Abstract
Background: Dermatofibrosarcoma protuberans (DFSP) is a superficial soft tissue sarcoma, and surgical excision is the first-line treatment. The aim of this systematic review is to provide an update about the current indications and clinical results regarding the use of postoperative radiotherapy in DSFP, considering both adjuvant and salvage setting. Methods: We conducted a systematic literature review using the main scientific database, including Cochrane library, Scopus, and PubMed, for any relevant article about the topic, and we considered all available papers without any time restriction. Results: Twenty-two papers, published between 1989 and 2023, were retrieved and considered eligible for inclusion in this review. Regarding the fractionation schedules, most authors reported using standard fractionation (2 Gy/die) with a wide total dose ranging from 50 to 70 Gy. The local control after postoperative radiotherapy was excellent (75–100%), with a median follow-up time of 69 months. Conclusions: After the primary surgical management of DFSP, postoperative radiotherapy may either be considered as adjuvant treatment (presence of risk factors, i.e., close margins, recurrent tumours, aggressive histological subtypes) or as salvage treatment (positive margins) and should be assessed within the frame of multidisciplinary evaluation.
1. Introduction
Dermatofibrosarcoma protuberans (DFSP) represents a superficial soft tissue sarcoma that involves the dermis, subcutaneous fat and, in rare cases, muscle and fascia [1].
It was first described in 1924 by Darier and Ferrand [2] as progressive and recurrent dermatofibroma, and was subsequently named DFSP by Hoffmann [3].
The incidence is 0.8–4.5 cases per one million persons and, despite being the most common cutaneous sarcoma, it constitutes less than 0.1% of all malignancies, and about 1.0% of all soft tissue sarcomas worldwide [4]. It mainly affects young and middle-aged adults, with a peak of incidence between the second and fifth decade of age [5].
Clinically, DFSP presents as a slow-growing, firm, multilobular nodule or plaque that ranges in colour from flesh-coloured to red and has irregular margins on the trunk (50%), preferentially on the shoulder girdle, upper and lower limbs (30–40%), and head-neck area (10–15%) [6,7,8]; rarely, DFSP has been reported to occur on toes [9,10,11,12,13], the scalp [14], breasts [15,16,17], and vulva [18].
In most patients, the age at diagnosis is between 20 to 59 years; however, 10–15% of lesions develop in children and adolescents. A definitive diagnosis requires a histological examination of a skin biopsy showing diffuse infiltration of the dermis and the subcutaneous fat by densely packed, spindle-shaped, CD34-positive tumour cells, arranged in a predominantly storiform pattern [19,20,21]. Less common histologic subtypes of DFSP are comprised of mixoid, pigmented (Bednar tumour), giant cell fibroblastoma, granular cell, sclerosing, atrophic, and fibrosarcomatous variants [22].
Dermoscopic features of DFSP have been reported so far in both case series and single case reports. The findings associated with this sarcoma included a pink-colored background, and structureless depigmented areas, and vessels (linear and arborizing); in addition, shiny white streaks and a fine pigment network were observed, although less specific for this condition [23]. In a large series, the median number of dermoscopic features was four per lesion, and the most common were a delicate pigmented network (87%), vessels (80%), structureless light brown areas (73%), shiny white streaks (67%), pink background coloration (67%), and structureless hypo- or depigmented areas (60%) [24].
DFSP is a locally aggressive tumour with a significant rate of local recurrence depending on treatment modalities (0–40%). It has a low metastastic potential; the 10-year disease-specific survival (DSS) is approximately 99% [25]. Fibrosarcomatous transformation within DFSP represents a rare event, which is characterized by exhibiting more aggressive behaviour, a higher rate of local recurrence, and distant metastases after surgery, as compared to the other histological subtypes [26]. Rare cases with lung, bone, or locoregional lymph nodes metastases have been described [27].
The first-line treatment of DFSP is surgical excision [28]. Mohs’ micrographic surgery (MMS), when available, might be preferrable, while a lateral safety margin of 3 cm is recommended using standard 2D surgical excision [29].
The role of integrated approaches, such as radiotherapy (RT) and/or systemic treatment (i.e., imatinib mesylate), has been reported, both in the neoadjuvant and adjuvant setting, as well as for the definitive treatment in patients not eligible for surgery [30,31,32].
The aim of this systematic review is to provide an update about current indications and clinical results regarding the use of postoperative RT in DSFP considering both adjuvant and salvage setting.
2. Materials and Methods
We conducted the search strategy in accordance with the Preferred Reporting Items for Systematic Reviews and Metanalysis (PRISMA) guidelines for systematic reviews, as reported in Figure 1.
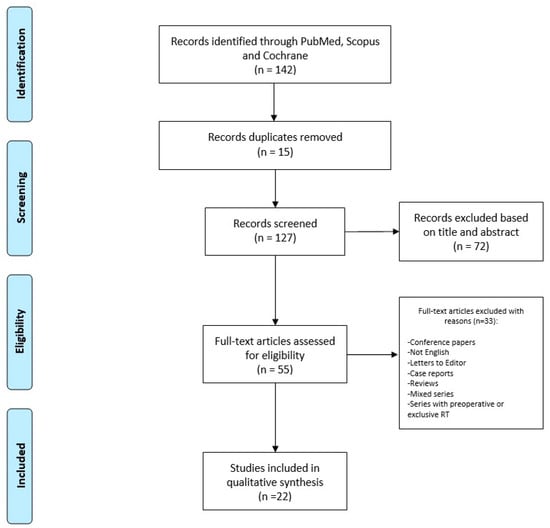
Figure 1.
