Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators
Abstract
1. Introduction
2. Materials and Methods
2.1. Dermoscopy
2.2. LC-OCT
2.3. RCM Device
2.4. Image Acquisition and Evaluation
3. Results
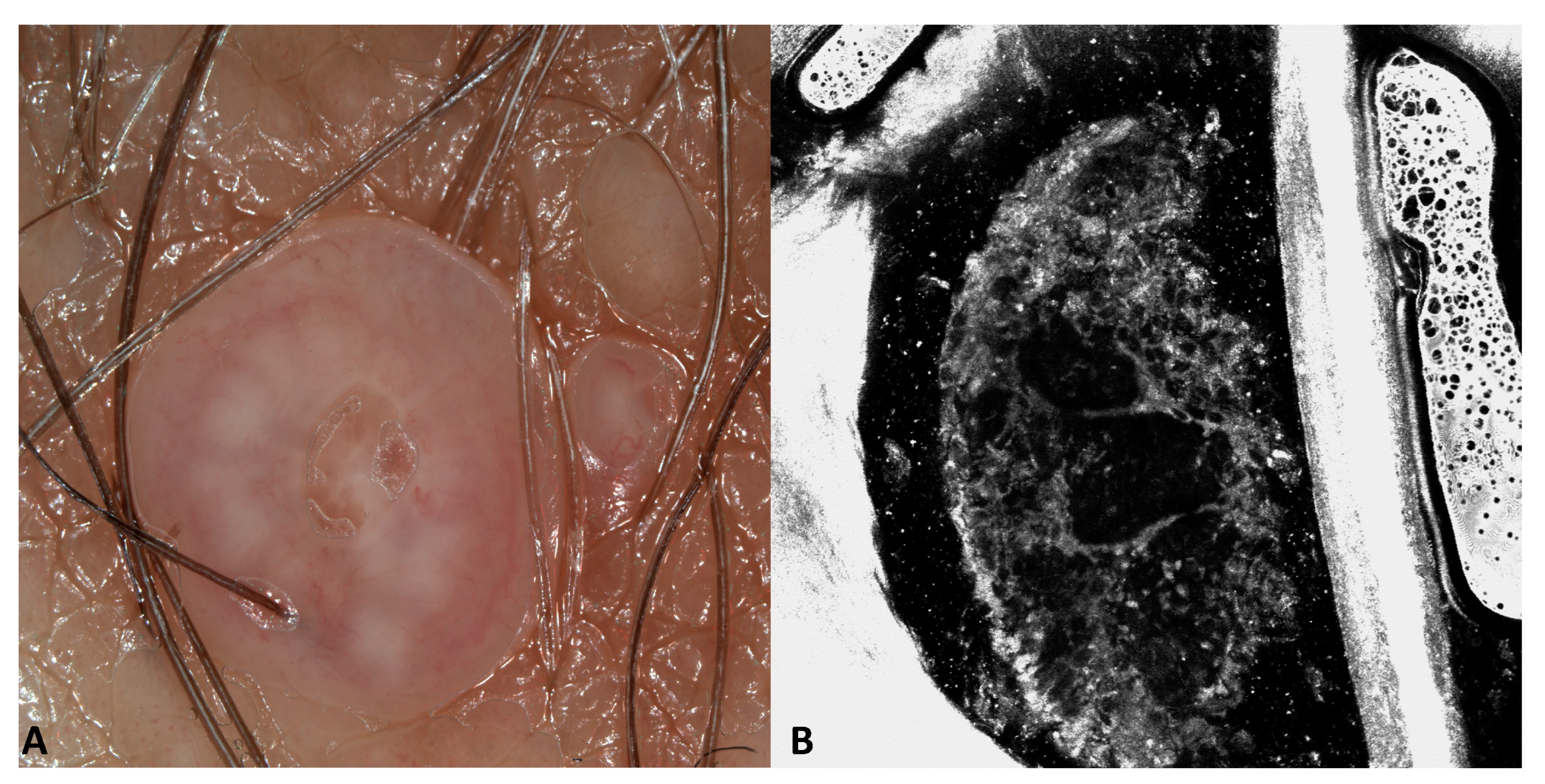
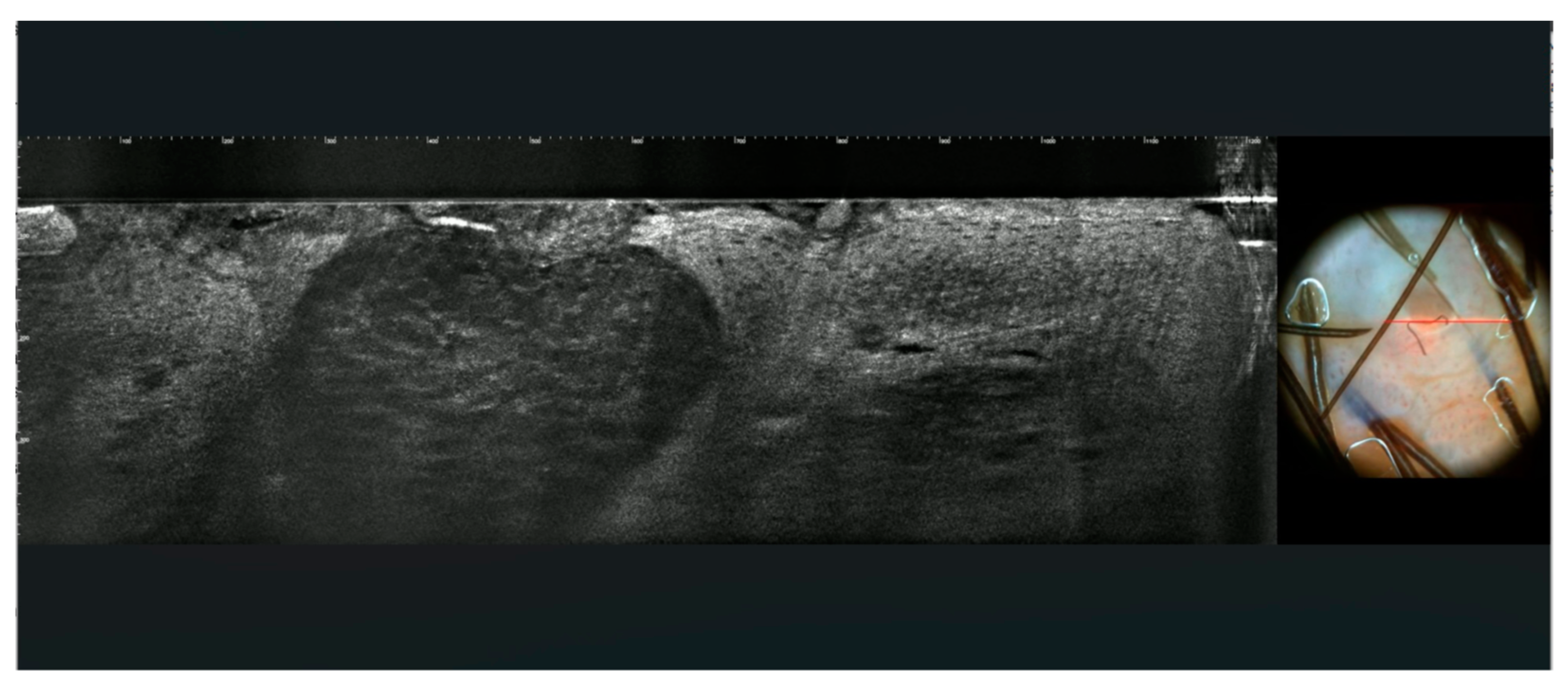
3.1. Images of the Genital Warts
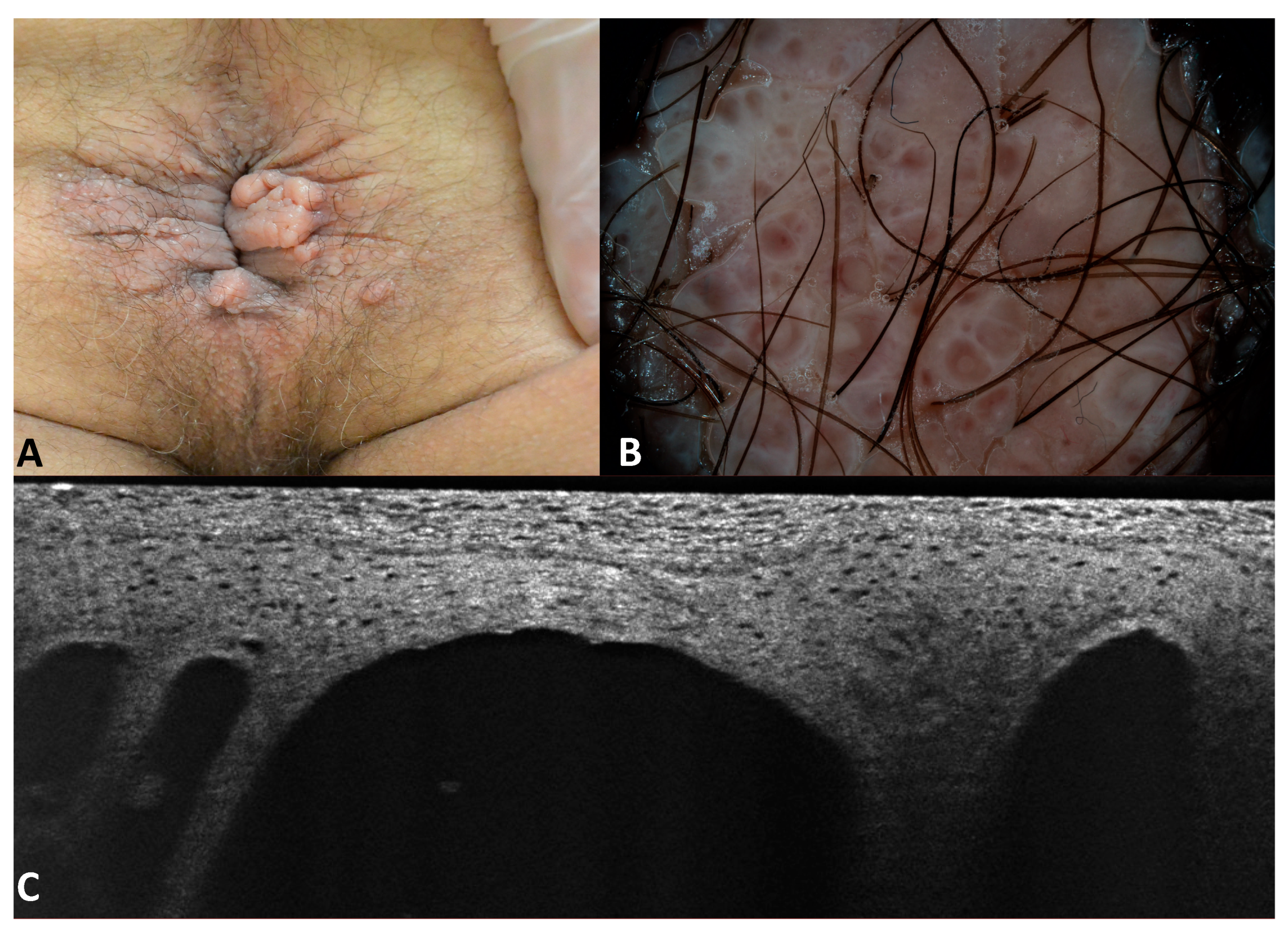
3.2. Images of the Imitators of Genital Warts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plotzker, R.E.; Vaidya, A.; Pokharel, U.; Stier, E.A. Sexually Transmitted Human Papillomavirus: Update in Epidemiology, Prevention, and Management. Infect. Dis. Clin. N. Am. 2023, 37, 289–310. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.E.; Werner, R.N.; Becker, J.C.; Brockmeyer, N.H.; Esser, S.; Hampl, M.; Hommel, S.; Jongen, J.; Mestel, D.S.; Meyer, T.; et al. S2k guideline: HPV-associated lesions of the external genital region and the anus—Anogenital warts and precancerous lesions of the vulva, the penis, and the peri- and intra-anal skin (short version). J. Dtsch. Dermatol. Ges. 2018, 16, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-field confocal optical coherence tomography for high-resolution noninvasive imaging of skin tumors. J. Biomed. Opt. 2018, 23, 106007–106009. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Framil, V.M.D.S.; Nadal, S.R.; Marta, A.C.; Lellis, R.F. Genital warts: Comparing clinical findings to dermatoscopic aspects, in vivo reflectance confocal features and histopathologic exam. An. Bras. Dermatol. 2014, 89, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Shu, D.; Campbell, T.M.; Frühauf, J.; Soyer, P.; Hofmann-Wellenhof, R. Dermatoscopy of genital warts. J. Am. Acad. Dermatol. 2011, 64, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Waddell, A.; Star, P.; Guitera, P. Advances in the use of reflectance confocal microscopy in melanoma. Melanoma Manag. 2018, 5, MMT04. [Google Scholar] [CrossRef]
- Cinotti, E.; Perrot, J.L.; Labeille, B.; Cambazard, F. Reflectance confocal microscopy for cutaneous infections and infestations. J. Eur. Acad. Dermatol. Venereol. 2015, 30, 754–763. [Google Scholar] [CrossRef]
- Reinholz, M.; Clanner-Engelshofen, B.M.; Heppt, M.V.; Hirai, Y.; Ruzicka, T.; Berking, C.; Von Braunmühl, T. Successful Treatment of Genital Warts with Ingenol Mebutate Monitored with Optical Coherence Tomography and Reflectance Confocal Microscopy. Ann. Dermatol. 2019, 31, 434–437. [Google Scholar] [CrossRef]
- Tang, Z.; Lu, J.; Huang, J.; Ding, S. Challenge of reflectance confocal microscopy in the diagnosis of genital warts. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 988–993. [Google Scholar]
