Short- and Long-Term Outcomes of Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy in Elderly Patients with Resectable and Borderline Resectable Pancreatic Cancer: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Neoadjuvant Chemoradiotherapy
2.3. Surgery
2.4. Follow-Up
2.5. Measurement of Results
2.6. Statistical Analyses
3. Results
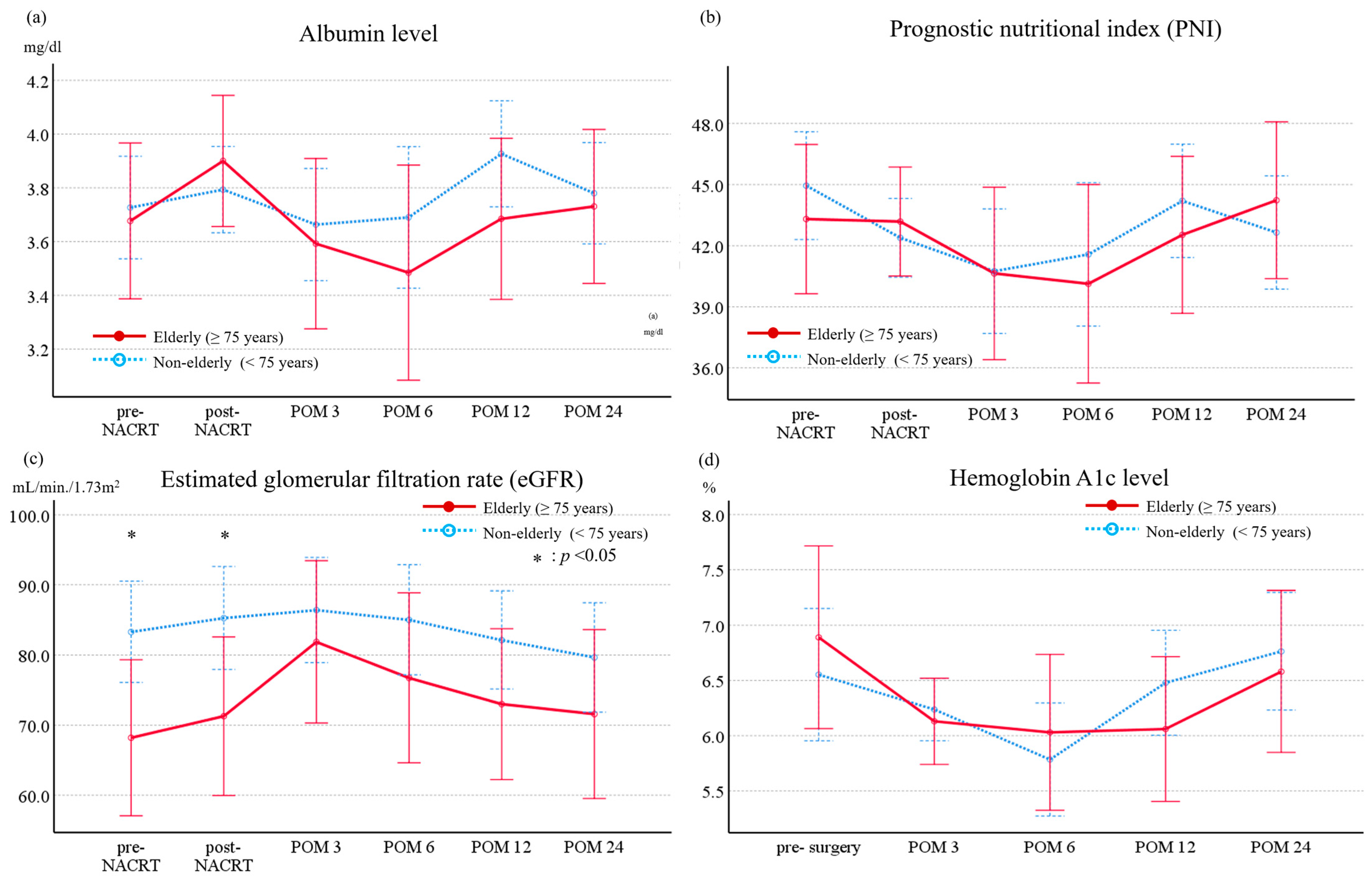
