Advancing Maxillary Reconstruction: A Systematic Review and Meta-Analysis of the Evolving Role of the Scapular Free Flap
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Collection Process
Outcomes Measures
- Early General Surgical Complications.
- Fistula Formation.
- Donor Site Morbidity.
- Upper Limb Function using the DASH score.
- Dental Restoration.
- Diet.
- Aesthetic Compromise.
- Eye problems.
2.4. Risk of Bias and Study Quality Assessment
2.5. Data Synthesis and Analysis
3. Results
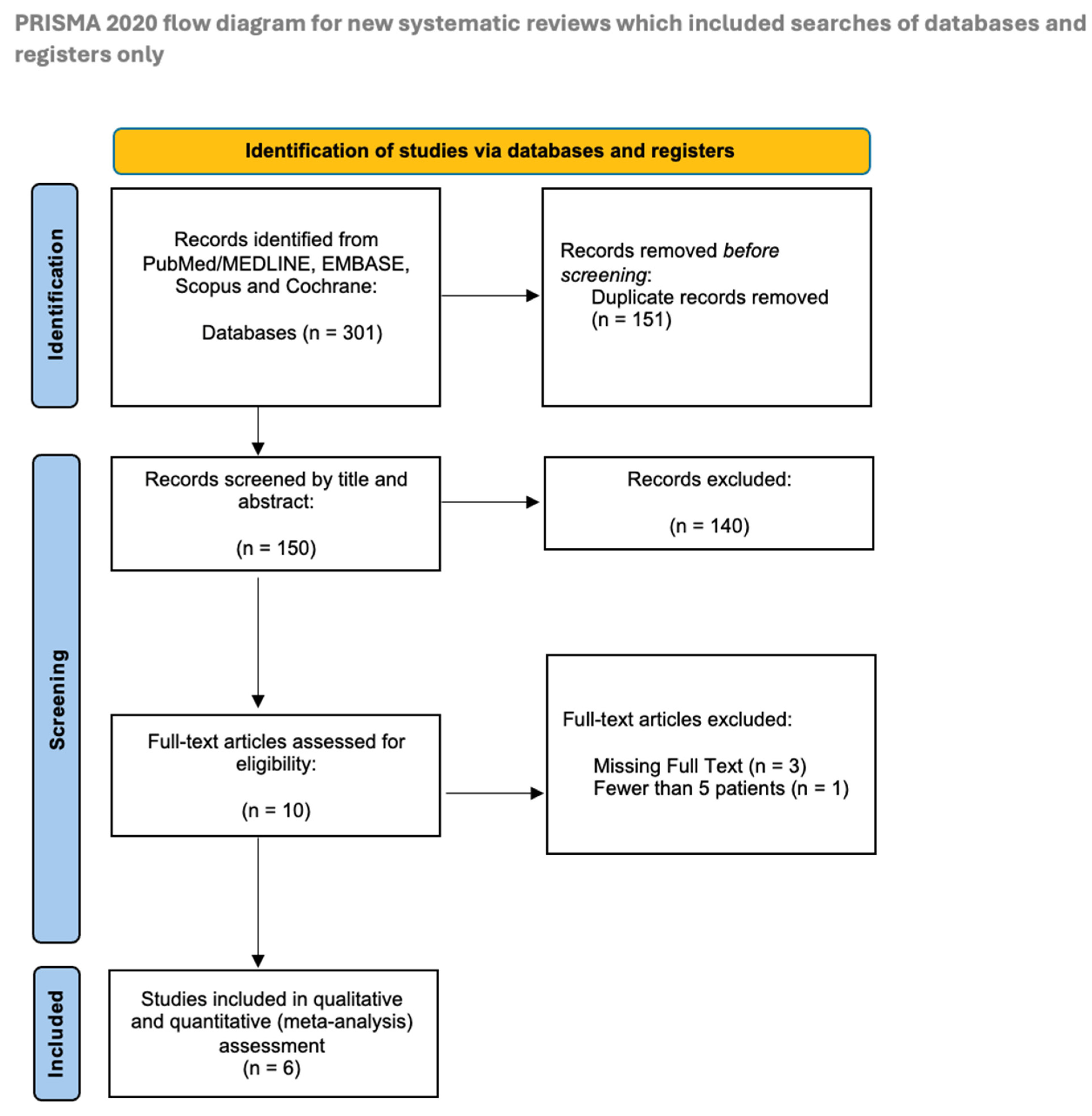
3.1. Study Selection
3.2. Description of the Studies
3.3. Study Results
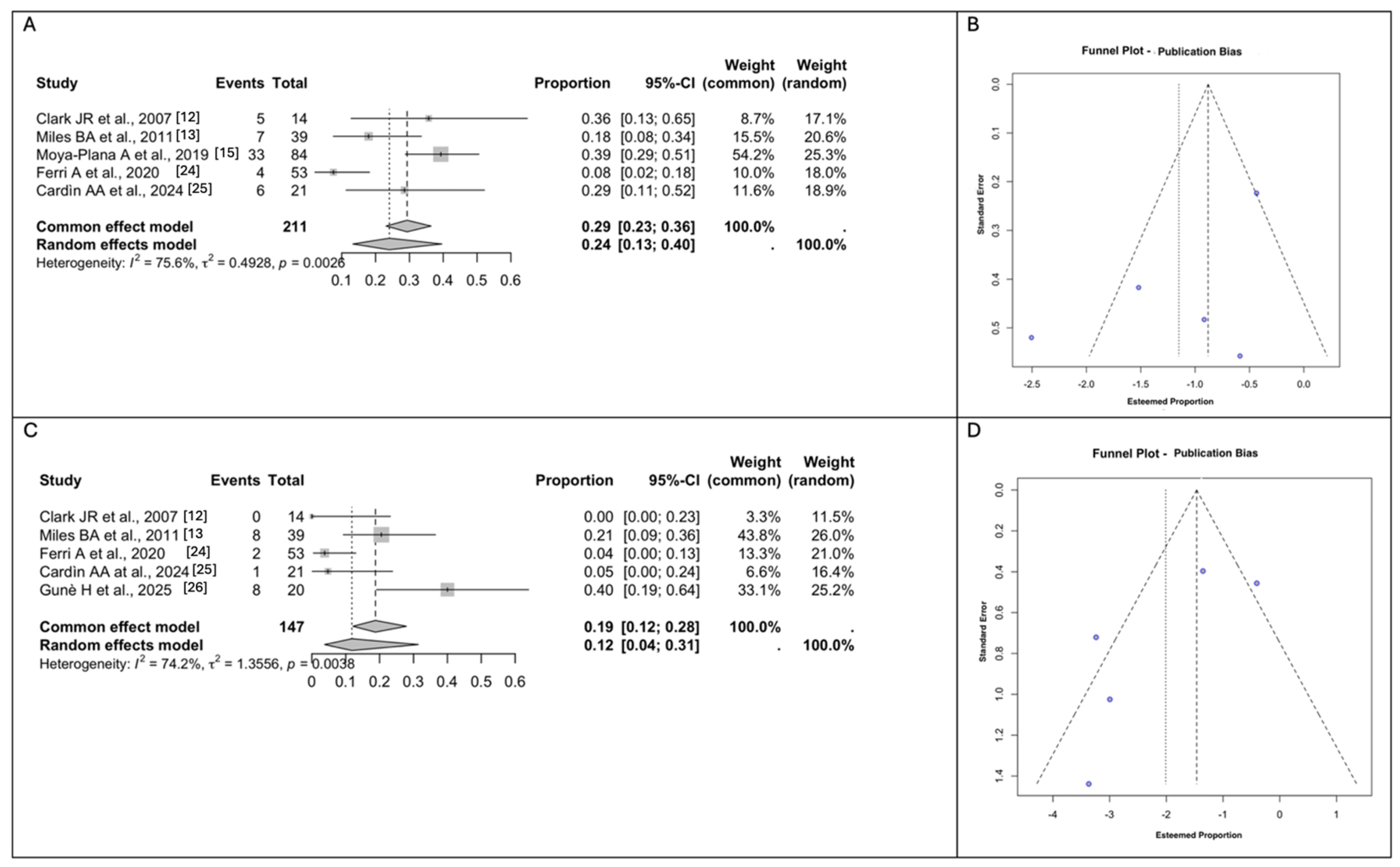
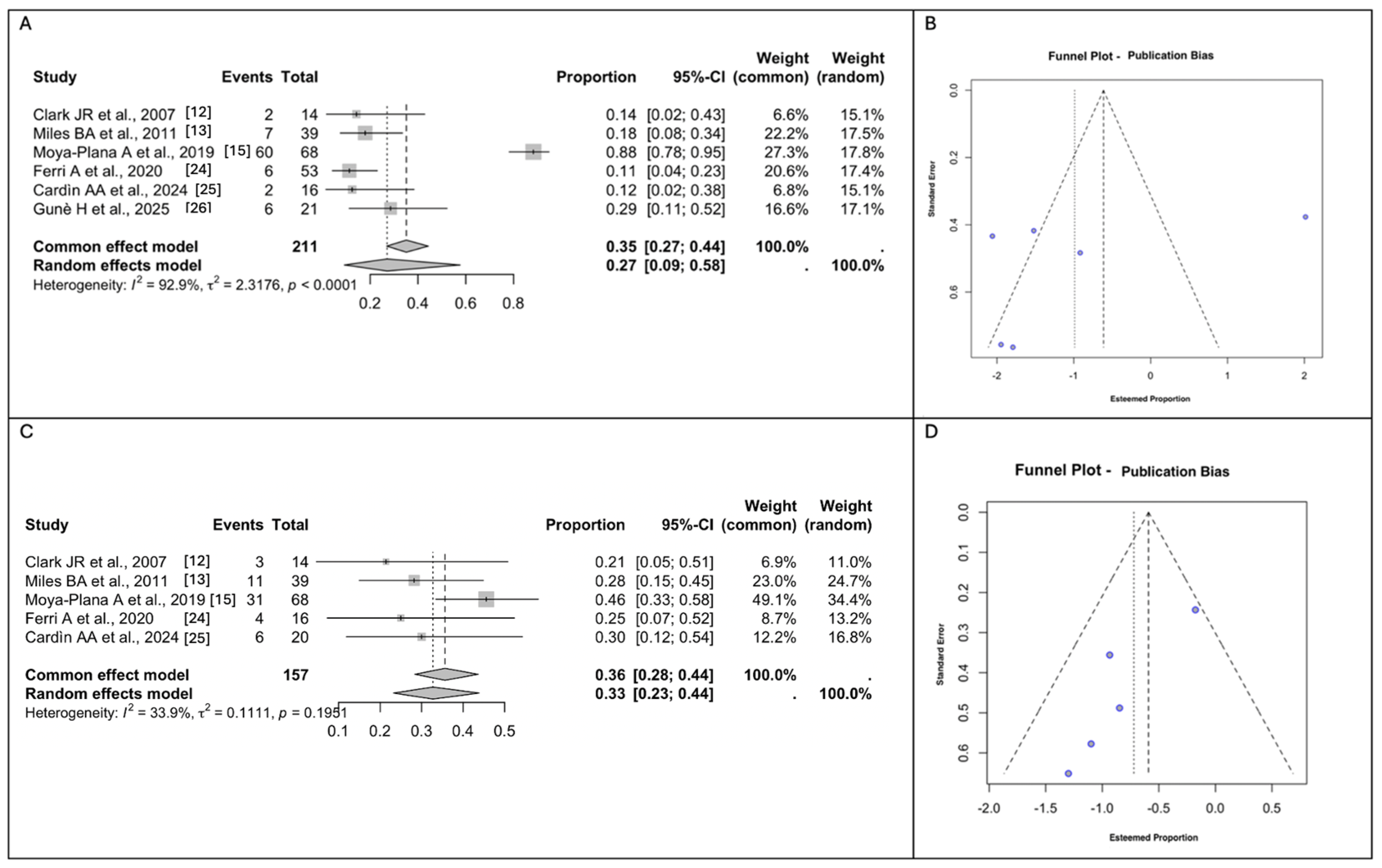
3.4. Meta-Analyses of the Surgical Outcomes and Complications
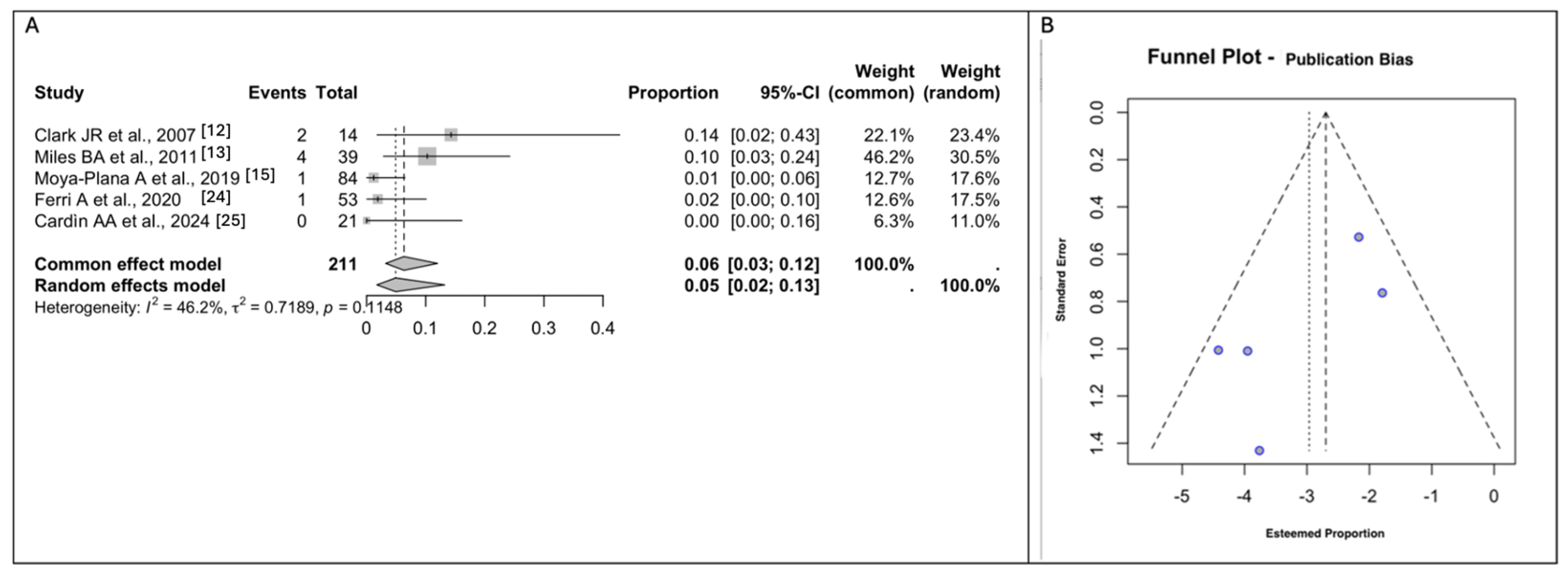
3.5. Meta-Analyses of Donor Site Morbidity