Search strategy.
Research was conducted in the main scientific database, including Cochrane library, Scopus, and PubMed, for any relevant article about the topic, and we considered all available papers starting from the inception of databases to June 2023. Several combinations of key terms were used to perform the search, including “Dermatofibrosarcoma” or “Dermatofibrosarcoma Protuberans”, and “Radiotherapy” or “Radiation Therapy”.
The inclusion criteria were original series focusing on DFSP and post-operative RT, with a retrospective and prospective setting. Conference papers, articles not in the English language, letters to the Editor, single case reports, reviews, articles including mixed series with other histologies, and series where RT was performed pre-operatively or as the only therapy were not included.
Two independent authors, a radiation oncologist (BF), and a surgeon (AL) made the study selection through screening titles and full abstracts retrieved from the searches, with the aim of identifying articles that met the inclusion criteria. Subsequently, all the articles were retrieved for full-text analysis to assess eligibility (SC, MD, TZ, AAC, and ER). In cases of uncertainties about their inclusion in the present review, articles were additionally examined by another team composed of expert specialists (ADS, AP, VL, and GS) who performed an independent check. Finally, a multidisciplinary Master committee, composed by senior experts in external beam RT and interventional RT, senior dermatologists, a senior surgeon, and a senior medical oncologist (SG, AGM, KP, and LT), conducted an additional review before the final approval of the definitive version.
3. Results
Twenty-two papers, published between 1989 and 2023, were retrieved and considered eligible for inclusion in this review. All investigated studies had a retrospective design and were published by North American, European, or Asian authors. Overall, 994 patients were included and reported upon in the present systematic review. A full and comprehensive list of all the collected variables is provided in Table 1.

Table 1.
Features of patients and relative treatment details.
The mean age of the patients who were referred for postoperative RT after the surgical removal of DFSP was 40 years (range 11–48) with a slight male prevalence (59%).
The most common site invovled was the trunk, followed by the extremities and the head-neck area.
The average size of the larger diameter of DFSP was 14 cm (with lesions up to 25 cm).
The surgical procedure performed in almost all of the patients before postoperative RT was WLE and, in particular, most series included patients treated both in the adjuvant (R0) and in the salvage (R1) setting; the most commonly reported reasons for adjuvant treatment included inadequate margins, multiple recurrences, or fibrosarcomatous differentiation.
There was a rather large amount of radiotherapy techniques used during the time period of the included articles’ publishing dates. Some authors reported using an electron beam or 2D techniques, whereas more recent papers used 3D and volumetric techniques; in a few cases they also utilized interventional radiotherapy (brachytherapy).
Regarding the fractionation schedules, most authors reported using standard fractionation (2 Gy/die) with a wide total dose ranging from 50 to 70 Gy.
The local control after RT was excellent (75–100%), with a median follow-up time ranging from 1 year to approximately 11 years (median: 69 months).
Only a few authors reported the side effects of their RT treatment; for example, Castle et al. reported breast implant asymmetry, oedema, xerostomia, osteoradionecrosis, and soft-tissue necrosis [37]; Sun et al. found only fibrosis or telangiectasia [43]; Suit et al. encountered atrophy and telangiectasia [47]; and Marks et al. encountered transient skin reaction, wound breakdown, and graft failure [48]. In the following paragraph, we describe, in detail, the result of the single studies described in Table 1.
Single Studies Results
Dai et al. [1] conducted a retrospective study of forty-nine patients with head and neck dermatofibrosarcoma protuberans. They collected patients affected by HNDFSP who had received surgical treatment. Eight patients (16.3% of all cases) were treated by postoperative radiotherapy (60 Gy), resulting in a remarkable effect on local disease control. Tumour size, patients’ age, and negative margins with enough safety width constituted the main independent factors affecting disease-free survival. The authors concluded that, despite HNDFSP being a rare disease, RT could improve the prognosis of those patients who were experiences significant challenges, as well as a worse prognosis, in using the current treatment strategies.
Mareş et al. [27] retrospectively reported about seven patients who were affected by DFSP, of whom four were males and three were females. They had a mean age of 38.2 years. All patients underwent surgical treatment with wide local excision, with a margin of 3 cm in five patients and 2 cm in the other two. Adjuvant radiotherapy (50–60 Gy doses) was performed in three patients (42.9% of all cases) who presented with deep fascial invasion after deep margin assessment. The authors concluded by emphasizing the importance of the multidisciplinary approach to good treatment, due to the high rate of DFSP recurrence.
Wang et al. [17] performed a retrospective analysis that included six breast DFSP patients (five female and one male with a mean age of 29.7 years). All patients underwent surgical treatment; specifically, five patients underwent a preoperative excisional biopsy folled by a wide local excision, while one patient underwent a wide local excision without a preoperative biopsy because of clinical suspicion of disease recurrence. Two patients were treated by RT. With a median follow-up of 36 months, all six patients survived without metastasis or recurrence. The authors concluded that DFSP of the breast is characterized by similar clinical features to DFSP at other sites, and the risk of recurrence can be reduced through surgical excision with margins of at least 2 cm.