- Lacarrubba, F.; Verzì, A.E.; Puglisi, D.F.; Micali, G. Line-field confocal optical coherence tomography: A novel, non-invasive imaging technique for a rapid, in-vivo diagnosis of herpes infection of the skin. J. Eur. Acad. Dermatol. Venereol. 2021, 35, E404–E406. [Google Scholar] [CrossRef]
- Suppa, M.; Fontaine, M.; Dejonckheere, G.; Cinotti, E.; Yélamos, O.; Diet, G.; Tognetti, L.; Miyamoto, M.; Cano, C.O.; Perez-Anker, J.; et al. Line-field confocal optical coherence tomography of basal cell carcinoma: A descriptive study. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 1099–1110. [Google Scholar] [CrossRef]
- Edwards, S.; Boffa, M.; Janier, M.; Calzavara-Pinton, P.; Rovati, C.; Salavastru, C.; Rongioletti, F.; Wollenberg, A.; Butacu, A.; Skerlev, M.; et al. 2020 European guideline on the management of genital molluscum contagiosum. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 17–26. [Google Scholar] [CrossRef]
- Wang, B.; Guo, S.; Yao, Y.; Zhang, G. Clinical and dermoscopic overlap of genital molluscum contagiosum with condyloma acuminate. SAGE Open Med. Case Rep. 2022, 10, 2050313X221086100. [Google Scholar] [CrossRef]
- Micali, G.; Lacarrubba, F. Augmented diagnostic capability using videodermatoscopy on selected infectious and non-infectious penile growths. Int. J. Dermatol. 2011, 50, 1501–1505. [Google Scholar] [CrossRef]