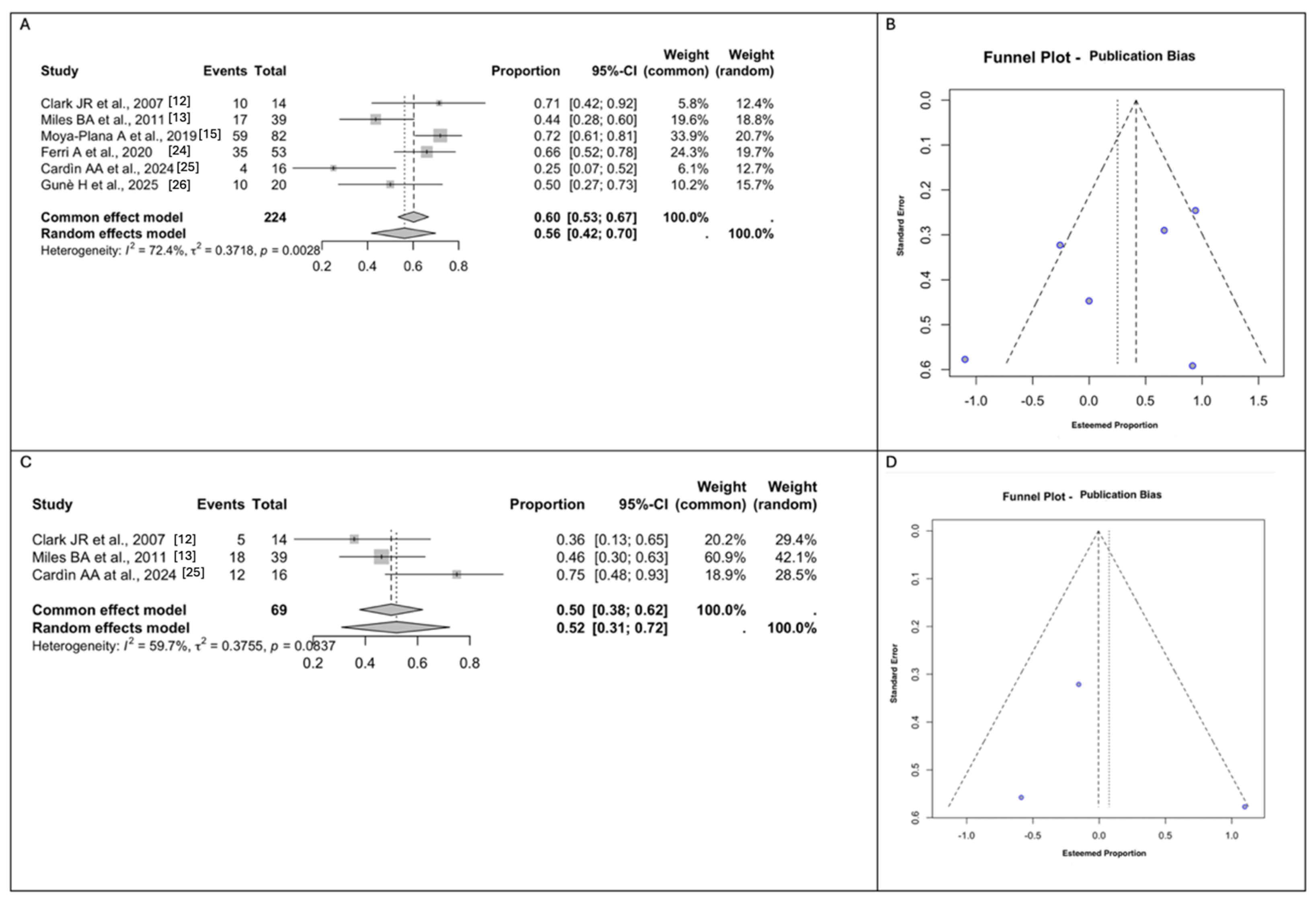
3.6. Meta-Analyses of Functional and Aesthetic Outcomes
3.7. Risk of Bias Assessment
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.S.; Shaw, R.J. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010, 11, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Dalgorf, D.; Higgins, K. Reconstruction of the midface and maxilla. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 303–311. [Google Scholar] [CrossRef]
- Futran, N.D.; Mendez, E. Developments in reconstruction of midface and maxilla. Lancet Oncol. 2006, 7, 249–258. [Google Scholar] [CrossRef]
- Lenox, N.D.; Kim, D.D. Maxillary reconstruction. Oral. Maxillofac. Surg. Clin. North Am. 2013, 25, 215–222. [Google Scholar] [CrossRef]
