Electromyographic Assessment of Muscle Activity in Children Undergoing Orthodontic Treatment—A Systematic Review
Abstract
1. Introduction
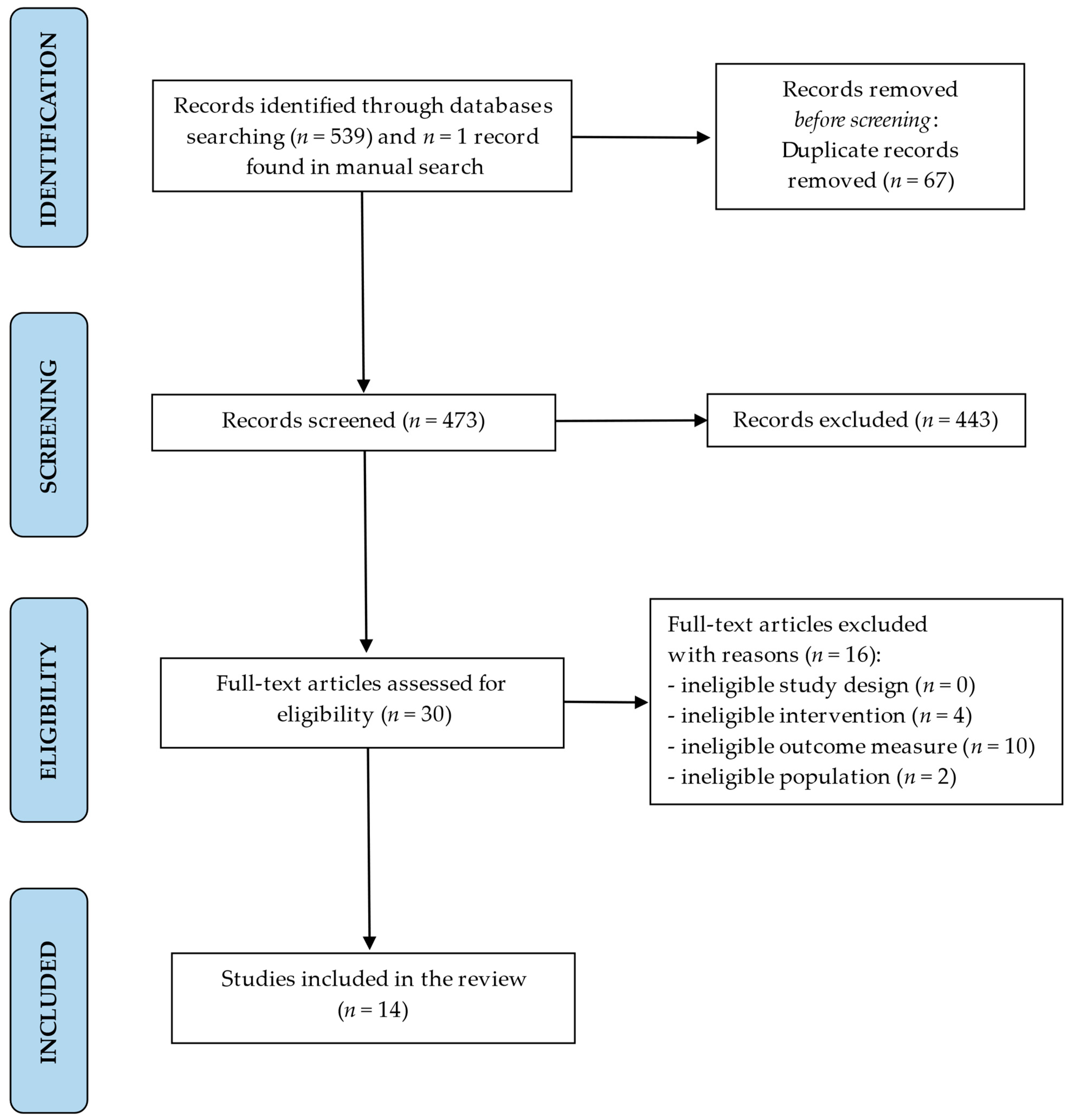
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
- -
- Study type: all types of controlled trials and observational studies on EMG muscle activity in children undergoing orthodontic treatment,
- -
- Outcome of interest: electromyographic analysis of alterations in muscle activity in children undergoing orthodontic treatment,
- -
- Object of the study: electromyographic assessment of muscle activity in orthodontically treated children compared with untreated children,
- -
- Participants: human subjects—children between the ages of 6 and 16 undergoing orthodontic treatment.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Certainty of Evidence
- -
- High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
- -
- Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
- -
- Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
- -
- Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of the effect.