Du et al. [33] assessed the role of postoperative radiotherapy in DFSP management through analyzing a total of 184 patients (140 male and 44 female) with a median age of 41. The most common site involved was the trunk (71.7%), followed by the head and neck (17.4%), and the extremities (10.9%). All patients underwent surgical resection, and 44 of them (23.9%) were treated with postoperative radiotherapy (50–66 Gy doses). The median follow-up time was 58 months, and the 3-year disease-free survival (DFS) was 94.6%. The authors concluded that postoperative RT can potentially improve DFS for high-risk DFSP. Moreover, the authors affirmed that Ki-67 might become a prognostic molecular marker in those patients.
Tsai et al. [22] described thirteen patients with DFSP (six male and seven female patients, median age of 11 years). In seven patients the lesion involved the trunk, in three cases the extremities were affected, in two patients the back was affected, and finally, in one case, the involved site was the head. All patients underwent a surgical wide excision, and three patients underwent adjuvant RT. The mean follow-up period was 129 months and there was no tumor recurrence reported.
Williams et al., 2014 [34] included fourteen patients with DFSP who were treated with radiotherapy (seven male and seven female, median age: 42 years). Regarding tumor location, in eight cases the site involved was the head and neck, in five patients the lesion affected the extremities and, finally, one patient had the lesion on the trunk. Thirteen out of fourteen patients underwent surgery before RT. The median follow-up was 126 months, and local control was described in 85.7% of cases.
Hamid et al., 2013 [35] described thirty-six patients diagnosed with DFSP (twenty-six male patients and ten female patients with a mean age of 38 years), of whom thirty patients underwent surgical treatment and postoperative adjuvant radiotherapy, and six patients were treated with radiotherapy alone. The head and neck were involved in three cases, the trunk was affected in twenty cases, twelve patients had the lesion in the extremities and, in one case, the external genitalia were involved. The median follow-up duration was 68 months and local control was achieved in 80% of patients.
Uysal et al., 2013 [36] retrospectively evaluated twenty-eight patients treated with radiotherapy for DFSP, of whom twenty-five subjects underwent postoperative adjuvant radiotherapy while three patients were treated with only radiotherapy. Eighteen patients were male and ten were female, with a median age of 26 years. Tumor location was 64% to the extremities, 22% to the trunk, and 14% to the head and neck district. The dose of RT delivered was between 50 and 70 Gy, and patients were followed for an average of 81 months. Regarding patients undergoing adjuvant surgery and radiotherapy, the five-year relapse-free survival (RFS) of the twenty patients treating with RT after wide excision was 89.6%, and the 5-year RFS of the five subjects undergoing adjuvant RT after limited excision was 74%, demonstrating a statistically significant difference between limited excision + RT and wide excision + RT groups.
Castle et al., 2013 [37] retrospectively collected fifty-three DFSP patients who were treated with surgery and preoperative or postoperative RT. Specifically, forty-six (87%) of the fifty-three patients were treated with postoperative RT (60–66 Gy). 57% of all cases were male and 43% were female, with a mean age of 41 years. In 36% of cases the lesion was on the trunk, in 21% of patients the tumor occurred on the scalp, in 15% of cases the lesion involved the head-neck district and, finally, in 28% the lesion affected the extremities. The median follow-up duration was 78 months and local control was achieved in 90% of patients.
Palmerini et al., 2012 [38] described a total of forty patients, 55% male and 45% female with a mean age of 43 years. The tumor involved the limbs in 60% and the trunk in 40%. Ninety percent of all patients received previous surgical treatment, and 27% underwent adjuvant RT. With a median follow-up of 49 months, the local control was achieved in 77% of patients.
Fields et al., 2011 [31] identified 244 patients treated for DFSP (50% male, 50% female, mean age 42 years). Extremities were the most commonly involved site (72%), followed by the trunk (31%), the head and neck district (14%), and other unspecified sites (2%). All patients underwent the wide surgical excision and 14 patients (5.7%) received posoperative RT. The median duration of follow-up was 50 months and, in all these cases, local control was achieved.
Archontaki et al., 2010 [39] reported the results of the treatment of sixteen patients affected by DFSP (nine females and seven males, with a mean age of 41 years). Tumour localization was to the trunk and proximal extremities in nine patients, to the lower extremities in two cases, and to the head-neck district in five patients. Primary treatment consisted of a surgical approach, and adjuvant RT was provided in two patients with local recurrence and one patient with massively extensive disease (18.75% of all cases). The median follow-up was about 44 months and all patients remained free of disease recurrence.
Heuvel et al., 2010 [40] described thirty-eight patients with DFSP (25 males and 13 females, with a median age of 38 years) in whom treatment consisted of surgery and, in cases of marginal or positive resection margins, adjuvant RT. The tumour involved the head and neck district in 16.7% of cases, the trunk in 66.6% of cases, and the extremities in 16.7% of patients. Adjuvant RT (50–70 Gy) was applied for eight patients (21%). The median follow-up was 89 months and the local control was achieved in 87.5% of patients.
Dagan et al., 2005 [41] reported a study of ten patients with DFSP that were treated with surgery and postoperative RT. There was an equal distribution between men and women and the mean age was 39 years. In six patients the lesion affected the head-neck district, in three patients the extremities and, finally, one patient had the tumor on the trunk. Postoperative RT was administered in doses ranging from 59.4 to 65 Gy, the local control was achieved in 90% of patients, and median duration of follow-up was 95 months.