3.1. Long-Term Evolution of Nutritional Markers, Renal Function, and Endocrine Function in Patients Undergoing Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy
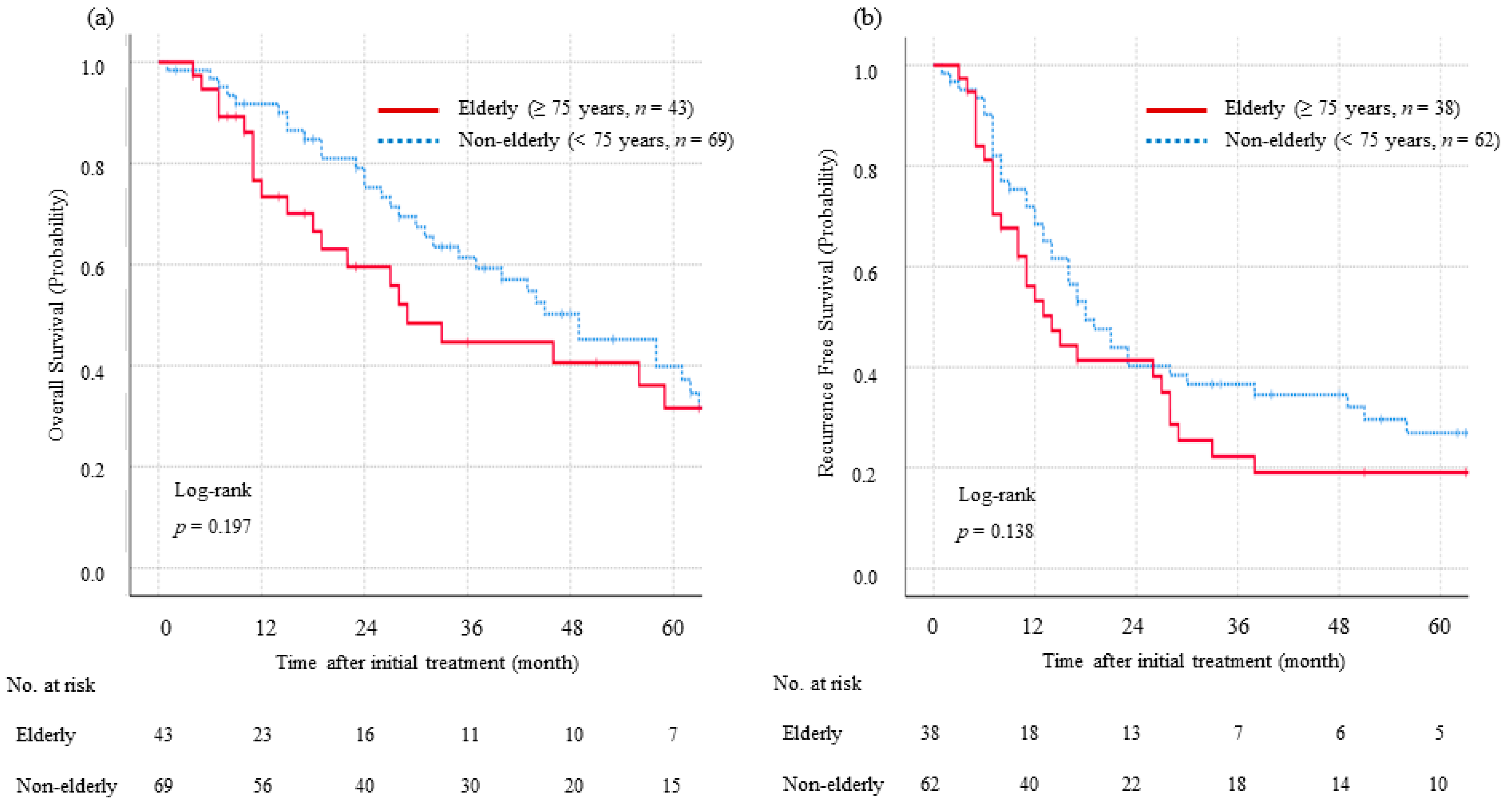
3.2. Long-Term Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Statistics 2023. Available online: https://apps.who.int/iris/bitstream/handle/10665/367912/9789240074323-eng.pdf?sequence=1&isAllowed=y (accessed on 7 November 2023).