2.6. Data Synthesis
3. Results
3.1. Results of the Quality Assessment
3.2. Findings of Certainty of Evidence
3.3. Characteristics of the Study Groups (Age, Malocclusion, and Orthodontic Method/Orthodontic Appliances)
3.4. Electromyographic (EMG) Muscle Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masci, C.; Ciarrocchi, I.; Spadaro, A.; Necozione, S.; Marci, M.C.; Monaco, A. Does orthodontic treatment provide a real functional improvement? a case control study. BMC Oral Health 2013, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Johal, A.; Alyaqoobi, I.; Patel, R.; Cox, S. The Impact of Orthodontic Treatment on Quality of Life and Self-Esteem in Adult Patients. Eur. J. Orthod. 2015, 37, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Silvola, A.S.; Rusanen, J.; Tolvanen, M.; Pirttiniemi, P.; Lahti, S. Occlusal Characteristics and Quality of Life Before and After Treatment of Severe Malocclusion. Eur. J. Orthod. 2012, 34, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Baldini, A.; Nota, A.; Cozza, P. The association between Occlusion Time and Temporomandibular Disorders. J. Electromyogr. Kinesiol. 2015, 25, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Kecik, D.; Kocadereli, I.; Saatci, I. Evaluation of the treatment changes of functional posterior crossbite in the mixed dentition. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yang, M.; Bai, S.; Zhang, S.; Huang, Y.; Gong, F.; Nong, X. Effects of orthodontic treatment on masticatory muscles activity: A meta-analysis. Ann. Hum. Biol. 2023, 50, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, K.; Piątkowska, D.; Lipski, M.; Mehr, K. Surface electromyography in orthodontics—A literature review. Med. Sci. Monit. 2013, 19, 416–423. [Google Scholar] [PubMed]
- Ferrario, V.F.; Tartaglia, G.M.; Galletta, A.; Grassi, G.P.; Sforza, C. The influence of occlusion on jaw and neck muscle activity: A surface EMG study in healthy young adults. J. Oral Rehabil. 2006, 33, 341–348. [Google Scholar] [CrossRef]
- Castroflorio, T.; Bracco, P.; Farina, D. Surface electromyography in the assessment of jaw elevator muscles. J. Oral Rehabil. 2008, 35, 638–645. [Google Scholar] [CrossRef]
- Ngo, C.; Munoz, C.; Lueken, M.; Hülkenberg, A.; Bollheimer, C.; Briko, A.; Kobelev, A.; Shchukin, S.; Leonhardt, S. A wearable, multi-frequency device to measure muscle activity combining simultaneous electromyography and electrical impedance myography. Sensors 2022, 22, 1941. [Google Scholar] [CrossRef]
- Hugger, S.; Schindler, H.J.; Kordass, B.; Hugger, A. Clinical relevance of surface EMG of the masticatory muscles. (Part 1): Resting activity, maximal and submaximal voluntary contraction, symmetry of EMG activity. Int. J. Comp. Dent. 2012, 15, 297–314. [Google Scholar]
- Szyszka-Sommerfeld, L.; Woźniak, K.; Matthews-Brzozowska, T.; Kawala, B.; Mikulewicz, M.; Machoy, M. The electrical activity of the masticatory muscles in children with cleft lip and palate. Int. J. Paediatr. Dent. 2018, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Klasser, G.D.; Okeson, J.P. The clinical usefulness of surface electromyography in the diagnosis and treatment of temporomandibular disorders. J. Am. Dent. Assoc. 2006, 137, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Szyszka-Sommerfeld, L.; Machoy, M.; Lipski, M.; Woźniak, K. The diagnostic value of electromyography in identifying patients with pain-related temporomandibular disorders. Front. Neurol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Szyszka-Sommerfeld, L.; Sycińska-Dziarnowska, M.; Spagnuolo, G.; Woźniak, K. Surface electromyography in the assessment of masticatory muscle activity in patients with pain-related temporomandibular disorders: A systematic review. Front. Neurol. 2023, 14, 1184036. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, K.; Piątkowska, D.; Szyszka-Sommerfeld, L.; Buczkowska-Radlińska, J. Impact of functional appliances on muscle activity: A surface electromyography study in children. Med. Sci. Monit. 2015, 21, 246–253. [Google Scholar] [PubMed]
- Saccucci, M.; Tecco, S.; Ierardoa, G.; Luzzi, V.; Festa, F.; Polimeni, A. Effects of interceptive orthodontics on orbicular muscle activity: A surface electromyographic study in children. J. Electromyogr. Kinesiol. 2011, 21, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Tran, J.; Castroflorio, T.; Tassi, A.; Cioffi, I. Evaluation of masticatory muscle response to clear aligner therapy using ambulatory electromyographic recording. Am. J. Orthod. Dentofac. Orthop. 2021, 159, e25–e33. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Kharbanda, O.P.; Mathur, R.; Duggal, R.; Parkash, H. Muscle response to the twin-block appliance: An electromyographic study of the masseter and anterior temporal muscles. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 405–414. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Tecco, S.; Caruso, S.; Marchetti, E.; Marzo, G.; Cutilli, T. Ultra-low-frequency transcutaneous electric nerve stimulation (ULF-TENS) in subjects with craniofacial pain: A retrospective study. Cranio 2020, 8, 396–401. [Google Scholar] [CrossRef]