- Yetzer, J.; Fernandes, R. Reconstruction of orbitomaxillary defects. J. Oral Maxillofac. Surg. 2013, 71, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Genden, E.M. Reconstruction of the mandible and the maxilla: The evolution of surgical technique. Arch. Facial Plast. Surg. 2010, 12, 87–90. [Google Scholar] [CrossRef]
- Mücke, T.; Hölzle, F.; Loeffelbein, D.J.; Ljubic, A.; Kesting, M.; Wolff, K.-D.; Mitchell, D.A. Maxillary reconstruction using microvascular free flaps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, B.; Ferri, A.; Ferrari, S.; Copelli, C.; Boni, P.; Sesenna, E. Iliac crest free flap for maxillary reconstruction. J. Oral Maxillofac. Surg. 2010, 68, 2706–2713. [Google Scholar] [CrossRef]
- Chana, J.S.; Odili, J. Perforator flaps in head and neck reconstruction. Semin. Plast. Surg. 2010, 24, 237–254. [Google Scholar] [CrossRef]
- Wilkman, T.; Husso, A.; Lassus, P. Clinical comparison of scapular, fibular, and iliac crest osseal free flaps in maxillofacial reconstructions. Scand. J. Surg. 2019, 108, 76–82. [Google Scholar] [CrossRef]
- Davison, S.P.; Sherris, D.A.; Meland, N.B. An algorithm for maxillectomy defect reconstruction. Laryngoscope 1998, 108, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Vesely, M.; Gilbert, R. Scapular angle osteomyogenous flap in postmaxillectomy reconstruction: Defect, reconstruction, shoulder function, and harvest technique. Head Neck 2007. [CrossRef]
- Miles, B.A.; Gilbert, R.W. Maxillary reconstruction with the scapular angle osteomyogenous free flap. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 1130–1135. [Google Scholar] [CrossRef]
- Seitz, A.; Papp, S.; Papp, C.; Maurer, H. The anatomy of the angular branch of the thoracodorsal artery. Cells Tissues Organs 1999, 164, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Veyrat, M.; Honart, J.F.; de Fremicourt, K.; Alkhashnam, H.; Sarfati, B.; Janot, F.; Leymarie, N.; Temam, S.; Kolb, F. Reconstruction of maxillectomy and midfacial defects using latissimus dorsi-scapular free flaps in a comprehensive cancer center. Oral Oncol. 2019, 99, 104468. [Google Scholar] [CrossRef]
- Franchi, A.; Perozzo, F.A.G.; Tiengo, C.; Walber, J.; Parisato, A.; Jandali, A.R.; Jung, F. SCIP/SIEA and PAP: The new workhorse flaps in soft tissue reconstruction for all body regions. J. Clin. Med. 2025, 14, 921. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. The Upper Extremity Collaborative Group (UECG). Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 May 2025).