DuBay et al., 2004 [42] described 62 patients with DFSP who underwent surgery. Thirty-nine subjects (63%) were female, twenty-three cases (37%) were male, and the mean age was 42 years. In forty-eight cases (76%) the lesion was located on the trunk or extremities, while in 15 cases (24%) the tumour was located on the head and neck region. Postoperative RT was administered in 5% of cases, with a 100% of local control, and median duration of follow-up was 53 months.
Sun et al., 2000 [43] collected 35 patients with DFSP that were treated with surgery with or without RT. The patients were 24 males and 11 females, with a median age of 37 years. The tumour locations were found on the trunk in 21 cases, the extremities in eight cases, and the head and neck district in six cases. Ten patients (28.5%) were treated with postoperative RT with a dose ranging from 46 to 68 Gy. With a median follow-up of 50 months, 81.8% of patients achieved local control.
Stojadinovic et al., 2000 [44] described 33 patients affected by head and neck DFSP, including 17 women and 16 men, with a mean age of 39 years. The anatomical distribution of tumours included 14 cases of scalp involvement, 6 neck injuries, and 13 face lesions. All patients underwent surgical treatment, and four patients (12.1% of cases) received adjuvant RT in doses ranging from 60 to 66 Gy. At median follow-up of 82 months, 75% of patients achieved local control.
Mentzel et al., 1998 [26] analysed 41 patients affected by the fibrosarcomatous variant of dermatofibrosarcoma protuberans (FS-DFSP), including 19 women and 22 men, with a mean age of 48 years. In twenty-five cases the lesion affected the trunk, in ten cases the tumour involved the extremities, and in five patients the lesion affected the head and neck district. Finally, there was one case in which the anatomical site of the tumour was unknown. In forty patients the tumour was surgically removed, while in one case the exact treatment was unknown. In addition, adjuvant RT was administered in three cases (7.4% of cases). The median follow-up was 90 months.
Ballo et al., 1998 [45] described 19 patients with DFSP who were treated with surgery and adjuvant RT to doses of 50–60 Gy. The patients included 12 males and 7 females with a median age of 40 years. Regarding anatomical distribution, the tumour affected the trunk in eight cases, the head and neck region in seven cases, and the extremities in four cases. At median follow-up of 72 months, 94.7% of patients achieved local control.
Haas et al., 1997 [46] performed a retrospective analysis on 38 patients affected by DFSP and who were surgically managed. A total of 22 females and 16 males with a mean age of 39 years were included. In 13 patients the tumour affected the extremities, in 11 patients the abdomen, in 6 patients the back, and in 5 patients the head and neck. RT was performed in 44.7% of patients in doses of 50–66 Gy, the median follow-up was 68 months, 82.4% of patients achieved local control.
Suit et al., 1996 [47] reported outcomes of eighteen patients affected by DFSP and who were treated with RT to doses of 50–67 Gy. In 15 patients, treatment included the combination of radiotherapy and surgery, while 3 patients received RT alone. Eleven patients were males and seven patients were females, with a mean age of 46 years. Regarding the anatomical distribution, in ten cases the lesion affected the head and neck, in five cases the trunk was affected, and in three patients it was the extremities. The median follow-up was 86 months, 83.3% of patients achieved local control.
Marks et al., 1989 [48] described ten patients with DFSP, including four women and six men, with a mean age of 44.4 years. The lesion involved the head and neck in five cases, in four cases the lesion affected the trunk, and in one case the tumour occurred to the extremities. RT was administered in 70% of patients, and the median follow up was 12 months, 90% of patients achieved local control.
4. Discussion
DFSP accounts for around 1–5% of all soft tissue sarcomas that affect adults, and account for 18% (the most common) of all cutaneous soft tissue sarcomas [49]. The most frequent localizations are on the trunk and extremities, while the head-neck region is less frequently involved [42].
Molecular studies have improved our knowledge on DFSP pathophysiology. Translocation involving chromosomes 17q22 and 22q13 leads to a fusion protein that promotes the continuous activation of PDGF receptor beta (PDGFR-beta) protein-tyrosine kinase, and it is found in more than 90% of DFSP. It seems to play a pivotal role in promoting tumour cell growth. Such a mechanism provides the rationale for the use of imatinib mesylate (IM) and other tyrosine kinase inhibitors (TKIs) in treating advanced DFSP [50].
This tumour is characterized by an infiltrative growth pattern and a high tendency of local recurrence (LR) after the primary resection [51,52]. However, DFSP has low metastatic potential and rarely causes death [31].
LR rates after surgical resection, as reported in the literature, greatly vary and range from 0 to 40% [53]. Although LR usually occur within three years after the surgical resection of the primary tumour, late recurrences are possible.
The most important factor that has a major impact on clinical outcomes for DFSP is the extent of resection. In fact, this high LR rate has been attributed to the missed excision of tumours that are subclinical and have horizontal finger-like extensions in the skin, as well as infiltration of deeper structures. They are clinically unapparent and evidenced only by microscopic examination of lesion margins. Indeed, on microscopic evaluation, multiple projections of neoplastic cells were found to extend laterally or deep up to 3 cm or more from the main lesion [44]. Therefore, the treatment of choice for DSFP is MMS or WLE with histologically confirmed free margins (R0 resection) [54].