- Nishi, S.E.; Basri, R.; Alam, M.K. Uses of electromyography in dentistry: An overview with meta-analysis. Eur. J. Dent. 2016, 10, 419–425. [Google Scholar] [CrossRef]
- Spolaor, F.; Mason, M.; De Stefani, A.; Bruno, G.; Surace, O.; Guiotto, A.; Gracco, A.; Sawacha, Z. Effects of Rapid Palatal Expansion on Chewing Biomechanics in Children with Malocclusion: A Surface Electromyography Study. Sensors 2020, 20, 2086. [Google Scholar] [CrossRef] [PubMed]
- Dellavia, C.P.B.; Begnoni, G.; Zerosi, C.; Guenza, G.; Khomchyna, N.; Rosati, R.; Musto, F.; Pellegrini, G. Neuromuscular Stability of Dental Occlusion in Patients Treated with Aligners and Fixed Orthodontic Appliance: A Preliminary Electromyographical Longitudinal Case-Control Study. Diagnostics 2022, 12, 2131. [Google Scholar] [CrossRef] [PubMed]
- De Felício, C.M.; Sidequersky, F.V.; Tartaglia, G.M.; Sforza, C. Electromyographic standardized indices in healthy Brazilian young adults and data reproducibility. J. Oral Rehabil. 2009, 36, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Svensson, P.; Wang, K.; Sessle, B.J.; Arendt-Nielsen, L. Associations between pain and neuromuscular activity in the human jaw and neck muscles. Pain 2004, 109, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hugger, A.; Hugger, S.; Schindler, H. Surface electromyography of the masticatory muscles for application in dental practice. Current evidence and future developments. Int. J. Comp. Dent. 2008, 11, 81–106. [Google Scholar]
- Nota, A.; Caruso, S.; Ehsani, S.; Ferrazzano, G.F.; Gatto, R.; Tecco, S. Short-Term Effect of Orthodontic Treatment with Clear Aligners on Pain and sEMG Activity of Masticatory Muscles. Medicina 2021, 57, 178. [Google Scholar] [CrossRef] [PubMed]
- Paes-Souza, S.A.; Garcia, M.A.C.; Souza, V.H.; Morais, L.S.; Nojima, L.I.; Nojima, M.D.C.G. Response of masticatory muscles to treatment with orthodontic aligners: A preliminary prospective longitudinal study. Dental Press J. Orthod. 2023, 28, e232198. [Google Scholar] [CrossRef] [PubMed]
- Nalamliang, N.; Thongudomporn, U. Effects of class II intermaxillary elastics on masticatory muscle activity balance, occlusal contact area and masticatory performance: A multicenter randomised controlled trial. J. Oral Rehabil. 2023, 50, 131–139. [Google Scholar] [CrossRef]
- Al-Dboush, R.; Al-Zawawi, E.; El-Bialy, T. Does short-term treatment with clear aligner therapy induce changes in muscular activity? Evid. Based Dent. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Satygo, E.A.; Silin, A.V.; Ramirez-Yañez, G.O. Electromyographic muscular activity improvement in Class II patients treated with the pre-orthodontic trainer. J. Clin. Pediatr. Dent. 2014, 38, 380–384. [Google Scholar] [CrossRef]
- Michelotti, A.; Rongo, R.; Valentino, R.; D’Antò, V.; Bucci, R.; Danzi, G.; Cioffi, I. Evaluation of masticatory muscle activity in patients with unilateral posterior crossbite before and after rapid maxillary expansion. Eur. J. Orthod. 2019, 41, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sackett, D.L.; Strauss, S.E.; Richardson, W.S.; Rosenberg, W.; Haynes, B.R. Evidence Based Medicine: How to Practice and Teach EBM, 2nd ed.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2000. [Google Scholar]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in metaanalyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Kilic, N.; Eröz, B. Changes in soft tissue profile and electromyographic activity after activator treatment. Aust. Orthod. J. 2009, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Palma, J.C.; Alamán, J.M.; Lopez-Quiñones, J.M.; Alarcón, J.A. Longitudinal evaluation of sEMG of masticatory muscles and kinematics of mandible changes in children treated for unilateral cross-bite. J. Electromyogr. Kinesiol. 2012, 22, 620–628. [Google Scholar] [CrossRef]
- Ocak, I.; Soylu, A.R.; Aksu, M. Changes in Orbicularis Oris Superior and Masseter Muscle Activities After Upper Incisor Protrusion in Class II Division 2 Malocclusion: An Electromyographic Study. Turk. J. Orthod. 2022, 35, 231–238. [Google Scholar] [CrossRef]
- Petrović, D.; Vujkov, S.; Petronijević, B.; Šarčev, I.; Stojanac, I. Examination of the bioelectrical activity of the masticatory muscles during Angle’s Class II division 2 therapy with an activator. Vojnosanit. Pregl. 2014, 71, 1116–1122. [Google Scholar] [CrossRef]
- Piancino, M.G.; Falla, D.; Merlo, A.; Vallelonga, T.; de Biase, C.; Dalessandri, D.; Debernardi, C. Effects of therapy on masseter activity and chewing kinematics in patients with unilateral posterior crossbite. Arch. Oral Biol. 2016, 67, 61–67. [Google Scholar] [CrossRef]