- Yoshioka, I.; Yamashita, Y.; Khanal, A.; Kodama, M.; Takahashi, T.; Tominaga, K. Maxillary reconstruction using a bipedicled osteocutaneous scapula flap. Int. J. Oral Maxillofac. Surg. 2009, 38, 1311–1315. [Google Scholar] [CrossRef]
- Granick, M.S.; Ramasastry, S.S.; Newton, E.D.; Solomon, M.P.; Hanna, D.C.; Kaltman, S. Reconstruction of complex maxillectomy defects with the scapular-free flap. Head Neck 1990, 12, 377–385. [Google Scholar] [CrossRef]
- Maier, E. Maxillary reconstruction with a scapula tip free flap and immediate implantation using virtual surgical planning and computer-aided design/computer-aided manufacturing technology. Int. J. Oral Maxillofac. Surg. 2024, 52, 71. [Google Scholar] [CrossRef]
- Leporati, M.; Ferrari, S.; Bianchi, B.; Ferri, A.; Copelli, C.; Sesenna, E. Scapular free flap for maxillary reconstruction. Int. J. Oral Maxillofac. Surg. 2011, 40, 1042. [Google Scholar] [CrossRef]
- Ferri, A.; Perlangeli, G.; Bianchi, B.; Zito, F.; Sesenna, E.; Ferrari, S. Maxillary reconstruction with scapular tip chimeric free flap. Microsurgery 2021, 41, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cardín, A.A.; Pereira, A.; Mendes, A.; Duarte, J.; Pereira, A. Maxillary reconstruction with horizontally oriented scapular tip free flap: Outcomes in orbital support and palate closure. J. Plast. Reconstr. Aesthetic Surg. 2024, 95, 221–230. [Google Scholar] [CrossRef]
- Guné, H.; Sjövall, J.; Becker, M.; Klasson, S. An evaluation of the scapular osseous free flap in maxillary reconstruction using the FACE-Q Head and Neck Cancer Module for patient-reported outcome measures. J. Plast. Surg. Hand Surg. 2025, 60, 21–26. [Google Scholar] [CrossRef]
- Okay, D.J.; Genden, E.; Buchbinder, D.; Urken, M. Prosthodontic guidelines for surgical reconstruction of the maxilla: A classification system of defects. J. Prosthet. Dent. 2001, 86, 352–363. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann Surg 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Paré, A.; Blanchard, P.; Rosellini, S.; Aupérin, A.; Gorphe, P.; Casiraghi, O.; Temam, S.; Bidault, F.; Page, P.; Kolb, F.; et al. Outcomes of multimodal management for sinonasal squamous cell carcinoma. J. Craniomaxillofac. Surg. 2017, 45, 1124–1132. [Google Scholar] [CrossRef]
- Gillespie, C.A.; Kenan, P.D.; Ferguson, B.J. Hard palate reconstruction in maxillectomy. Laryngoscope 1986, 96, 443–444. [Google Scholar] [CrossRef]
- Rogers, S.N.; Lowe, D.; McNally, D.; Brown, J.S.; Vaughan, E. Health-related quality of life after maxillectomy: A comparison between prosthetic obturation and free flap. Br. J. Oral. Maxillofac. Surg. 2003, 61, 174–181. [Google Scholar] [CrossRef]
- Genden, E.M.; Wallace, D.I.; Okay, D.; Urken, M.L. Reconstruction of the hard palate using the radial forearm free flap: Indications and outcomes. Head Neck 2004, 26, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Carreirao, S.; Lessa, S. Tongue flaps and the closing of large fistulas of the hard palate. Ann. Plast. Surg. 1980, 4, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.F. Palatal defect reconstruction with hinged nasal septum flap. Ann. Plast. Surg. 1983, 10, 167–171. [Google Scholar] [CrossRef]
- Biorklund, A.; Koch, H.; Pettersson, K.I. A new method for strengthening palatal closure defects of the hard palate. Acta Otolaryngol. 1976, 82, 147–150. [Google Scholar] [CrossRef]
- Lissaois, B. Immediate reconstruction of the hard palate following maxillectomy for carcinoma. Int. Surg. 1975, 60, 477–478. [Google Scholar] [PubMed]
- Colmenero, C.; Martorell, V.; Colmenero, B.; Sierra, I. Temporalis myofascial flap for maxillofacial reconstruction. J. Oral Maxillofac. Surg. 1991, 49, 1067–1073. [Google Scholar] [CrossRef]
- Konno, A.; Togawa, K.; Iizuka, K. Primary reconstruction after total or extended total maxillectomy for maxillary cancer. Plast. Reconstr. Surg. 1981, 67, 440–448. [Google Scholar] [CrossRef]
- Butterworth, C.J.; Lowe, D.; Rogers, S.N. The Zygomatic Implant Perforated (ZIP) flap reconstructive technique for the management of low-level maxillary malignancy—Clinical & patient related outcomes on 35 consecutively treated patients. Head Neck. 2021, 44, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Shestak, K.C.; Schusterman, M.A.; Jones, N.F.; Johnson, J.T. Immediate microvascular reconstruction of combined palatal and midfacial defects using soft tissue only. Microsurgery 1988, 9, 128–131. [Google Scholar] [CrossRef]