- Cinotti, E.; Labeille, B.; Douchet, C.; Chovet, M.; Habougit, C.; Cambazard, F.; Perrot, J.L.; Groupe Imagerie Cutanée Non Invasive de la Société Française de Dermatologie. Role of in vivo and ex vivo confocal microscopy and of optical coherence tomography as aids in the diagnosis of molluscum contagiosum. Ann. Dermatol. Venereol. 2016, 143, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Verzì, A.E.; Micali, G.; Lacarrubba, F. Line-field confocal optical coherence tomography in molluscum contagiosum: A case series. J. Eur. Acad. Dermatol. Venereol. 2021, 35, E934–E936. [Google Scholar] [CrossRef] [PubMed]
- Karaarslan, I.; Oraloglu, G.; Yaman, B. A case of lymphangioma circumscriptum with new reflectance confocal microscopy findings. Australas. J. Dermatol. 2021, 62, E283–E285. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Umegaki-Arao, N.; Futei, Y.; Iwabuchi, E.; Ishizaki, S.; Tanaka, M. Two cases of acquired lymphangioma: Dermoscopic-histopathologic correlation between irregular vessels on lacunae. J. Dermatol. 2021, 48, E506–E507. [Google Scholar] [CrossRef] [PubMed]
- Dermoscopic Features of Pearly Penile Papules|Dermatology|Karger Publishers. Available online: https://karger.com/drm/article/217/1/21/112681/Dermoscopic-Features-of-Pearly-Penile-Papules (accessed on 2 January 2024).