MMS is the more frequently recommended approach for DFSP, particularly in tumours developing in sensitive skin areas or for recurrent DSFPs [55]. In MMS, tissue layers are surgically removed sequentially and examined under a microscope during the surgery to define the extent of tumour invasion. Sequential layers are removed until the neoplasm is completely removed. When the tissue sections have been cryostat-frozen, it may be challenging for the pathologist to distinguish the scattered malignant spindle cells from normal fibroblasts. A variant of MMS, named Slow-Mohs, is commonly used. In this MMS variant, tissue samples are not analysed extemporaneously but are fixed and paraffin embedded for a subsequent histological and immunohistochemical evaluation [56,57,58]. However, MMS and its variants are a time-consuming technique, and not widely diffused as standard surgery procedure.
For such reasons, several authors have proposed WLE (with at least 3 cm including the underlying fascia) as a valid therapeutic option to MMS [59].
In some cases, such as facial DFSP, the optimal wide surgical margins of 3 cm are limited by anatomical structures. Postoperative radiotherapy should be considered in cases of close and positive resection margins, but also in cases of several recurrences, in which additional surgical resections could result in potentially disfiguring or functional sequelae [40,41].
The value of RT in the management of DFSP has been mainly assessed in retrospective series [60].
Recently, a series reporting 184 patients highlighted that a free margin <2 cm, as well as the presence of multiple lesions, are both strong predictors of recurrence after a surgical approach and, in both cases, it is advisable to consider adjuvant RT [33].
A smaller analysis coming from another institution was able to identify the initial tumour size >5 cm, the presence of multiple recurrence or of high-grade sarcomatous changes to be clinically relevant indications for adjuvant RT [61].
Considering the salvage setting, which may include either microscopic residual (R1) or macroscopic residual (R2) tumour, RT should be taken into account in all cases where an additional surgery stage could be potentially disfiguring or leading to functional sequelae.
When analysing, in detail, the doses and fractionations schedules, the vast majority of authors use a conventional fractionation (2 Gy/die) with dose ranging from 50 up to 70 Gy in their clinical practice. The clinical target volume should consider the primary anatomical location of the tumour and size of the surgical scar with an additional margin of 3–5 cm, according to the constraints of the surrounding organ at risk.
Considering all the papers reported in this systematic review, only a very few articles had their authors reported adverse events and, in addition, the great variety of the primary anatomical sites involved, which further contributes to the difficulty of providing a comprehensive assessment of the side effects related to adjuvant radiotherapy treatment in DFSP [62].
There are several limitations to our review, in fact, most of the available evidence comes from retrospective series; there are only a few series that include a considerable number of patients. In some series, it is not possible to extrapolate doses and fractionations used for the postoperative RT; in many series, the clinical indication for postoperative RT if adjuvant (presence of risk factors) or salvage (positive margins); in several reports, the number of surgical procedures before postoperative RT is not accurately described; additionally, most data come from single institutions studies.
Prospective larger and multidisciplinary studies are desirable, for all of these reasons, in order to better elucidate these points and improve the clinical knowledge about the role of postoperative RT in DFSP.
5. Conclusions
After primary surgical management, postoperative RT may either be considered as adjuvant treatment (presence of risk factors, i.e., close margins, recurrent tumours, aggressive histological subtypes) or as salvage treatment (positive margins), and should be assessed within the frame of multidisciplinary evaluation.
Author Contributions
Conceptualization, B.F. and A.D.S.; methodology, V.L. and S.C.; validation, S.G., A.R., K.P. A.G.M. and L.T.; data curation, A.P. and G.S.; writing—original draft preparation, A.L.; writing—review and editing, E.R., A.A.C., T.Z. and M.D.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dai, Z.; He, Y.; Zhang, X.; Tian, Z.; Zhu, G.; Ren, Z.; Ye, L.; Liu, Z.; Ma, C.; Cao, W.; et al. Head-and-neck dermatofibrosarcoma protuberans: Survival analysis and Clinically relevant immunohistochemical indicators. Oral. Dis. 2023; early view. [Google Scholar] [CrossRef]