- Uysal, T.; Yagci, A.; Kara, S.; Okkesim, S. Influence of pre-orthodontic trainer treatment on the perioral and masticatory muscles in patients with Class II division 1 malocclusion. Eur. J. Orthod. 2012, 34, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Wasinwasukul, P.; Nalamliang, N.; Pairatchawan, N.; Thongudomporn, U. Effects of anterior bite planes fabricated from acrylic resin and thermoplastic material on masticatory muscle responses and maximum bite force in children with a deep bite: A 6-month randomised controlled trial. J. Oral Rehabil. 2022, 49, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Yuen, S.W.; Hwang, J.C.; Poon, P.W. Changes in power spectrum of electromyograms of masseter and anterior temporal muscles during functional appliance therapy in children. Am. J. Orthod. Dentofac. Orthop. 1990, 97, 301–307. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Mills, C.; Antonarakis, G. Masseter muscle thickness as a predictive variable in treatment outcome of the twin- block appliance and masseteric thickness changes during treatment. Orthod. Craniofac. Res. 2010, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, G.; Kiliaridis, S. Predictive value of masseter muscle thickness and bite force on class II functional appliance treatment: A prospective controlled study. Eur. J. Orthod. 2015, 37, 570–577. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomonari, H.; Kubota, T.; Yagi, T.; Kuninori, T.; Kitashima, F.; Uehara, S.; Miyawaki, S. Posterior scissors-bite: Masticatory jaw movement and muscle activity. J. Oral Rehabil. 2014, 41, 257–265. [Google Scholar] [CrossRef] [PubMed]
- English, J.D.; Buschang, P.H.; Throckmorton, G.S. Does malocclusion affect masticatory performance? Angle Orthod. 2002, 72, 21–27. [Google Scholar] [PubMed]
- Alarcón, J.A.; Martín, C.; Palma, J.C. Effect of unilateral posterior crossbite on the electromyographic activity of human masticatory muscles. Am. J. Orthod. Dentofac. Orthop. 2000, 118, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.A.; Martín, C.; Palma, J.C.; Menendez-Nunez, M. Activity of jaw muscles in unilateral cross-bite without mandibular shift. Arch. Oral Biol. 2009, 54, 108–114. [Google Scholar] [CrossRef]
- Bakke, M.; Michler, L.; Moller, E. Occlusal control of mandibular elevator muscles. Scand. J. Dent. Res. 1992, 100, 284–291. [Google Scholar] [CrossRef]
- Bakke, M.; Moller, E. Craniomandibular disorders and masticatory muscle function. Scand. J. Dent. Res. 1992, 100, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, L.; Arnrup, K.; Bondemark, L. The impact of malocclusion on the quality of life among children and adolescents: A systematic review of quantitative studies. Eur. J. Orthod. 2015, 37, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, A.; Almotairy, N.; Kumar, A.; Grigoriadis, A. Effect of malocclusion on jaw motor function and chewing in children: A systematic review. Clin. Oral Investig. 2022, 26, 2335–2351. [Google Scholar] [CrossRef] [PubMed]
- Janson, G.; Branco, N.C.; Fernandes, T.M.F.; Sathler, R.; Garib, D.; Lauris, J.R.P. Influence of orthodontic treatment, midline position, buccal corridor and smile arc on smile attractiveness. Angle Orthod. 2011, 81, 153–161. [Google Scholar] [CrossRef] [PubMed]
- de Paula, J.D.F.; Santos, N.C.M.; da Silva, É.T.; Nunes, M.F.; Leles, C.R. Psychosocial impact of dental esthetics on quality of life in adolescents. Angle Orthod. 2009, 79, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Stocka, A.; Kuc, J.; Sierpinska, T.; Golebiewska, M.; Wieczorek, A. The Influence of Emotional State on the Masticatory Muscles Function in the Group of Young Healthy Adults. BioMed Res. Int. 2015, 2015, 174013. [Google Scholar]
- Zieliński, G.; Ginszt, M.; Zawadka, M.; Rutkowska, K.; Podstawka, Z.; Szkutnik, J.; Majcher, P.; Gawda, P. The Relationship between Stress and Masticatory Muscle Activity in Female Students. J. Clin. Med. 2021, 10, 3459. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Suwała, M.; Ginszt, M.; Szkutnik, J.; Majcher, P. Bioelectric activity of mastication muscles and the functional impairment risk groups concerning the masticatory muscles. Acta Bioeng. Biomech. 2018, 20, 161–166. [Google Scholar] [PubMed]
- Augusto, V.G.; Perina, K.C.B.; Penha, D.S.G.; Dos Santos, D.C.A.; Oliveira, V.A.S. Temporomandibular Dysfunction, Stress and Common Mental Disorder in University Students. Acta Ortop. Bras. 2016, 24, 330–333. [Google Scholar] [CrossRef]
- Castroflorio, T.; Talpone, F.; Deregibus, A.; Piancino, M.G.; Bracco, P. Effects of a functional appliance on masticatory muscles of young adults suffering from muscle-related temporomandibular disorders. J. Oral Rehabil. 2004, 31, 524–529. [Google Scholar] [CrossRef]