- Futran, N.D.; Wadsworth, J.T.; Villaret, D.; Farwell, D.G. Midface reconstruction with the fibula free flap. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 161–166. [Google Scholar] [CrossRef]
- Yu, P.; Chang, D.W.; Miller, M.J.; Reece, G.; Robb, G.L. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck 2009, 31, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hunsaker, F.G.; Cioffi, D.A.; Amadio, P.C.; Wright, J.G.; Caughlin, B. The American Academy of Orthopaedic Surgeons outcomes instruments—Normative values from the general population. J. Bone Joint. Surg. Am. 2002, 84, 208–215. [Google Scholar] [CrossRef]
- Sampathirao, L.M.C.S.R.; Thankappan, K.; Duraisamy, S.; Hedne, N.; Sharma, M.; Mathew, J.; Iyer, S. Orbital floor reconstruction with free flaps after maxillectomy. Craniomaxillofac. Trauma Reconstr. 2013, 6, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Suárez, C.; Ferlito, A.; Lund, V.J.; Silver, C.E.; Fagan, J.J.; Rodrigo, J.P.; Llorente, J.L.; Cantù, G.; Politi, M.; Wei, W.I.; et al. Management of the orbit in malignant sinonasal tumors. Head Neck 2008, 30, 242–250. [Google Scholar] [CrossRef]
- Connolly, T.M.; Sweeny, L.; Greene, B.; Morlandt, A.; Carroll, W.R.; Rosenthal, E.L. Reconstruction of midface defects with the osteocutaneous radial forearm flap: Evaluation of long-term outcomes including patient-reported quality of life. Microsurgery 2017, 37, 752–762. [Google Scholar] [CrossRef]
- Cracchiolo, J.R.; Klassen, A.F.; Young-Afat, D.A.; Albornoz, C.R.; Cano, S.J.; Patel, S.G.; Pusic, A.L.; Matros, E. Leveraging patient-reported outcomes data to inform oncology clinical decision making: Introducing the FACE-Q Head and Neck Cancer Module. Cancer 2019, 125, 863–872. [Google Scholar] [CrossRef] [PubMed]
| Database | Search Terms | Filters Applied | Last Search Date |
|---|---|---|---|
| PubMed/MEDLINE | (“scapular free flap” OR “SFF” OR “scapular flap” OR “free flap” AND (“maxillary reconstruction” OR “maxillectomy” OR “maxillary defect”) AND (“surgical outcome” OR “functional outcome” OR “aesthetic outcome” OR “complication” OR “fistula” OR “morbidity” OR “dental rehabilitation” OR “upper limb function” OR “eye problem” OR “revision” OR “flap survival”) AND (“cohort study” OR “case–control study” OR “cross-sectional study” OR “randomized clinical trial” OR “RCT”) | Full text, English language, Published in a peer-reviewed journal | 10th February 2025 |
| Cochrane Library | (“scapular free flap” OR “SFF” OR “scapular flap”) AND (“maxillary reconstruction” OR “maxillectomy”) AND (“surgical outcome” OR “functional outcome” OR “aesthetic outcome” OR “complication” OR “fistula” OR “morbidity” OR “dental rehabilitation” OR “upper limb function”) AND (“cohort” OR “case–control” OR “RCT”) | Peer-reviewed articles, English language | 8th February 2025 |
| Google Scholar | “scapular free flap” AND “maxillary reconstruction” AND (“surgical outcome” OR “functional outcome” OR “aesthetic outcome” OR “fistula” OR “morbidity” OR “dental rehabilitation” OR “upper limb function”) AND (“cohort study” OR “case–control” OR “randomized trial” OR “RCT”) | Peer-reviewed articles, English language | 10th February 2025 |
| Scopus | (“scapular free flap” OR “SFF” OR “scapular flap”) AND (“maxillary reconstruction” OR “maxillectomy”) AND (“surgical outcome” OR “functional outcome” OR “aesthetic outcome” OR “complication” OR “fistula” OR “morbidity” OR “dental rehabilitation” OR “upper limb function” OR “flap survival”) AND (“cohort” OR “case–control” OR “cross-sectional” OR “RCT” OR “clinical trial”) | Full text, English language, Published in a peer-reviewed journal | 10th February 2025 |
| Inclusion Criteria | |
|---|---|
| Patients (P) | Studies including adult patients (≥18 years) who underwent SFF reconstruction following a post-ablative maxillectomy for any cause (e.g., cancer, trauma). |
| Intervention (I) | Studies where the primary intervention was SFF reconstruction following maxillectomy, including both total and partial maxillectomy procedures. |
| Outcomes (O) | Studies must have reported on at least one of the following outcomes:
|
| Study design (S) | Retrospective and prospective cohort studies, case–control and cross-sectional studies and RCTs. |
| Exclusion Criteria | |
| Non-Full Text availability | To ensure access to complete methodology, data, and results. |
| Studies that included fewer than 5 patients | To minimize the risk of bias and ensure the robustness of the analysis. |
