Intraoperative Predictors and Proposal for a Novel Prognostic Risk Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN Score)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Thoracoabdominal/suprarenal/pararenal aortic aneurysms;
- Symptomatic patients without CT signs of rupture;
- Isolated iliac aneurysms;
- Previous open/endovascular AAA repair;
- Death during operation time.
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Intraoperative Factors: Univariate Analysis
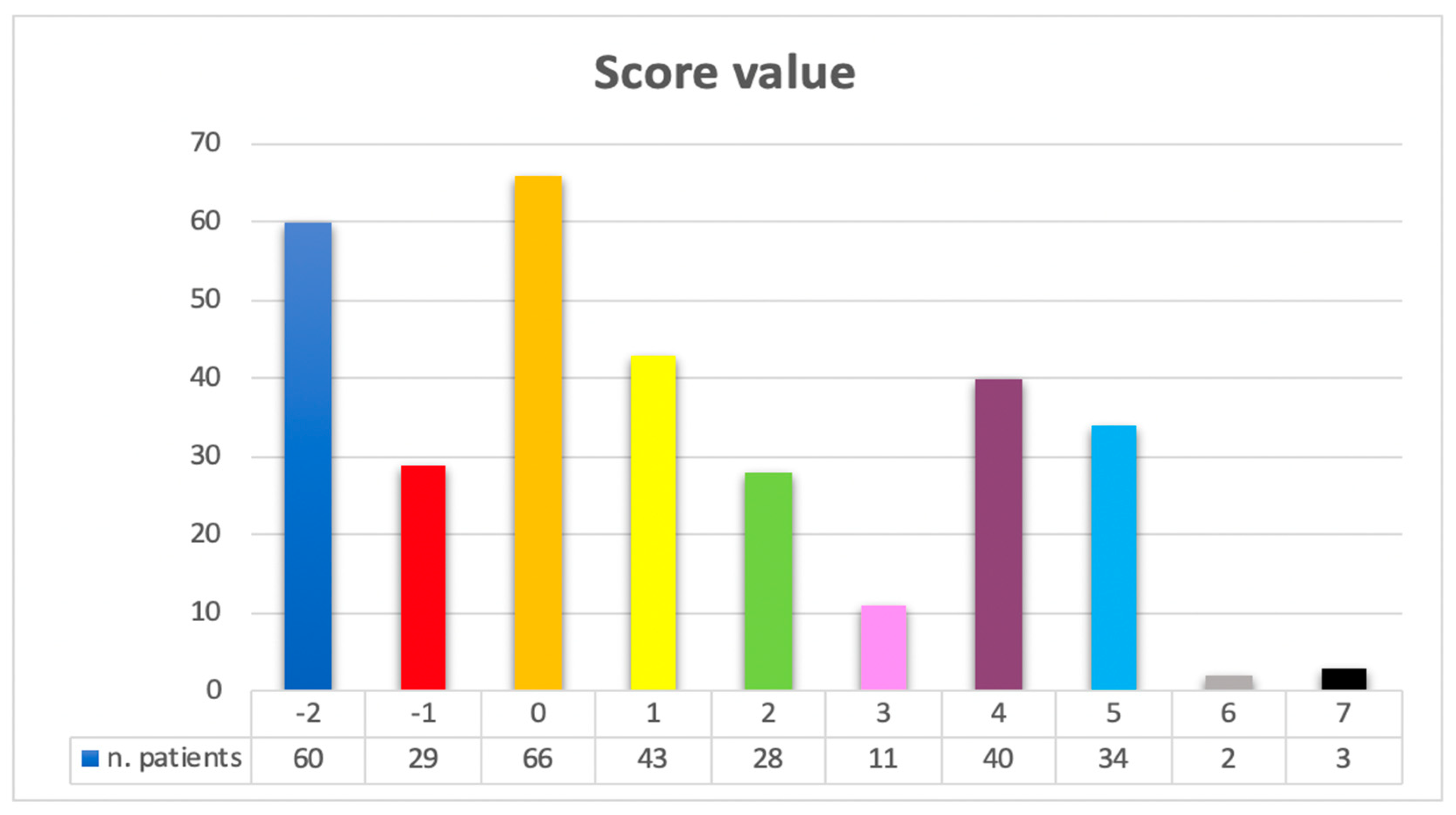
3.3. Prognostic Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN)
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reimerink, J.J.; van der Laan, M.J.; Koelemay, M.J.; Balm, R.; Legemate, D.A. Systematic review and meta-analysis of population-based mortality from ruptured abdominal aortic aneurysm. Br. J. Surg. 2013, 11, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 12, 2805. [Google Scholar] [CrossRef] [PubMed]
- Karthikesalingam, A.; Holt, P.J.; Vidal-Diez, A.; Ozdemir, B.A.; Poloniecki, J.D.; Hinchliffe, R.J.; Thompson, M.M. Mortality from ruptured abdominal aortic aneurysms: Clinical lessons from a comparison of outcomes in England and the USA. Lancet 2014, 383, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Dias-Neto, M.; Castro-Ferreira, R.; Mani, K.; Freitas, A.; Leite-Moreira, A.; Sampaio, S.M. Nationwide Analysis of Ruptured Abdominal Aortic Aneurysm in Portugal (2000–2015). Eur. J. Vasc. Endovasc. Surg. 2020, 1, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kwan, S.; Colvard, B.D.; d’Audiffret, A.; Kashyap, V.S.; Cho, J.S. Impact of interfacility transfer of ruptured abdominal aortic aneurysm patients. J. Vasc. Surg. 2022, 6, 1548–1554. [Google Scholar] [CrossRef]
- Hemingway, J.F.; French, B.; Caps, M.; Benyakorn, T.; Quiroga, E.; Tran, N.; Singh, N.; Starnes, B.W. Preoperative risk score accuracy confirmed in a modern ruptured abdominal aortic aneurysm experience. J. Vasc. Surg. 2021, 5, 1508–1518. [Google Scholar] [CrossRef]
- Samy, A.K.; Murray, G.; MacBain, G. Glasgow aneurysm score. Cardiovasc. Surg. 1994, 2, 41–44. [Google Scholar]
- Chen, J.C.; Hildebrand, H.D.; Salvian, A.J.; Taylor, D.C.; Strandberg, S.; Myckatyn, T.M.; Hsiang, Y.N. Predictors of death in nonruptured and ruptured abdominal aortic aneurysms. J. Vasc. Surg. 1996, 24, 614–620. [Google Scholar] [CrossRef]
- Tambyraja, A.; Murie, J.; Chalmers, R. Predictors of outcome after abdominal aortic aneurysm rupture: Edinburgh Ruptured Aneurysm Score. World J. Surg. 2007, 31, 2243–2247. [Google Scholar] [CrossRef]
- Hardman, D.T.; Fisher, C.M.; Patel, M.I.; Neale, M.; Chambers, J.; Lane, R.; Appleberg, M. Ruptured abdominal aortic aneurysms: Who should be offered surgery? J. Vasc. Surg. 1996, 23, 123–129. [Google Scholar] [CrossRef]
- Prytherch, D.R.; Sutton, G.L.; Boyle, J.R. Portsmouth POSSUM models for abdominal aortic aneurysm surgery. Br. J. Surg. 2001, 7, 958–963. [Google Scholar] [CrossRef]