- Darier, S.; Ferrand, M. Dermatofibrosarcomes progressives etricidivantes on fibrosarcomes de la peau. Ann. Dermatol. Venereol. 1924, 5, 545–562. [Google Scholar]
- Hoffmann, E.D.I. Über das knollentreibende Fibrosarkom der Haut (Dermatofibrosarkoma protuberans). Dermatology 1925, 43, 1–28. [Google Scholar] [CrossRef]
- Trofymenko, O.; Bordeaux, J.S.; Zeitouni, N.C. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J. Am. Acad. Dermatol. 2018, 78, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Kreicher, K.L.; Kurlander, D.E.; Gittleman, H.R.; Barnholtz-Sloan, J.S.; Bordeaux, J.S. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. Dermatol. Surg. 2016, 42, S24–S31. [Google Scholar] [CrossRef] [PubMed]
- Bowne, W.B.; Antonescu, C.R.; Leung, D.H.; Katz, S.C.; Hawkins, W.G.; Woodruff, J.M.; Brennan, M.F.; Lewis, J.J. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer 2000, 88, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Jacobs, I.A.; Salti, G.I. Outcomes of surgery for dermatofibrosarcoma protuberans. Eur. J. Surg. Oncol. 2004, 30, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Gloster, H.M., Jr. Dermatofibrosarcoma protuberans. J. Am. Acad. Dermatol. 1996, 35, 355–374. [Google Scholar] [CrossRef]
- LeBlanc, J.; Chan, C.; Zedlitz, A. Dermatofibrosarcoma protuberans. Cutis 2017, 100, E6–E7. [Google Scholar]
- Behfar, K.N.; Mendeszoon, M.J.; Chrzan, J.S.; Habershaw, G.M. Dermatofibrosarcoma protuberans of the hallux. J. Am. Podiatr. Med. Assoc. 1996, 86, 126–128. [Google Scholar] [CrossRef]
- Assassa, G.S.; Siegel, M.E.; Chen, D.C.; Ansari, A.N. Dermatofibrosarcoma protuberans of the toe. Findings on multiple imaging modalities. Clin. Nucl. Med. 1993, 18, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.; Spector, A.; Siddiqui, S.; Mirkin, G.; Yim, J.; Hao, X. Dermatofibrosarcoma Protuberans on Adult Toes: A Case Report and Review of the Literature. Anticancer Res. 2019, 39, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- Takahira, T.; Oda, Y.; Tamiya, S.; Higaki, K.; Yamamoto, H.; Kobayashi, C.; Izumi, T.; Tateishi, N.; Iwamoto, Y.; Tsuneyoshi, M. Detection of COL1A1-PDGFB fusion transcripts and PDGFB/PDGFRB mRNA expression in dermatofibrosarcoma protuberans. Mod. Pathol. 2007, 20, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.G.; Gonda, P.; Sheppard, P.; Keohane, S. Dermatofibrosarcoma Protuberans of the Scalp: A Challenging Tumor with a Proposed Modification to the Slow Mohs Technique. Dermatol. Surg. 2020, 46, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Bouhani, M.; Fertani, Y.; Zemni, I.; Adouni, O.; Bouida, A.; Chargui, R.; Khaled, R. Dermatofibrosarcoma Protuberans of the Breast in Man: An Extremely Rare Entity with a Review of the Literature. J. Investig. Med. High. Impact Case Rep. 2019, 7, 2324709619875634. [Google Scholar] [CrossRef]
- Vecchio, G.M.; Broggi, G.; Mulè, A.; Piombino, E.; Magro, G. Dermatofibrosarcoma protuberans: A tumor in the wide spectrum of the bland-looking spindle cell lesions of the breast. Pathologica 2019, 111, 87–91. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, R.; Tang, Z.; Liu, S. A Rare Malignant Disease, Dermatofibrosarcoma Protuberans of the Breast: A Retrospective Analysis and Review of Literature. Biomed. Res. Int. 2020, 2020, 8852182. [Google Scholar] [CrossRef] [PubMed]
- Edelweiss, M.; Malpica, A. Dermatofibrosarcoma protuberans of the vulva: A clinicopathologic and immunohistochemical study of 13 cases. Am. J. Surg. Pathol. 2010, 34, 393–400. [Google Scholar] [CrossRef]
- Ugurel, S.; Kortmann, R.D.; Mohr, P.; Mentzel, T.; Garbe, C.; Breuninger, H.; Bauer, S.; Grabbe, S. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—Update 2018. J. Dtsch. Dermatol. Ges. 2019, 17, 663–668. [Google Scholar] [CrossRef]
- Ionescu, S.; Nicolescu, A.C.; Madge, O.L.; Simion, L.; Marincas, M.; Ceausu, M. Intra-Abdominal Malignant Melanoma: Challenging Aspects of Epidemiology, Clinical and Paraclinical Diagnosis and Optimal Treatment-A Literature Review. Diagnostics 2022, 12, 2054. [Google Scholar] [CrossRef]
- Gheoca Mutu, D.E.; Avino, A.; Balcangiu-Stroescu, A.E.; Mehedințu, M.; Bălan, D.G.; Brîndușe, L.A.; Popescu, A.M.; Ionescu, D.; Cristea, B.M.; Tomescu, L.F.; et al. Histopathological evaluation of cutaneous malignant melanoma: A retrospective study. Exp. Ther. Med. 2022, 23, 402. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Lin, P.Y.; Chew, K.Y.; Chiang, Y.C. Dermatofibrosarcoma protuberans in children and adolescents: Clinical presentation, histology, treatment, and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1222–1229. [Google Scholar] [CrossRef]
- Apalla, Z.; Liopyris, K.; Kyrmanidou, E.; Fotiadou, C.; Sgouros, D.; Patsatsi, A.; Trakatelli, M.G.; Kalloniati, E.; Lallas, A.; Lazaridou, E. Clinical and Dermoscopic Characteristics of Cutaneous Sarcomas: A Literature Review. Diagnostics 2023, 13, 1822. [Google Scholar] [CrossRef]