| Non-original research | Review articles, case reports, conference abstracts, letters to the editor, and book chapters. |
| First Author, Year | Country | Study Design | No. (Male) | Type of Scapular Flap | Classification of Maxillectomy Defects | Median Follow-Up | Objective Outcomes | PROMS Measures |
|---|---|---|---|---|---|---|---|---|
| Clark JR et al., 2007 [12] | Canada | Retrospective | 14 (8) | Scapular angle osteomyogenous flap | Okay | 12 | Reconstructive Outcomes: approach, Muscle/other combined with scapular tip, bone orientation/other, LOS day, dental restoration, morbidity, revision, diet | Upper limb function: DASH score |
| Miles BA et al., 2011 [13] | Canada | Retrospective | 39 (N/A) | Scapular angle osteomyogenous flap | Okay | 12.5 | Reconstructive Outcomes: early and late postoperative complications, dental restoration, revision, diet | Upper limb function: DASH score |
| Moya-Plana A et al., 2019 [15] | France | Retrospective | 84 (46) | Latissimus dorsi-scapular free | Kolb | 45 | Post- operative complications, and oncologic and functional outcomes (speech and swallowing disorders, dental rehabilitation, rhinologic dysfunction, oculopalpebral disorders, and esthetic outcomes: good, acceptable, or mediocre) | Upper limb function: DASH score |
| Ferri A et al., 2020 [24] | Italy | Retrospective | 53 (29) | STFF: scapular tip free flap | Brown I: 16; II: 21; IIIa: 13; IIIb: 3 | N/A | Complications. Functional results: oronasal fistula, achievement of dental rehabilitation, and the extent of mouth opening (good (>3 cm), partially limited (2–3 cm), or limited (<2 cm)) | Esthetic outcomes: excellent, good, poor, or unacceptable |
| Cardìn AA at al., 2024 [25] | Portugal | Retrospective | 21 (7) | STFF: scapular tip free flap | Brown IIa: 5; IIb: 5; IIIa: 8; IIIb: 3 | 41 | Surgical outcomes: days of admission, short- and long-term complications, follow-up months, and disease | Functional assessment: speech, eating or swallowing, rhinologic dysfunction, eye or eyelid disorders, orofacial pain, breathing, and esthetic or social discomfort). Upper limb function: QuickDASH score |
| Gunè H et al., 2025 [26] | Sweden | Prospective cohort | 20 (16) | SOFF: osseous flap, osseous flap with ± latissimus dorsi muscle ± skin island flap | Brown I: 1; II: 12; III: 6; IV: 1 | 6 | Postoperative outcomes and complications: general surgical complications according to Clavien–Dindo, dental rehabilitation, oronasal fistula and eye-related problems | Functional, psycho- social, and experiential outcomes: FACE-Q |
| Study | Selection | Comparison | Outcome |
|---|---|---|---|
| Clark JR et al., 2007 [12] | xxx | x | xxx |
| Miles BA et al., 2011 [13] | xxx | x | xxx |
| Moya-Plana A et al., 2019 [15] | xxx | x | xxx |
| Ferri A et al., 2020 [24] | xxx | x | xx |
| Cardìn AA et al., 2024 [25] | xxx | x | xxx |
| Gunè H et al., 2025 [26] | xxx | x | xx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, G.; Scocca, V.; Troise, S.; Abbate, V.; Bonavolontà, P.; Vaira, L.A.; Scarpa, A.; Lechien, J.R.; De Fazio, G.; Carraturo, E.; et al. Advancing Maxillary Reconstruction: A Systematic Review and Meta-Analysis of the Evolving Role of the Scapular Free Flap. J. Clin. Med. 2025, 14, 3278. https://doi.org/10.3390/jcm14103278
Salzano G, Scocca V, Troise S, Abbate V, Bonavolontà P, Vaira LA, Scarpa A, Lechien JR, De Fazio G, Carraturo E, et al. Advancing Maxillary Reconstruction: A Systematic Review and Meta-Analysis of the Evolving Role of the Scapular Free Flap. Journal of Clinical Medicine. 2025; 14(10):3278. https://doi.org/10.3390/jcm14103278
Chicago/Turabian StyleSalzano, Giovanni, Veronica Scocca, Stefania Troise, Vincenzo Abbate, Paola Bonavolontà, Luigi Angelo Vaira, Alfonso Scarpa, Jerome R. Lechien, Gianluca De Fazio, Emanuele Carraturo, and et al. 2025. "Advancing Maxillary Reconstruction: A Systematic Review and Meta-Analysis of the Evolving Role of the Scapular Free Flap" Journal of Clinical Medicine 14, no. 10: 3278. https://doi.org/10.3390/jcm14103278
APA StyleSalzano, G., Scocca, V., Troise, S., Abbate, V., Bonavolontà, P., Vaira, L. A., Scarpa, A., Lechien, J. R., De Fazio, G., Carraturo, E., & Dell’Aversana Orabona, G. (2025). Advancing Maxillary Reconstruction: A Systematic Review and Meta-Analysis of the Evolving Role of the Scapular Free Flap. Journal of Clinical Medicine, 14(10), 3278. https://doi.org/10.3390/jcm14103278