- Zhu, X.H.; Wu, Y.F.; Qiu, Y.D.; Jiang, C.P.; Ding, Y.T. Effect of early enteral combined with parenteral nutrition in patients undergoing pancreaticoduodenectomy. World J. Gastroenterol. 2013, 19, 5889–5896. [Google Scholar] [CrossRef] [PubMed]
- Hatzaras, I.; Schmidt, C.; Klemanski, D.; Muscarella, P.; Melvin, W.S.; Ellison, E.C.; Bloomston, M. Pancreatic resection in the octogenarian: A safe option for pancreatic malignancy. J. Am. Coll. Surg. 2011, 212, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Sclabas, G.; Lombardo, K.R.; Sarr, M.G.; Nagorney, D.; Kendrick, M.L.; Donohue, J.H.; Que, F.G.; Farnell, M.B. Pancreatoduodenectomy for ductal adenocarcinoma in the very elderly; is it safe and justified? J. Gastrointest. Surg. 2010, 14, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Winter, J.M.; Schafer, M.; Cunningham, S.C.; Cameron, J.L.; Yeo, C.J.; Clavien, P.A. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann. Surg. 2006, 244, 931–937; discussion 937–939. [Google Scholar] [CrossRef] [PubMed]
- Tani, M.; Kawai, M.; Hirono, S.; Ina, S.; Miyazawa, M.; Nishioka, R.; Shimizu, A.; Uchiyama, K.; Yamaue, H. A pancreaticoduodenectomy is acceptable for periampullary tumors in the elderly, even in patients over 80 years of age. J. Hepatobiliary Pancreat. Surg. 2009, 16, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, M.; Aoki, H.; Nagahisa, S.; Une, Y.; Kimura, Y.; Watanabe, M.; Taniguchi, F.; Arata, T.; Katsuda, K.; Tanakaya, K. Nutritional assessment and surgical outcomes in very elderly patients undergoing pancreaticoduodenectomy: A retrospective study. Surg. Today 2021, 51, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Oguro, S.; Shimada, K.; Kishi, Y.; Nara, S.; Esaki, M.; Kosuge, T. Perioperative and long-term outcomes after pancreaticoduodenectomy in elderly patients 80 years of age and older. Langenbecks Arch. Surg. 2013, 398, 531–538. [Google Scholar] [CrossRef]
- Belyaev, O.; Herzog, T.; Kaya, G.; Chromik, A.M.; Meurer, K.; Uhl, W.; Müller, C.A. Pancreatic surgery in the very old: Face to face with a challenge of the near future. World J. Surg. 2013, 37, 1013–1020. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology Pancreatic Adenocarcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 17 October 2023).
- Suto, H.; Oshima, M.; Ando, Y.; Matsukawa, H.; Takahashi, S.; Shibata, T.; Kamada, H.; Kobara, H.; Masaki, T.; Kumamoto, K.; et al. Efficacy of neoadjuvant chemoradiotherapy followed by pancreatic resection for older patients with resectable and borderline resectable pancreatic ductal adenocarcinoma. HPB 2023, 25, 136–145. [Google Scholar] [CrossRef]
- Okano, K.; Suto, H.; Oshima, M.; Maeda, E.; Yamamoto, N.; Kakinoki, K.; Kamada, H.; Masaki, T.; Takahashi, S.; Shibata, T.; et al. A Prospective Phase II Trial of Neoadjuvant S-1 with Concurrent Hypofractionated Radiotherapy in Patients with Resectable and Borderline Resectable Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 2777–2784. [Google Scholar] [CrossRef]