- Bernard, J.; Poulalhon, N.; Argenziano, G.; Debarbieux, S.; Dalle, S.; Thomas, L. Dermoscopy of dermatofibrosarcoma protuberans: A study of 15 cases. Br. J. Dermatol. 2013, 169, 85–90. [Google Scholar] [CrossRef]
- Liang, C.A.; Jambusaria-Pahlajani, A.; Karia, P.S.; Elenitsas, R.; Zhang, P.D.; Schmults, C.D. A systematic review of outcome data for dermatofibrosarcoma protuberans with and without fibrosarcomatous change. J. Am. Acad. Dermatol. 2014, 71, 781–786. [Google Scholar] [CrossRef]
- Mentzel, T.; Beham, A.; Katenkamp, D.; Dei Tos, A.P.; Fletcher, C.D. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: Clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am. J. Surg. Pathol. 1998, 22, 576–587. [Google Scholar] [CrossRef]
- Mareş, T.; Răducu, L.; Avino, A.; Gheoca-Mutu, D.E.; Teodoreanu, R.N.; Jecan, C.R. Dermatofibrosarcoma Protuberans: One Centre Experience. Chirurgia 2022, 117, 601–617. [Google Scholar] [CrossRef]
- Saiag, P.; Grob, J.J.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Stratigos, A.; Middelton, M.; Basholt, L.; et al. Diagnosis and treatment of dermatofibrosarcoma protuberans. European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, A.; Abeni, D.; Rusciani, A.; Cigna, E.; Wolter, M.; Scuderi, N.; Rusciani, L.; Kaufmann, R.; Podda, M. Dermatofibrosarcoma protuberans: Wide local excision vs. Mohs micrographic surgery. Cancer Treat. Rev. 2008, 34, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Metgudmath, R.B.; Metgudmath, A.R.; Das, A.T.; Malur, P.R. Dermatofibrosarcoma Protuberans of Face: A Rare Entity and Review of Literature. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74, 5469–5472. [Google Scholar] [CrossRef]
- Fields, R.C.; Hameed, M.; Qin, L.X.; Moraco, N.; Jia, X.; Maki, R.G.; Singer, S.; Brennan, M.F. Dermatofibrosarcoma protuberans (DFSP): Predictors of recurrence and the use of systemic therapy. Ann. Surg. Oncol. 2011, 18, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Henry, O.S.; Platoff, R.; Cerniglia, K.S.; Batchu, S.; Goodwin, B.J.; Sandilos, G.; Adams, A.; Hong, Y.K. Tyrosine kinase inhibitors versus radiation therapy in unresectable dermatofibrosarcoma protuberans (DFSP): A narrative systematic review. Am. J. Surg. 2023, 225, 268–274. [Google Scholar] [CrossRef]
- Du, K.; Li, J.; Tang, L.; Lin, X.; Kong, X.; Liao, X.; Peng, Q.; Dong, Y.; He, J.; Huang, Y.; et al. Role of postoperative radiotherapy in dermatofibrosarcoma protuberans: A propensity score-matched analysis. Radiat. Oncol. 2019, 14, 20. [Google Scholar] [CrossRef]
- Williams, N.; Morris, C.G.; Kirwan, J.M.; Dagan, R.; Mendenhall, W.M. Radiotherapy for dermatofibrosarcoma protuberans. Am. J. Clin. Oncol. 2014, 37, 430–432. [Google Scholar] [CrossRef]
- Hamid, R.; Hafeez, A.; Darzi, M.A.; Zaroo, I.; Rasool, A.; Rashid, H. Outcome of wide local excision in dermatofibrosarcoma protuberans and use of radiotherapy for margin-positive disease. Indian. Dermatol. Online J. 2013, 4, 93–96. [Google Scholar] [CrossRef]
- Uysal, B.; Sager, O.; Gamsiz, H.; Cicek, A.; Demiral, S.; Dincoglan, F.; Surenkok, S.; Demiriz, M.; Beyzadeoglu, M. Evaluation of the role of radiotherapy in the management of dermatofibrosarcoma protuberans. J. BUON 2013, 18, 268–273. [Google Scholar] [PubMed]
- Castle, K.O.; Guadagnolo, B.A.; Tsai, C.J.; Feig, B.W.; Zagars, G.K. Dermatofibrosarcoma protuberans: Long-term outcomes of 53 patients treated with conservative surgery and radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 585–590. [Google Scholar] [CrossRef]
- Palmerini, E.; Gambarotti, M.; Staals, E.L.; Zanella, L.; Sieberova, G.; Longhi, A.; Cesari, M.; Bonarelli, S.; Picci, P.; Ruggieri, P.; et al. Fibrosarcomatous changes and expression of CD34+ and apolipoprotein-D in dermatofibrosarcoma protuberans. Clin. Sarcoma Res. 2012, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Archontaki, M.; Korkolis, D.P.; Arnogiannaki, N.; Konstantinidou, C.; Georgopoulos, S.; Dendrinos, P.; Zarkadas, G.; Kokkalis, G. Dermatofibrosarcoma protuberans: A case series of 16 patients treated in a single institution with literature review. Anticancer Res. 2010, 30, 3775–3779. [Google Scholar] [PubMed]
- Heuvel, S.T.; Suurmeijer, A.; Pras, E.; Van Ginkel, R.J.; Hoekstra, H.J. Dermatofibrosarcoma protuberans: Recurrence is related to the adequacy of surgical margins. Eur. J. Surg. Oncol. 2010, 36, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Morris, C.G.; Zlotecki, R.A.; Scarborough, M.T.; Mendenhall, W.M. Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am. J. Clin. Oncol. 2005, 28, 537–539. [Google Scholar] [CrossRef]