- Kim, S.H.; Seo, S.H.; Ko, H.C.; Kwon, K.S.; Kim, M.B. The use of dermatoscopy to differentiate vestibular papillae, a normal variant of the female external genitalia, from condyloma acuminata. J. Am. Acad. Dermatol. 2009, 60, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, I.; Brito, J.; Alos, L.; Malvehy, J.; Puig, S. In vivo characterization of solitary angiokeratoma by reflectance confocal microscopy and high definition optical coherence tomography. J. Am. Acad. Dermatol. 2015, 72 (Suppl. S1), S43–S44. [Google Scholar] [CrossRef]
- Tognetti, L.; Cinotti, E.; Coriolani, G.; Suppa, M.; Perrot, J.L.; Vascotto, M.; Grosso, S.; Rubegni, P. Cutaneous lesions of Anderson-Fabry disease examined with a novel technique: Line-field confocal optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E371–E373. [Google Scholar] [CrossRef] [PubMed]
- Ürün, Y.G.; Ürün, M.; Fıçıcıoğlu, S. A case of perianal bowenoid papulosis: Dermoscopic features and a review of previous cases. Acta Dermatovenerol. Alp. Pannonica Adriat. 2021, 30, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Mazzilli, S.; Gamo-Villegas, R.; Pampin-Franco, A.; Estebaran, J.L.L.; Pinedo, F.; Vollono, L.; Di Prete, M.; Campione, E.; Gonzalez, S. Reflectance Confocal Microscopy of Pigmented Bowen’s Disease: A Case Series of Difficult to Diagnose Lesions. Case Rep. Dermatol. 2020, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cozma, E.C.; Celarel, A.M.; Stoenica, I.V.; Lupu, M.; Banciu, L.M.; Voiculescu, V.M. Correlations between Histopathological and Confocal Reflectance Microscopy Aspects in a Patient with Bowenoid Papulosis. Diagnostics 2023, 13, 1531. [Google Scholar] [CrossRef]
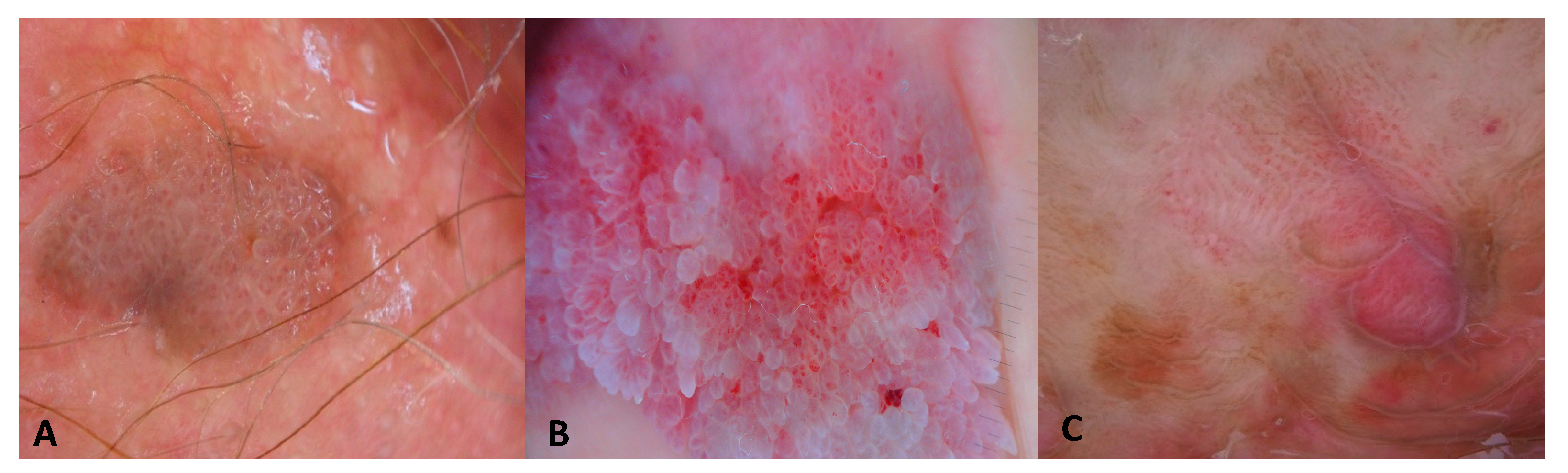
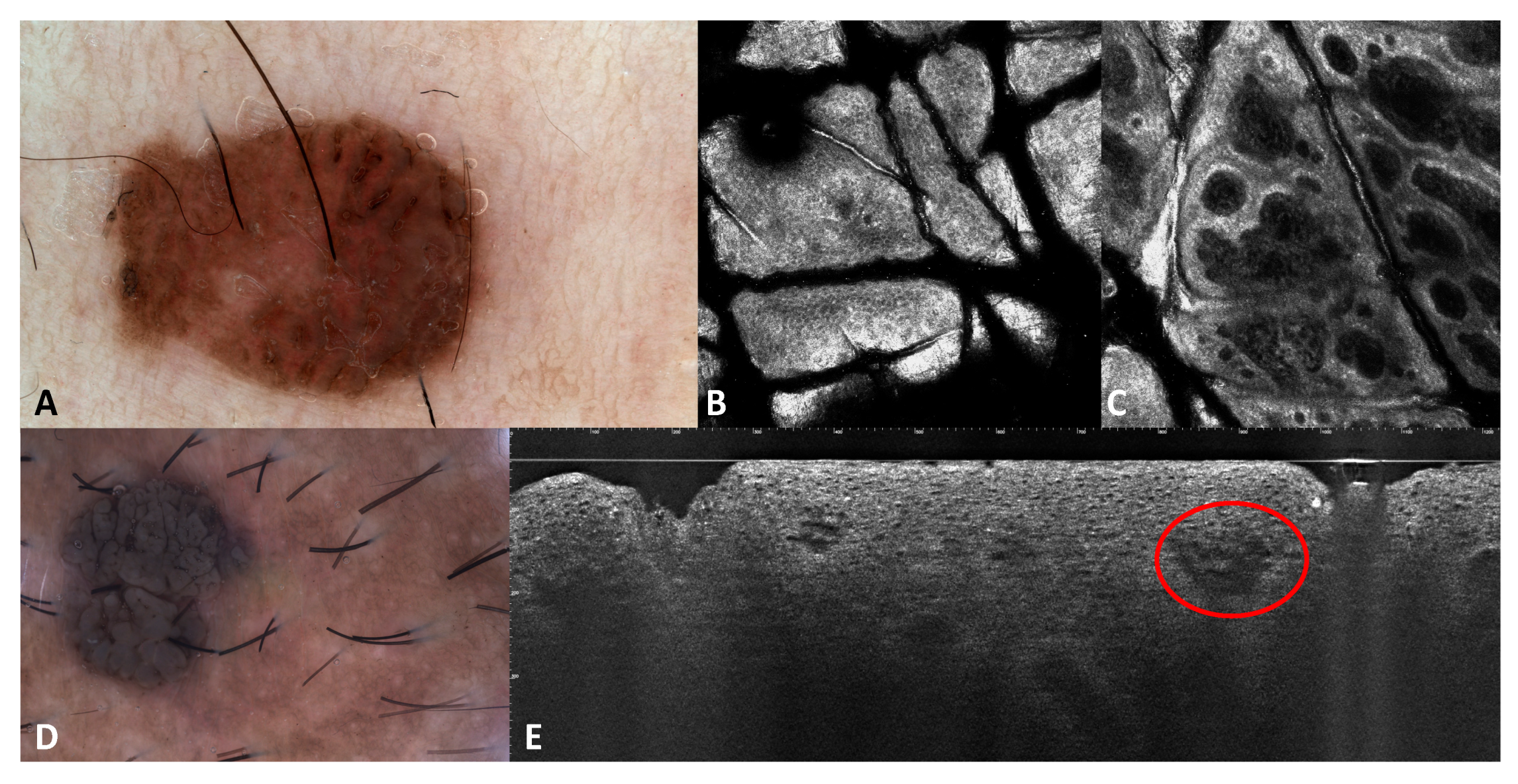

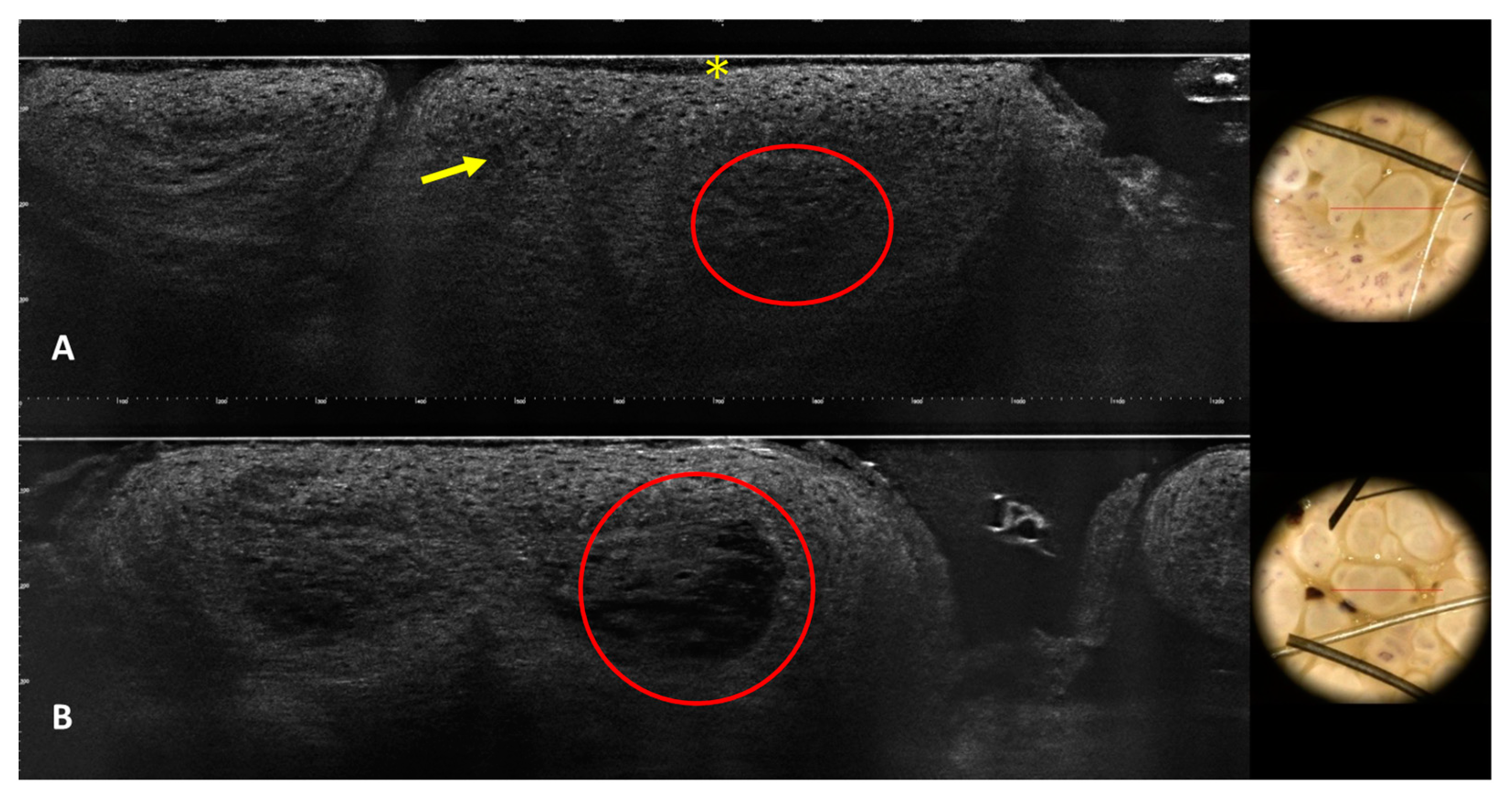
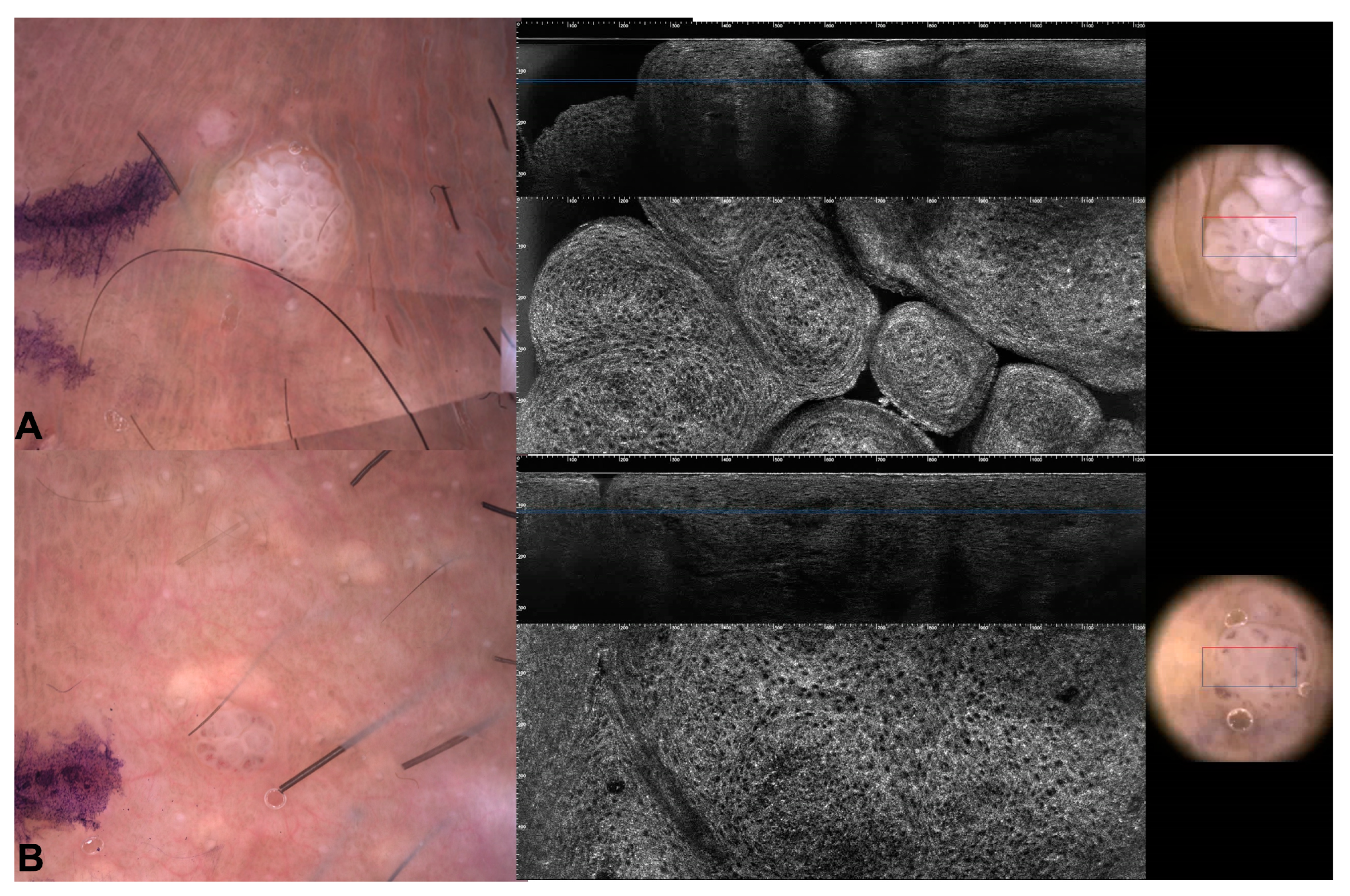


| Patient Number | N. of Lesions | Sex | Age | RCM Images | LC-OCT Images | Site | Clinical Diagnosis | Dermoscopy Diagnosis | Histological Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | M | 44 | yes | yes | inguinal region | SK vs. wart | Wart | wart |