- Patil, S.R.; Doni, B.R.; Patil, C.; Nawab, S.; Khursheed Alam, M. Role of Electromyography in Dental Research: A Review. J. Res. Dent. Maxillofac. Sci. 2023, 8, 71–78. [Google Scholar] [CrossRef]
- Ferrario, V.F.; Sforza, C.; Colombo, A.; Ciusa, V. An electromyographic investigation of masticatory muscles symmetry in normo-occlusion subjects. J. Oral Rehabil. 2000, 27, 33–40. [Google Scholar] [CrossRef] [PubMed]
| Authors, Year | Study Participants (Number, Age, Malocclusion, Orthodontic Treatment) | Outcomes | Results |
|---|---|---|---|
| Erdem et al., 2009 [38] | n = 25 subjects with CII/1 malocclusions who were randomly assigned to either a treatment group (n = 15; 9 girls, 6 boys; mean age 11.3 ± 1.1 years) or a control group (n = 10; 4 girls, 6 boys; mean age 11.0 ± 1.3 years). The subjects in the treatment group were treated with activators and the subjects in the control group were untreated. | The EMG recordings of the TA and MM muscles during clenching, chewing, and swallowing and the EMG activity of the OO muscle during whistling were obtained at the start of the study and 12 months later. The EMG recordings were performed with a Disa Neuromatic 2000 electromyograph device (Dantec DISA, Scovlunde, Denmark). | The EMG activity of the TA and MM muscles during clenching, chewing, and swallowing increased significantly in both groups, especially in the TA muscle during maximal clenching in the treatment group (p < 0.001). The EMG activity in the OO muscles during whistling increased significantly in the treatment group. All between-group EMG differences were statistically significant (p < 0.001 for the electrical activity of the masticatory muscles during maximal clenching and chewing, and p < 0.05 for the EMG activity of the OO muscles during whistling), except in the case of the EMG activity of the TA and MM muscles during swallowing (p > 0.05). |
| Kecik et al., 2007 [5] | n = 35 patients (20 girls, 15 boys) with FPXB at a mean age of 10.6 ± 1.4 years consisted of the experimental group; the control group consisted of 31 normo-occlusive subjects (18 girls, 13 boys) at a mean age of 9.8 ± 1.6 years. The subjects in the FPXB group were treated with the QH appliance. | The EMG activity of the TA, MM, SC, and DA muscles of both sides was recorded during rest, swallowing, and maximum clenching. The data were collected at 1 time point in the controls, and before treatment and 6 months after treatment (3 months of expansion and 3 months of retention) in the FPXB group. | In the FBCX group, TA and MM activity was significantly higher on the crossbite side at rest before treatment (p < 0.001). After treatment, differences between the two sides had been eliminated and no significant divergences between the FPXB and control groups were observed (p > 0.05). Significant differences were found between both groups during clenching (p < 0.001). EMG values in the TA muscle were significantly higher on the crossbite side before treatment (p < 0.001). The right and left sides of the control group showed no significant difference during clenching (p > 0.05). MM muscle activity was considerably higher on the non-crossbite side before treatment, and the difference between the groups was significant (p < 0.001). Following treatment, no significant differences were observed between the groups in terms of MM activity (p > 0.05). |
| Martín et al., 2012 [39] | n = 25 children (10 boys, 15 girls), aged 10 to 14 years old diagnosed with UPXB and functional mandibular lateral shift. n = 30 age-matched children with normal occlusion (15 boys, 15 girls) served as a control group. Orthodontic therapy consisted of expansion with the QH appliance. | The EMG activity of the TA, TP, MM, and SH muscle areas was evaluated at rest and during swallowing, mastication, and clenching. The study was performed using an EM2 electromyograph (K6-I Diagnostic System, Myotronics-Noromed, Kent, WA, USA). | At the beginning of the study, EMG activity in non-crossbite side TA during swallowing was considerably higher in a UPXB group than in controls (p < 0.01). During clenching, EMG activity in crossbite side MM was lower in the UPXB group (p < 0.05). After treatment, crossbite side TA and MM activity increased significantly (p < 0.001) and remained stable after retention. A significant reduction in the resting EMG activity of crossbite side TA (p < 0.001) and MM (p < 0.05) was noted post-treatment. During mastication, MM activity increased significantly (p < 0.001), and its asymmetry was corrected post-treatment. |