- Suto, H.; Okano, K.; Oshima, M.; Ando, Y.; Matsukawa, H.; Takahashi, S.; Shibata, T.; Kamada, H.; Kobara, H.; Tsuji, A.; et al. Efficacy and Safety of Neoadjuvant Chemoradiation Therapy Administered for 5 Versus 2 Weeks for Resectable and Borderline Resectable Pancreatic Cancer. Pancreas 2022, 51, 269–277. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zulke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2016. [Google Scholar]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B.; Rich, T.A.; Byrd, D.R.; Cleary, K.R.; Connelly, J.H.; Levin, B.; Charnsangavej, C.; Fenoglio, C.J.; Ames, F.C. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch. Surg. 1992, 127, 1335–1339. [Google Scholar] [CrossRef]
- Takahashi, S.; Ohno, I.; Ikeda, M.; Konishi, M.; Kobayashi, T.; Akimoto, T.; Kojima, M.; Morinaga, S.; Toyama, H.; Shimizu, Y.; et al. Neoadjuvant S-1 With Concurrent Radiotherapy Followed by Surgery for Borderline Resectable Pancreatic Cancer: A Phase II Open-label Multicenter Prospective Trial (JASPAC05). Ann. Surg. 2022, 276, e510–e517. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.; Shi, Q.; Ahmad, S.A.; Herman, J.M.; Marsh Rde, W.; Collisson, E.; Schwartz, L.; Frankel, W.; Martin, R.; Conway, W.; et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016, 151, e161137. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: A phase 2 clinical trial. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Sulpice, L.; Rayar, M.; D’Halluin, P.N.; Harnoy, Y.; Merdrignac, A.; Bretagne, J.F.; Meunier, B.; Boudjema, K. Impact of age over 75 years on outcomes after pancreaticoduodenectomy. J. Surg. Res. 2012, 178, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, R.; Spaggiari, M.; Di Benedetto, F.; Montalti, R.; Masetti, M.; De Ruvo, N.; Romano, A.; Guerrini, G.P.; De Blasiis, M.G.; Gerunda, G.E. Do not deny pancreatic resection to elderly patients. J. Gastrointest. Surg. 2009, 13, 341–348. [Google Scholar] [CrossRef]
- Okadome, K.; Baba, Y.; Yagi, T.; Kiyozumi, Y.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann. Surg. 2020, 271, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Nakata, K.; Kibe, S.; Mori, Y.; Miyasaka, Y.; Ohuchida, K.; Ohtsuka, T.; Oda, Y.; Nakamura, M. Prognostic Value of Preoperative Nutritional and Immunological Factors in Patients with Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 3996–4003. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; Tsujita, E.; Fukuzawa, K.; Sugimachi, K.; Iguchi, T.; Ninomiya, M.; Maeda, T.; Kajiyama, K.; Adachi, E.; Uchiyama, H.; et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: A multi-institutional retrospective study. Pancreatology 2021, 21, 1356–1363. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hendry, P.O.; Coolsen, M.M.; Bemelmans, M.H.; Lassen, K.; Revhaug, A.; Fearon, K.C.; Garden, O.J.; Dejong, C.H.; Enhanced Recovery After Surgery (ERAS) Group. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br. J. Surg. 2008, 95, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, C.; Zhao, Y.; Chen, W.; Huang, Y. Enhanced recovery after surgery for pancreaticoduodenectomy: Review of current evidence and trends. Int. J. Surg. 2018, 50, 79–86. [Google Scholar] [CrossRef]
- Balzano, G.; Zerbi, A.; Braga, M.; Rocchetti, S.; Beneduce, A.A.; Di Carlo, V. Fast-track recovery programme after pancreaticoduodenectomy reduces delayed gastric emptying. Br. J. Surg. 2008, 95, 1387–1397. [Google Scholar] [CrossRef]
| Elderly (≥75 Years, n = 43) | Non-Elderly (<75 Years, n = 69) | p Value | ||
|---|---|---|---|---|