- Robinson, W.P.; Schanzer, A.; Li, Y.; Goodney, P.P.; Nolan, B.W.; Eslami, M.H.; Cronenwett, J.L.; Messina, L.M. Derivation and validation of a practical risk score for prediction of mortality after open repair of ruptured abdominal aortic aneurysms in a US regional cohort and comparison to existing scoring systems. J. Vasc. Surg. 2013, 57, 354–361. [Google Scholar] [CrossRef]
- Garland, B.T.; Danaher, P.J.; Desikan, S.; Tran, N.T.; Quiroga, E.; Singh, N.; Starnes, B.W. Preoperative risk score for the prediction of mortality after repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2018, 68, 991–997. [Google Scholar] [CrossRef]
- von Meijenfeldt, G.C.; van Beek, S.C.; Bastos Goncalves, F.; Verhagen, H.J.; Zeebregts, C.J.; Vahl, A.C.; Wisselink, W.; van der Laan, M.; Balm, R. Development and external validation of a model predicting death after surgery in patients with a ruptured abdominal aortic aneurysm: The Dutch Aneurysm Score. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hirzalla, O.; Emous, M.; Ubbink, D.T.; Legemate, D. External validation of the Glasgow Aneurysm Score to predict outcome in elective open abdominal aortic aneurysm repair. J. Vasc. Surg. 2006, 4, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Tambyraja, A.L.; Fraser, S.C.; Murie, J.A.; Chalmers, R.T. Validity of the Glasgow Aneurysm Score and the Hardman Index in predicting outcome after ruptured abdominal aortic aneurysm repair. Br. J. Surg. 2005, 5, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Montoya, S.B.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. European society for Vasculòar Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Troisi, N.; Isernia, G.; Bertagna, G.; Adami, D.; Baccani, L.; Parlani, G.; Berchiolli, R.; Simonte, G. Preoperative factors affecting long-term mortality in patients survived to ruptured abdominal aortic aneurysm repair. Int. Angiol. 2023, 4, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.M.; Shahin, Y.; Din, W.; Akomolafe, B.; Johnson, B.F.; Renwick, P.; Chetter, I.; McCollum, P. Perioperative, Postoperative, and Long-Term Outcomes Following Open Surgical Repair of Ruptured Abdominal Aortic Aneurysm. Angiology 2020, 7, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Arici, V.; Bozzani, A.; Rossi, M.; Corbetta, R.; Brunetto, M.B.; Scudeller, L.; Ticozzelli, G.; Rossini, R.; Rota, M.; Ragni, F. Contemporary Early and Long-Term Results of Open Repair for Ruptured and Symptomatic Unruptured Infrarenal AAA. Single Center Experience. Ann. Vasc. Surg. 2020, 64, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.G.; de Vries, J.P.; Werson, D.A.; van Dongen, E.P.; Schreve, M.A.; Ünlü, Ç. Evaluation of five different aneurysm scoring systems to predict mortality in ruptured abdominal aortic aneurysm patients. J. Vasc. Surg. 2016, 6, 1609–1616. [Google Scholar] [CrossRef]