- DuBay, D.; Cimmino, V.; Lowe, L.; Johnson, T.M.; Sondak, V.K. Low recurrence rate after surgery for dermatofibrosarcoma protuberans: A multidisciplinary approach from a single institution. Cancer 2004, 100, 1008–1016. [Google Scholar] [CrossRef]
- Sun, L.M.; Wang, C.J.; Huang, C.C.; Leung, S.W.; Chen, H.C.; Fang, F.M.; Huang, E.Y.; Lee, S.P. Dermatofibrosarcoma protuberans:treatment results of 35 cases. Radiother. Oncol. 2000, 57, 175–181. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Karpoff, H.M.; Antonescu, C.R.; Shah, J.P.; Singh, B.; Spiro, R.H.; Dumornay, W.; Shaha, A.R. Dermatofibrosarcoma protuberans of the head and neck. Ann. Surg. Oncol. 2000, 7, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Ballo, M.T.; Zagars, G.K.; Pisters, P.; Pollack, A. The role of radiation therapy in the management of dermatofibrosarcoma protuberans. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Haas, R.L.; Keus, R.B.; Loftus, B.M.; Rutgers, E.J.; van Coevorden, F.; Bartelink, H. The role of radiotherapy in the local management of dermatofibrosarcoma protuberans. Soft Tissue Tumours Working Group. Eur. J. Cancer 1997, 33, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.; Spiro, I.; Mankin, H.J.; Efird, J.; Rosenberg, A.E. Radiation in management of patients with dermatofibrosarcoma protuberans. J. Clin. Oncol. 1996, 14, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Suit, H.D.; Rosenberg, A.E.; Wood, W.C. Dermatofibrosarcoma protuberans treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Yamamoto, H.; Oda, Y. Current Update on the Molecular Biology of Cutaneous Sarcoma: Dermatofibrosarcoma Protuberans. Curr. Treat. Options Oncol. 2019, 20, 29. [Google Scholar] [CrossRef]
- McArthur, G. Molecularly targeted treatment for dermatofibrosarcoma protuberans. Semin. Oncol. 2004, 31, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lemm, D.; Mügge, L.O.; Mentzel, T.; Höffken, K. Current treatment options in dermatofibrosarcoma protuberans. J. Cancer Res. Clin. Oncol. 2009, 135, 653–665. [Google Scholar] [CrossRef]
- Sundram, U.N. Review: Dermatofibrosarcoma protuberans: Histologic approach and updated treatment recommendations. Clin. Adv. Hematol. Oncol. 2009, 7, 406–408. [Google Scholar] [PubMed]
- Monnier, D.; Vidal, C.; Martin, L.; Danzon, A.; Pelletier, F.; Puzenat, E.; Algros, M.P.; Blanc, D.; Laurent, R.; Humbert, P.H.; et al. Dermatofibrosarcoma protuberans: A population-based cancer registry descriptive study of 66 consecutive cases diagnosed between 1982 and 2002. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.P.; Galante, J.M.; Bold, R.J.; Schneider, P.D.; Ramsamooj, R.; Goodnight, J.E., Jr. Dermatofibrosarcoma protuberans: Reappraisal of wide local excision and impact of inadequate initial treatment. Ann. Surg. Oncol. 2003, 10, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; La Rosa, G.; Di Buduo, A.; Paradisi, A.; Soglia, S.; Calzavara-Pinton, P.; Amerio, P. Conventional surgery compared with formalin-fixed tissue Mohs surgery (slow Mohs) for DFSP: A comparative analysis of 83 cases. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1393–e1395. [Google Scholar] [CrossRef]
- Snow, S.N.; Gordon, E.M.; Larson, P.O.; Bagheri, M.M.; Bentz, M.L.; Sable, D.B. Dermatofibrosarcoma protuberans: A report on 29 patients treated by Mohs micrographic surgery with long-term follow-up and review of the literature. Cancer 2004, 101, 28–38. [Google Scholar] [CrossRef]
- Massey, R.A.; Tok, J.; Strippoli, B.A.; Szabolcs, M.J.; Silvers, D.N.; Eliezri, Y.D. A comparison of frozen and paraffin sections in dermatofibrosarcoma protuberans. Dermatol. Surg. 1998, 24, 995–998. [Google Scholar] [CrossRef]
- Khaddaj, L.; Martinot-Duquennoy, V.; Guerreschi, P.; Mortier, L.; Calibre, C. Mohs micrographic surgery for skin cancers: A 10 year-single-center series of 548 patients treated by formalin-fixed tissue Mohs surgery assessing the impact of reduced margins. Ann. Chir. Plast. Esthet. 2021, 6, 429–439. [Google Scholar] [CrossRef]
- Sondak, V.K.; Cimmino, V.M.; Lowe, L.M.; Dubay, D.A.; Johnson, T.M. Dermatofibrosarcoma protuberans: What is the best surgical approach? Surg. Oncol. 1999, 8, 183–189. [Google Scholar] [CrossRef]
- Rutkowski, P.; Debiec-Rychter, M. Current treatment options for dermatofibrosarcoma protuberans. Expert. Rev. Anticancer Ther. 2015, 15, 901–909. [Google Scholar] [CrossRef]
- Verma, H.; Sehgal, K.; Panchal, K.B.; Chakraborty, S.; Biswas, B.; Mukherjee, G.; Midha, D.; Biswas, G. Presentation and Management of Dermatofibrosarcoma Protuberans: A Single Center Protocol. Indian. J. Surg. Oncol. 2020, 11, 35–40. [Google Scholar] [CrossRef]
- Buck, D.W., 2nd; Kim, J.Y.; Alam, M.; Rawlani, V.; Johnson, S.; Connor, C.M.; Dumanian, G.A.; Wayne, J.D. Multidisciplinary approach to the management of dermatofibrosarcoma protuberans. J. Am. Acad. Dermatol. 2012, 67, 861–866. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).