| Masci et al., 2023 [1] | This study enrolled 30 patients (20 females, 10 males, mean age: 15.78 years) with a CII/1 malocclusion treated with fixed multibracket appliances. A group of 30 subjects (19 females, 11 males; mean age: 16.15 years), selected among subjects with CII/1 malocclusion who underwent no treatment, served as the control group. | The EMG activity of the MM, TA, DA, and SC muscles of both sides was examined in closed and open eyes conditions. The EMG activity was recorded with an eight-channel K7 system (Myotronic Inc.; Seattle, WA, USA). | The EMG activity of the TA muscles was significantly higher in treated subjects compared to untreated patients in the open eyes condition (p < 0.05). |
| Michelotti et al., 2019 [32] | n = 29 children with UPXB (UPXB group, 13 boys, 16 girls; mean age 9.6 ± 1.6 years) and n = 40 UPXB-free controls (control group, 17 boys, 23 girls; mean age 10.5 ± 1.1 years) were recruited. The UPXB group was treated with RME. | The EMG activity of the left and right TA and MM muscles was recorded during MVC in intercuspal position, and MVC in intercuspal position on cotton rolls and chewing. In the UPXB group, data were collected before treatment (T0), after the correction of the UPXB (T1), and 6 months later (T2). A wireless EMG device (TMJOINT, BTS SpA, Garbagnate Milanese, Italy) was used. | Before treatment, both groups were characterized by asymmetric activity of the TA and MM muscles, with no differences between groups (p > 0.05). The treatment effected a decrease in EMG muscle activity (p = 0.040) and a more asymmetric pattern of muscle activation during chewing after correcting crossbite (p = 0.040), which returned to values similar to baseline at T2 (all p > 0.05). |
| Ocak et al., 2022 [40] | n = 20 patients (8 girls, 12 boys; mean age 10.29 ± 0.90 years) with CII/2 malocclusion were selected for the study group. n = 15 patients (5 girls, 10 boys; mean age 10.56 ± 1.06 years) with Angle Class I malocclusion were recruited as controls. Upper incisors were protruded with utility arch in the study group. | The EMG activity of the SOO and left and right MM was recorded during the rest position, tightening the lips, and clenching teeth, with a Biopac MP150 sEMG device (Biopac Systems Inc., Goleta, CA, USA), before and after upper incisor protrusion and at the 6-month retention. | A significant change over time in SOO (p < 0.001) and in both right (p < 0.05) and left MM (p < 0.01) m-EMG in a group of treated patients was observed. In the CII/2 group, SOO m-EMG values increased following upper incisor protrusion (p < 0.001), and this increase remained stable (p < 0.05). MM m-EMG measurements decreased after protrusion (p < 0.01) and then increased significantly after retention (p < 0.05). No differences were observed between the groups following protrusion, and considerable differences were noted in left MM m-EMG at the end of the retention (p < 0.05). |
| Petrović et al., 2014 [41] | The sample consisted of 100 subjects of both sexes, divided into the control group (n = 30, mean age 11.23 ± 1.56 years) with neutral dental arches and Class I occlusion, and the study group (n = 70, mean age 11.68 ± 1.21) of patients with CII/2 malocclusions treated with an activator. | The EMG recordings of the TA and MM muscles were conducted in the physiologic rest position, central mandible occlusion, and during MVC and saliva swallowing prior to treatment, after one year of the orthodontic treatment, and after the treatment with an activator. | There was no significant difference in the left TA muscle between the groups, while in the right TA muscle the significant difference was noted in all positions at the beginning of the treatment (p < 0.05). Intergroup analysis of the EMG activity of the MM muscles showed significant differences in both left and right muscles in all positions (p < 0.05), except CO. The results for the first year of treatment showed an increase in electrical activity in the TA and MM muscles (p < 0.05). |
| Piancino et al., 2016 [42] | n = 50 children (mean age 9.1 ± 2.3 years) with UPXB and n = 20 children (mean age: 9.5 ± 2.6 years) with normal occlusion were selected for the study. Each patient was treated with the functional appliance “Function Generating Bite”. | The mandibular motion and the muscle activity of the MM muscles during chewing were simultaneously recorded, before and after correction of UPXB, after a mean treatment time of 7.3 ± 2.4 months plus the retention time of 5–6 months, using a multichannel EMG amplifier (a part of the K7-I WIN Diagnostic System K7-I; Myotronics, Tukwila, WA, USA). | Before treatment, the difference between electromyography envelope peaks in treated patients was less than in controls (p < 0.01) and increased significantly after treatment (p < 0.05), reaching values close to the reference normal value. |