- Grandi, A.; Bertoglio, L.; Lepidi, S.; Kölbel, T.; Mani, K.; Budtz-Lilly, J.; DeMartino, R.; Scali, S.; Hanna, L.; Troisi, N.; et al. Risk Prediction Models for Peri-Operative Mortality in Patients Undergoing Major Vascular Surgery with Particular Focus on Ruptured Abdominal Aortic Aneurysms: A Scoping Review. J. Clin. Med. 2023, 17, 5505. [Google Scholar] [CrossRef]
- Troisi, N.; Bertagna, G.; Saratzis, A.; Guadagni, S.; Minichilli, F.; Adami, D.; Ferrari, M.; Berchiolli, R. Intraoperative predictors of in-hospital mortality after open repair of ruptured abdominal aortic aneurysms. Int. Angiol. 2023, 4, 310–317. [Google Scholar] [CrossRef]
- Ozen, A.; Hanedan, M.O.; Songur, Ç.M.; Boysan, E.; Unal, E.U.; Mola, S.; Erkengel, H.I.; Kubat, E.; Iscan, Z.; Tutun, U.; et al. Risk Factors for Survival following Open Surgical Repair of Ruptured Abdominal Aortic Aneurysms: A 13-Year Experience. J. Tehran Heart Cent. 2015, 3, 117–121. [Google Scholar]
- Kunishige, H.; Ishibashi, Y.; Kawasaki, M.; Morimoto, K.; Inoue, N. Risk factors affecting survival after surgical repair of ruptured abdominal aortic aneurysm. Ann. Vasc. Dis. 2013, 3, 631–636. [Google Scholar] [CrossRef]
- Acher, C.; Acher, C.W.; Castello Ramirez, M.C.; Wynn, M. Operative Mortality and Morbidity in Ruptured Abdominal Aortic Aneurysms in the Endovascular Age. Ann. Vasc. Surg. 2020, 66, 70–76. [Google Scholar] [CrossRef]
- Reitz, K.M.; Phillips, A.R.; Tzeng, E.; Makaroun, M.S.; Leeper, C.M.; Liang, N.L. Characterization of immediate and early mortality after repair of ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2022, 6, 1578–1587.e5. [Google Scholar] [CrossRef]
- Kim, S.D.; Hwang, J.K.; Park, S.C.; Kim, J.I.; Moon, I.S.; Park, J.S.; Yun, S.S. Predictors of postoperative mortality of ruptured abdominal aortic aneurysm: A retrospective clinical study. Yonsei Med. J. 2012, 4, 772–780. [Google Scholar] [CrossRef][Green Version]
- Davidović, L.; Marković, M.; Kostić, D.; Cinara, I.; Marković, D.; Maksimović, Z.; Cvetković, S.; Sindjelic, R.; Ille, T. Ruptured abdominal aortic aneurysms: Factors influencing early survival. Ann. Vasc. Surg. 2005, 1, 29–34. [Google Scholar] [CrossRef]
- Georgakis, P.; Paraskevas, K.I.; Bessias, N.; Mikhailidis, D.P.; Andrikopoulos, V.; Katsouli-Liapis, I. Duration of aortic cross-clamping during elective open abdominal aortic aneurysm repair operations and postoperative cardiac/renal function. Int. Angiol. 2010, 29, 244–248. [Google Scholar] [PubMed]
- Mufarrih, S.H.; Schaefer, M.S.; Sharkey, A.; Fassbender, P.; Qureshi, N.Q.; Quraishi, I.; Fatima, H.; Schermerhorn, M.; Mahmood, F.; Matyal, R. Open Abdominal Aortic Aneurysm Surgery and Renal Dysfunction; Association of Demographic and Clinical Variables with Proximal Clamp Location. Ann. Vasc. Surg. 2022, 84, 239–249. [Google Scholar] [CrossRef]