| Age, year, median (range, IQR) | 79 (75–84, 77–81) | 68 (44–74, 63–71) | <0.001 | |
| Sex, male/female, n (%) | 22 (51)/21 (49) | 43 (62)/26 (38) | 0.245 | |
| Body mass index, kg/m2, median (range, IQR) | 22.0 (15.0–27.9, 19.9–23.7) | 22.3 (14.0–27.9, 19.9–23.9) | 0.818 | |
| Hemoglobin level, g/dL, median (range, IQR) | 11.1 (7.8–15.5, 10.0–12.6) | 11.8 (8.3–15.0, 11.0–13.2) | 0.040 | |
| Total bilirubin level, mg/dl, median (range, IQR) | 0.6 (0.3–1.0, 0.5–0.8) | 0.6 (0.2–6.5, 0.5–0.8) | 0.690 | |
| Performance status *, 0/1, n (%) | 22 (51)/21 (49) | 55 (80)/14 (20) | 0.002 | |
| Comorbidity, n (%) | ||||
| Cardiac disease Chronic kidney disease Diabetes mellitus Pulmonary disease Use of anticoagulants Use of steroid | 3 (7) 4 (9) 19 (44) 3 (7) 10 (23) 4 (9) | 4 (6) 1 (1) 21 (30) 2 (3) 6 (9) 2 (3) | 0.802 0.050 0.140 0.309 0.032 0.143 | |
| Preoperative biliary drainage, n (%) | 22 (51) | 47 (68) | 0.073 | |
| Resectability †, n (%) | ||||
| Resectable Borderline resectable | 31 (72) 12 (28) | 40 (58) 29 (42) | 0.131 | |
| NACRT protocol, n (%) | ||||
| 2-week S1 regimen 5-week S1 regimen 2-week Gemcitabine with S1 regimen | 15 (35) 15 (35) 13 (30) | 27 (39) 24 (35) 18 (26) | 0.864 | |
| Completion of NACRT protocol, n (%) | 39 (91) | 56 (81) | 0.171 | |
| 2-week S1 regimen 5-week S1 regimen 2-week Gemcitabine with S1 regimen | 15 (100) 13 (87) 11 (85) | 23 (85) 19 (79) 14 (78) | 0.117 0.553 0.634 | |
| Adverse events ‡ during NACRT, ≥Grade 3, n (%) | 10 (23) | 12 (17) | 0.447 | |
| 2-week S1 regimen 5-week S1 regimen 2-week Gemcitabine with S1 regimen | 2 (13) 3 (20) 5 (38) | 2 (7) 4 (17) 6 (33) | 0.531 0.792 0.768 | |
| Resection rate, n (%) | 38 (88) | 62 (90) | 0.805 | |
| Parameter | Elderly (≥75 Years, n = 43) | Non-Elderly (<75 Years, n = 69) | p | ||
|---|---|---|---|---|---|
| Complete blood count, median (range, IQR) | |||||
| White blood cell count,/mm3 | Pre Post | 4990 (2690–11560, 4170–6160) 4220 (2580–8060, 3250–5060) | 5670 (2930–12030, 4685–6795) 4110 (1690–10460, 3285–5345) | 0.053 0.924 | |
| Neutrophil count,/mm3 | Pre Post | 2911 (1400–8033, 2615–4050) 2420 (1201–6650, 1892–3606) | 3613 (1509–9107, 2777–4348) 2810 (989–8954, 2118–3631) | 0.044 0.572 | |
| Lymphocyte count,/mm3 | Pre Post | 1238 (668–2750, 1001–1743) 797 (269–2623, 561–1020) | 1431 (601–3167, 1221–1779) 879 (169–2370, 603–1134) | 0.062 0.446 | |
| Monocyte count,/mm3 | Pre Post | 332 (129–682, 271–384) 329 (132–648, 289–436) | 365 (102–1376, 278–427) 350 (106–743, 235–471) | 0.100 0.964 | |
| Platelet count, ×104/mm3 | Pre Post | 20.3 (10.1–34.7, 15.7–25.7) 18.3 (7.9–35.5, 13.4–24.0) | 23.2 (11.3–43.2, 20.2–26.5) 19.2 (7.9–37.8, 14.1–23.8) | 0.006 0.680 | |
| eGFR, mL/min./1.73m2, median (range, IQR) | Pre Post | 74.0 (36.8–112.4, 61.8–81.4) 72.9 (42.2–114.6, 59.0–91.3) | 83.4 (47.1–135.5, 70.3–96.9) 84.5 (33.1–144.2, 74.3–100.7) | 0.001 0.004 | |
| Nutritional indices, median (range, IQR) | |||||
| Albumin level, g/dL | Pre Post | 3.6 (2.6–4.6, 3.3–3.9) 3.6 (2.5–4.4, 3.3–3.9) | 3.7 (2.3–4.6, 3.5–4.1) 3.7 (1.8–4.9, 3.3–4.0) | 0.003 0.373 | |
| Total cholesterol level, mg/dL | Pre Post | 166 (21–268, 144–184) 152 (84–259, 126–176) | 175 (89–256, 154–204) 161 (98–283, 141–188) | 0.082 0.190 | |
| Prognostic nutritional index (PNI) | Pre Post | 43.0 (31.0–52.9, 38.3–46.5) 40.8 (28.2-51.1, 35.6-44.4) | 46.1 (27.8-56.7, 42.1-49.1) 42.0 (20.3-53.6, 36.9-45.7) | 0.017 0.199 | |