| Saccucci et al., 2011 [17] | n = 13 patients (9 males, 4 females; mean age 9.0 ± 1.5 years) with Class II malocclusion and deep bite treated with an orthodontic/functional device (Occlus-o-Guide™Ortho-Tain Inc., Toa Alta, Puerto Rico). n = 15 children (9 males, 6 females; mean age 9.5 ± 0.8 years) with normal occlusion were recruited as a control group. | The electrical potentials of the OO muscle were investigated by EMG (Bio-pak EMG, BIOEMG 800™, Bio research Assoc. Inc., Goleta, CA, USA), during the rest position, kissing, swallowing, opening of mouth, clenching of teeth, and protrusion of the mandible at T0 (before therapy for the treated group), and after three (T1) (only for the treated group) and six (T2) months of treatment for both groups. | Before treatment, except when swallowing, the EMG activity of the lower OO muscle was lower in treated patients compared to a control group, with significant differences noted at rest and during mandibular protrusion (p < 0.05). A significant increase in muscle activity was observed in the lower OO muscle at rest in the treated group from T0 to T1 (p = 0.004). A significant increase in the activity of the upper OO muscle during protrusion of the mandible was observed from T1 to T2 (p = 0.004). No significant differences in EMG activity were observed in the control group during the follow-up (p > 0.05). The muscular contractility of the treated patients was at a similar level to the control group at T2. |
| Satygo et al., 2014 [31] | n = 36 CII/1 malocclusion patients (mean age 7.6 ± 1.3 years) composed the treated group and wore the POT functional appliance. n = 22 children with a similar age and malocclusion composed the untreated controls. n = 20 children with no dental malocclusion participated as normal controls. | The EMG activity of the right and left MM and TA muscles at clench was recorded using an electromyograph (DuoTrode; Myotronics Inc., Seattle, WA, USA) before and after 12 months of treatment. | Children in the treatment group reported a significant bilateral increase in TA and MM electrical activity (p < 0.001). After treatment, they recorded EMG values similar to those measured in normal controls, whereas the values noted for the untreated controls remained lower than those recorded at the beginning of the study. |
| Spolaor et al., 2020 [22] | n = 53 children (26 girls and 27 boys) divided into 2 groups: n = 43 patients with malocclusion and n = 10 healthy controls. n = 10 patients with BPXB, n = 15 with UPXB, and n = 18 with NOXB were treated with RME. | The EMG activity of the right and left MM and TA muscles during chewing tasks was collected using the 8-channel Free EMG system, 1000 Hz (BTS Bioengineering, Quincy, MA, USA), before and after RME application and 3 months after removal. | The EMG measurements before treatment revealed significant differences in muscle activity in terms of mean activation and occurrence during chewing tasks in the case of all malocclusions and when compared with a control group. In particular, symmetrical malocclusions (BPXB and NOXB) were characterized by symmetrical and similar muscle function, while patients affected by UPXB presented with lower activity in terms of PoE and earlier activation with regard to PPoE on the contralateral side. The EMG activity in the case of BPXB and NOXB increased slightly compared to the control group. Three months after RME removal, the activity of all the muscles had decreased in terms of PoE, with the exception of RM in UPXB patients. In terms of PPoE, the biggest improvement was observed in NOXB, with a significant reduction occurring in the RM, LT, and LM muscles. |
| Uysal et al., 2012 [43] | n = 20 patients (10 boys, 10 girls; mean age: 9.8 ± 2.2 years) with a CII/1 malocclusion and incompetent lips. Patients were treated with POT (Myofunctional Research Co., Queensland, Australia). n = 15 subjects (6 boys, 9 girls; mean age: 9.2 ± 0.9 years) with untreated CII/1 malocclusions were used as a control group. | The EMG recordings of the TA, mental, OO, and MM muscles during clenching, sucking, and swallowing were performed in the treatment group at the beginning and at the end of the POT therapy (mean treatment period: 7.43 ± 1.06 months). Follow-up records of the control group were taken after 8 months of the first records. The EMG activity was taken using the Biopac-MP150 unit (BIOPAC Systems Inc., Goleta, CA, USA). | During the course of POT treatment, the electrical activity of the TA (p < 0.001), mental (p < 0.05), and MM (p < 0.001) muscles decreased significantly, while OO activity (p < 0.01) increased during clenching when compared with a control group. During sucking, the EMG activity of the TA muscle decreased in the treatment group and remained unchanged in the controls (p < 0.05). The EMG activity of the OO muscle increased in the treatment group during sucking (p < 0.05). Intergroup comparisons of the EMG activity of the MM muscles showed significant differences during swallowing (p < 0.01) and sucking (p < 0.01). |