| 2 | 4 | M | 50 | yes | yes | pubis and penis | SK vs. wart | Wart | wart |
| 3 | 1 | F | 40 | yes | no | Pubis | SK | SK | wart |
| 4 | 2 | M | 50 | no | yes | Pubis | wart | wart | wart |
| 5 | 1 | F | 35 | no | yes | Lip | wart | wart | wart |
| 6 | 2 | M | 30 | no | yes | pubis | SK | SK | wart |
| 7 | 3 | F | 50 | no | no | vagina | SK vs. wart | wart | wart |
| 8 | 3 | M | 60 | no | no | perianal region | wart | wart | wart |
| 9 | 1 | M | 61 | no | no | urethra | fibroma | Fibroma vs. wart | wart |
| 10 | 8 | F | 23 | yes | no | labia majora | MC vs. wart | MC vs. wart | MC |
| 11 | 3 | F | 43 | yes | yes | labia majora | MC vs. wart | MC vs. wart | MC |
| 12 | 1 | M | 52 | no | no | pubis | MC vs. wart | MC vs. wart | MC |
| 13 | 1 | F | 48 | no | no | labia minora | wart | MC vs. Fordyce’s spot | Fordyce’s spot |
| 14 | multiple | M | 51 | yes | yes | perianal area | wart | lymphangiectasia | lymphangiectasia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinotti, E.; Barbarossa, L.; Cortonesi, G.; Lamberti, A.; La Marca, F.; Tognetti, L.; Rubegni, P.; Perrot, J.L. Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators. J. Clin. Med. 2024, 13, 1345. https://doi.org/10.3390/jcm13051345
Cinotti E, Barbarossa L, Cortonesi G, Lamberti A, La Marca F, Tognetti L, Rubegni P, Perrot JL. Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators. Journal of Clinical Medicine. 2024; 13(5):1345. https://doi.org/10.3390/jcm13051345
Chicago/Turabian StyleCinotti, Elisa, Lorenzo Barbarossa, Giulio Cortonesi, Arianna Lamberti, Francesca La Marca, Linda Tognetti, Pietro Rubegni, and Jean Luc Perrot. 2024. "Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators" Journal of Clinical Medicine 13, no. 5: 1345. https://doi.org/10.3390/jcm13051345
APA StyleCinotti, E., Barbarossa, L., Cortonesi, G., Lamberti, A., La Marca, F., Tognetti, L., Rubegni, P., & Perrot, J. L. (2024). Non-Invasive Imaging for the Diagnosis of Genital Warts and Their Imitators. Journal of Clinical Medicine, 13(5), 1345. https://doi.org/10.3390/jcm13051345