- Fransson, M.; Rydningen, H.; Henriksson, A.E. Early coagulopathy in patients with ruptured abdominal aortic aneurysm. Clin. Appl. Thromb. Hemost. 2012, 18, 96–99. [Google Scholar] [CrossRef]
- Welborn, M.B., 3rd; Seeger, J.M. Prevention and management of sigmoid and pelvic ischemia associated with aortic surgery. Semin. Vasc. Surg. 2001, 4, 255–265. [Google Scholar] [CrossRef]
- Farivar, B.S.; Kalsi, R.; Drucker, C.B.; Goldstein, C.B.; Sarkar, R.; Toursavadkohi, S. Implications of concomitant hypogastric artery embolization with endovascular repair of infrarenal abdominal aortic aneurysms. J. Vasc. Surg. 2017, 66, 95–101. [Google Scholar] [CrossRef][Green Version]
- de Athayde Soares, R.; Campos, A.B.C.; Figueiredo, P.W.S.; Vaz, J.H.L.G.; Brienze, C.S.; Waisberg, J.; Sacilotto, R. The Importance of the Hypogastric Artery Preservation during Treatment for Aortoiliac Aneurysms: A Prospective Single-Center Study. Ann. Vasc. Surg. 2023, 92, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, F.; Troëng, T. Treatment choice and survival after ruptured abdominal aortic aneurysm: A population-based study. J. Vasc. Surg. 2020, 72, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Sweeting, M.J.; Thompson, M.M.; Ashleigh, R.; Bell, R.; Gomes, M.; Greenhalgh, R.M.; Grieve, R.; Heatley, F.; Hinchliffe, R.J.; et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ 2014, 348, f7661. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Antoniou, S.A.; Torella, F. Editor’s Choice-Endovascular vs. Open Repair for Abdominal Aortic Aneurysm: Systematic Review and Meta-analysis of Updated Peri-operative and Long Term Data of Randomised Controlled Trials. Eur. J. Vasc. Endovasc. Surg. 2020, 3, 385–397. [Google Scholar] [CrossRef]
- D’Oria, M.; Scali, S.; Neal, D.; DeMartino, R.; Beck, A.W.; Mani, K.; Lepidi, S.; Huber, T.S.; Stone, D.H. Center Volume and Failure to Rescue after Open or Endovascular Repair of Ruptured Abdominal Aortic Aneurysms. J. Vasc. Surg. 2022, 76, 1565–1576. [Google Scholar] [CrossRef]
- D’Oria, M.; Hansen, K.; Schermerhorn, M.; Bower, T.C.; Mendes, B.C.; Shuja, F.; Oderich, G.S.; DeMartino, R.R. Editor’s Choice—Short-term and long-term outcomes after endovascular or open repair for ruptured infrarenal abdominal aortic aneurysms in the Vascular Quality Initiative. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 703–716. [Google Scholar] [CrossRef]
- Menges, A.L.; D’Oria, M.; Zimmermann, A.; Dueppers, P. Ruptured abdominal aorto-iliac aneurysms: Diagnosis, treatment, abdominal compartment syndrome, and role of simulation-based training. Semin. Vasc. Surg. 2023, 36, 163–173. [Google Scholar] [CrossRef] [PubMed]
| Demographic Features | n. Patients = 316 |
|---|---|
| Age (mean ± SD) | 77.3 ± 8.5 |
| Age > 80 years | 131 (41.5%) |
| Male sex | 208 (65.8%) |
| Comorbidities | |
| 237 (75%) |
| 78 (24.7%) |
| 42 (13.3%) |
| 94 (29.7%) |
| 236 (11.4%) |
| 63 (19.9%) |