| Wasinwasukul et al., 2022 [44] | n = 66 children (35 boys and 31 girls) aged 9–13 years were randomly assigned to the ABP, TBP, or untreated control groups. The treated group of children had a deep bite. | The EMG activity of the TA and MM muscles was assessed at rest and during clenching via an 8-channel BioEMG III and BioPAK Measurement System (BioResearch, Inc., Brown Deer, WI, USA) before, immediately after appliance insertion, and after 2 weeks and 1, 3, and 6 months of treatment. | No statistical differences between two treatment groups and a control group during maximum clenching, in terms of the %MVC of all muscles before treatment (p ≥ 0.05), were observed. Immediately after appliance placement, MM activity was significantly lower in the ABP group compared with the TBP group (p < 0.05). At 1 month, the %MVC vales of all muscles in both treatment groups were not significantly different from the control group, with the exception of the TA of the TBP group (p < 0.05). From 3 months onward, no significant differences were observed between any of the groups in terms of the %MVC of any of the muscles (p ≥ 0.05). |
| Yuen et al., 1990 [45] | n = 18 children (9 boys and 9 girls) aged between 9.6 and 10.7 were divided into 3 groups receiving either Bionator, Fränkel type I, or Fränkel type III therapy. A fourth group consisting of n = 6 children (3 boys and 3 girls) aged 10.1 ± 0.8 years who underwent no treatment served as a control. | The EMG recordings of the TA and MM muscles were performed during MVC in the position of maximum intercuspation before and after 3, 6, and 12 months of therapy. | There was a greater downward shift in mean frequency that occurred in those subjects treated with functional appliances than untreated children. Changes in mean frequency in children treated with Bionator and Fränkel type I appliances were greater than in those treated with the Fränkel type III appliance. |
| The Quality Assessment of the Non-Randomized Studies (NOS) | ||||||
| Authors, Year | Selection | Comparability | Outcome | Total Score | ||
| Kecik et al., 2007 [5] | ** | ** | ** | 6 | ||
| Martín et al., 2012 [39] | *** | ** | ** | 7 | ||
| Masci et al., 2023 [1] | *** | ** | ** | 7 | ||
| Michelotti et al., 2019 [32] | *** | ** | ** | 7 | ||
| Ocak et al., 2022 [40] | ** | ** | ** | 6 | ||
| Petrović et al., 2014 [41] | *** | ** | ** | 7 | ||
| Piancino et al., 2016 [42] | *** | ** | ** | 7 | ||
| Saccucci et al., 2011 [17] | ** | ** | ** | 6 | ||
| Satygo et al., 2013 [31] | ** | ** | ** | 6 | ||
| Spolaor et al., 2014 [22] | ** | ** | ** | 6 | ||
| Uysal et al., 2012 [43] | ** | ** | ** | 6 | ||
| Yuen et al., 1990 [45] | * | ** | ** | 5 | ||
| The Quality Assessment of the Randomized Studies (RoB2) | ||||||
| Authors, Year | Randomization process | Deviations from the intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall |
| Erdem et al., 2009 [38] | Some concerns | Low risk | Some concerns | Some concerns | Some concerns | Some concerns |
| Wasinwasukul et al., 2022 [44] | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Outcomes | Impact | Number of Participants (Studies) | Certainty of Evidence (GRADE) | Comments |
|---|---|---|---|---|
| Muscle activity RSs | Significant impact reported in two studies. | 91 (2 RS) | MODERATE a (downgraded by 1 level due to risk of bias) | Orthodontic treatment may affect muscle activity. |
| Muscle activity NRSs | Significant impact reported in eleven studies. | 673 (12 NRS) | LOW b | Orthodontic treatment may affect muscle activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szyszka-Sommerfeld, L.; Sycińska-Dziarnowska, M.; Cernera, M.; Esposito, L.; Woźniak, K.; Spagnuolo, G. Electromyographic Assessment of Muscle Activity in Children Undergoing Orthodontic Treatment—A Systematic Review. J. Clin. Med. 2024, 13, 2051. https://doi.org/10.3390/jcm13072051
Szyszka-Sommerfeld L, Sycińska-Dziarnowska M, Cernera M, Esposito L, Woźniak K, Spagnuolo G. Electromyographic Assessment of Muscle Activity in Children Undergoing Orthodontic Treatment—A Systematic Review. Journal of Clinical Medicine. 2024; 13(7):2051. https://doi.org/10.3390/jcm13072051
Chicago/Turabian StyleSzyszka-Sommerfeld, Liliana, Magdalena Sycińska-Dziarnowska, Mariangela Cernera, Luigi Esposito, Krzysztof Woźniak, and Gianrico Spagnuolo. 2024. "Electromyographic Assessment of Muscle Activity in Children Undergoing Orthodontic Treatment—A Systematic Review" Journal of Clinical Medicine 13, no. 7: 2051. https://doi.org/10.3390/jcm13072051
APA StyleSzyszka-Sommerfeld, L., Sycińska-Dziarnowska, M., Cernera, M., Esposito, L., Woźniak, K., & Spagnuolo, G. (2024). Electromyographic Assessment of Muscle Activity in Children Undergoing Orthodontic Treatment—A Systematic Review. Journal of Clinical Medicine, 13(7), 2051. https://doi.org/10.3390/jcm13072051







