [0.09–0.72] | 0.25 [0.10–0.80] | 0.02 |
| Parameters | Odds Ratio (95% Confidence Interval) | p |
|---|---|---|
| A | ||
| Malignancy | 3.53 (1.45–8.58) | <0.01 |
| CFS | 1.23 (1.03–1.45) | 0.02 |
| IMV on admission | 44.12 (17.99–108.17) | <0.01 |
| mNUTRIC ≥ 4 | 1.49 (1.23–1.88) | 0.02 |
| B | ||
| Malignancy | 3.81 (1.55–9.34) | <0.01 |
| CFS | 1.24 (1.05–1.47) | <0.01 |
| IMV on admission | 43.21 (17.90–104.34) | <0.01 |
| NRS 2002 ≥ 4 | 1.03 (0.42–2.54) | 0.94 |
| C | ||
| Malignancy | 3.43 (1.39–8.47) | <0.01 |
| CFS | 1.23 (1.04–1.45) | 0.02 |
| IMV on admission | 43.31 (17.97–104.39) | <0.01 |
| MUST ≥ 3 | 1.90 (0.67–5.33) | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, M.; Halacli, B.; Kaya, E.K.; Ulusoydan, E.; Ortac Ersoy, E.; Topeli, A. Prognostic Accuracy of Nutritional Assessment Tools in Critically-Ill COVID-19 Patients. J. Clin. Med. 2025, 14, 3382. https://doi.org/10.3390/jcm14103382
Yildirim M, Halacli B, Kaya EK, Ulusoydan E, Ortac Ersoy E, Topeli A. Prognostic Accuracy of Nutritional Assessment Tools in Critically-Ill COVID-19 Patients. Journal of Clinical Medicine. 2025; 14(10):3382. https://doi.org/10.3390/jcm14103382
Chicago/Turabian StyleYildirim, Mehmet, Burcin Halacli, Esat Kivanc Kaya, Ege Ulusoydan, Ebru Ortac Ersoy, and Arzu Topeli. 2025. "Prognostic Accuracy of Nutritional Assessment Tools in Critically-Ill COVID-19 Patients" Journal of Clinical Medicine 14, no. 10: 3382. https://doi.org/10.3390/jcm14103382
APA StyleYildirim, M., Halacli, B., Kaya, E. K., Ulusoydan, E., Ortac Ersoy, E., & Topeli, A. (2025). Prognostic Accuracy of Nutritional Assessment Tools in Critically-Ill COVID-19 Patients. Journal of Clinical Medicine, 14(10), 3382. https://doi.org/10.3390/jcm14103382