| 24 (7.6%) |
| 32 (10.1%) |
| Preoperative clinical status | |
| 43 (13.6%) |
| 104 (32.9%) |
| 68 (21.5%) |
| 115 (36.4%) |
| 152 (48.1%) |
| Variables | n. Patients Alive (219) | n. Patients Dead (97) | p |
|---|---|---|---|
| Hemoperitoneum | |||
| - yes | 61 (27.9%) | 51 (52.6%) | <0.001 |
| Operation time > 240 min | |||
| - yes | 73 (33.3%) | 47 (48.5%) | 0.008 |
| Suprarenal clamping | |||
| - yes | 115 (52.5%) | 69 (71.1%) | 0.001 |
| Supraceliac clamping | |||
| - yes | 27 (12.3%) | 17 (17.5%) | 0.146 |
| Tube graft | |||
| - yes | 106 (48.4%) | 45 (46.4%) | 0.957 |
| Patency of at least one hypogastric artery | |||
| - yes | 210 (95.9%) | 85 (87.6%) | 0.008 |
| Inflammatory aneurysm | |||
| - yes | 15 (6.8%) | 2 (2.1%) | 0.132 |
| Mycotic aneurysm | |||
| - yes | 6 (2.7%) | 1 (1%) | 0.094 |
| Variables | B | Standard Error | Odds Ratio | p | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|---|---|
| Hemoperitoneum | 0.973 | 0.262 | 13.788 | <0.001 | 2.645 | 1.583 | 4.420 |
| Operation time > 240 min | 0.553 | 0.264 | 4.381 | 0.036 | 1.738 | 1.036 | 2.917 |
| Suprarenal clamping | 0.689 | 0.277 | 6.187 | 0.013 | 1.991 | 1.157 | 3.426 |
| Patency of at least one hypogastric artery | −1.236 | 0.487 | 6.450 | 0.011 | 0.290 | 0.112 | 0.754 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berchiolli, R.; Troisi, N.; Bertagna, G.; D’Oria, M.; Mezzetto, L.; Malquori, V.; Artini, V.; Motta, D.; Grosso, L.; Grando, B.; et al. Intraoperative Predictors and Proposal for a Novel Prognostic Risk Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN Score). J. Clin. Med. 2024, 13, 1384. https://doi.org/10.3390/jcm13051384
Berchiolli R, Troisi N, Bertagna G, D’Oria M, Mezzetto L, Malquori V, Artini V, Motta D, Grosso L, Grando B, et al. Intraoperative Predictors and Proposal for a Novel Prognostic Risk Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN Score). Journal of Clinical Medicine. 2024; 13(5):1384. https://doi.org/10.3390/jcm13051384
Chicago/Turabian StyleBerchiolli, Raffaella, Nicola Troisi, Giulia Bertagna, Mario D’Oria, Luca Mezzetto, Vittorio Malquori, Valerio Artini, Duilio Motta, Lorenzo Grosso, Beatrice Grando, and et al. 2024. "Intraoperative Predictors and Proposal for a Novel Prognostic Risk Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN Score)" Journal of Clinical Medicine 13, no. 5: 1384. https://doi.org/10.3390/jcm13051384
APA StyleBerchiolli, R., Troisi, N., Bertagna, G., D’Oria, M., Mezzetto, L., Malquori, V., Artini, V., Motta, D., Grosso, L., Grando, B., Badalamenti, G., Calvagna, C., Mastrorilli, D., Veraldi, G. F., Adami, D., & Lepidi, S. (2024). Intraoperative Predictors and Proposal for a Novel Prognostic Risk Score for In-Hospital Mortality after Open Repair of Ruptured Abdominal Aortic Aneurysms (SPARTAN Score). Journal of Clinical Medicine, 13(5), 1384. https://doi.org/10.3390/jcm13051384