| Inflammatory biomarkers, median (range, IQR) | |||||
| C-reactive protein, mg/dL | Pre Post | 0.20 (0.02-2.82, 0.08-0.46) 0.11 (0.02-3.70, 0.04-0.44) | 0.18 (0.01-3.73, 0.08-0.46) 0.14 (0.01-4.54, 0.05-0.39) | 0.940 0.495 | |
| Neutrophil-to-lymphocyte ratio (NLR) | Pre Post | 2.46 (0.97-7.83, 1.92-2.94) 3.61 (0.92-10.69, 2.33-5.10) | 2.37 (0.99-7.83, 1.87-2.98) 3.30 (0.86-16.60, 2.20-4.77) | 0.781 0.532 | |
| Platelet-to-lymphocyte ratio (PLR) | Pre Post | 151.7 (49.1–405.6, 117.3–199.7) 218.7 (74.0–636.2, 163.4–349.4) | 162.8 (57.3–363.1, 134.0–193.3) 211.0 (63.8–1053.3, 161.4–303.0) | 0.438 0.528 | |
| Lymphocyte-to-monocyte ratio (LMR) | Pre Post | 4.41 (2.25–7.75, 3.31–4.94) 2.21 (0.70–6.31, 1.51–3.29) | 4.22 (1.27–12.22, 3.31–5.40) 2.43 (0.76–9.40, 1.96–3.52) | 0.863 0.228 | |
| C-reactive protein-to-albumin ratio (CRP/Alb) | Pre Post | 0.051 (0.005–1.007, 0.022–0.132) 0.029 (0.005–1.240, 0.012–0.147) | 0.044 (0.003–1.243, 0.019–0.136) 0.041 (0.003–1.376, 0.014–0.101) | 0.841 0.564 | |
| Tumor markers, median (IQR, range) | |||||
| CEA level, ng/ml | Pre Post | 3.2 (1.0–56.4, 1.8–4.9) 3.5 (0.9–34.7, 2.3–5.1) | 3.6 (0.5–158.3, 2.5–5.4) 3.2 (0.8–11.6, 2.2–4.4) | 0.306 0.513 | |
| CA19-9 level, U/mL | Pre Post | 140 (2–18159, 66–813) 83 (2–52480, 25–469) | 213 (2–70555, 19–813) 68 (2–2500, 10–209) | 0.677 0.130 | |
| SUVmax level in FDG-PET, median (IQR, range) | Pre Post | 6.91 (2.10–30.49, 4.97–10.61) 4.60 (1.44–11.14, 2.79–6.95) | 7.55 (2.00–45.71, 4.97–10.61) 4.26 (1.56–47.57, 3.07–6.02) | 0.185 0.438 | |
| Elderly (≥75 Years, n = 38) | Non-Elderly (<75 Years, n = 62) | p | ||
|---|---|---|---|---|
| Operation time, min, median (range, IQR) | 499 (357–733, 437–555) | 503 (349–816, 468–584) | 0.416 | |
| Blood loss, ml, median (range, IQR) | 1376 (305–6970, 764–2165) | 1059 (88–9268, 565–1830) | 0.091 | |
| Intraoperative transfusion, n (%) | 20 (53) | 19 (31) | 0.029 | |
| Portal vein resection, n (%) | 17 (45) | 30 (48) | 0.723 | |
| Mortality, within 90 days, n (%) | 1 (3) | 1 (2) | 0.724 | |
| Morbidity ‡, ≥Grade 3, n (%) | 11 (29) | 8 (13) | 0.047 | |
| Pancreatic fistula *, Grade B or C, n (%) | 2 (5) | 4 (6) | 0.808 | |
| Delayed gastric emptying †, Grade B or C, n (%) | 13 (34) | 10 (16) | 0.037 | |
| Postoperative hospital stay, day, median (range, IQR) | 28 (12–203, 16–56) | 22 (11–96, 16–32) | 0.022 | |
| Resection status, R0/R1, n (%) | 34 (89)/4 (11) | 62 (100)/0 (0) | 0.009 | |
| Node positive pathology, n (%) | 13 (34) | 32 (52) | 0.090 | |
| Pathological tumor response to NACRT §, n (%) | ||||
| Grade I-IIA Grade IIB-IV | 24 (63) 14 (37) | 38 (56) 26 (42) | 0.614 | |
| TNM stage ¶, n (%) | ||||
| 0 IA IB IIA IIB III IV | 1 (3) 7 (18) 14 (37) 3 (8) 13 (34) 0 (0) 0 (0) | 3 (5) 15 (24) 9 (15) 2 (3) 25 (40) 5 (8) 3 (5) | 0.064 | |
| Postoperative adjuvant chemotherapy received, n (%) | 27 (71) | 52 (84) | 0.127 | |
| S-1 Gemcitabine | 27 (100) 0 (0) | 49 (94) 3 (6) | 0.203 | |
| Cause of death (n = 55 cases) | ||||
| Pancreatic cancer specific Non-pancreatic cancer specific | 15 (68) 7 (32) | 30 (88) 4 (12) | 0.065 | |
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR | p | HR | 95% CI | p | |
| Monocyte count after NACRT, ≥323/mm3 | 2.37 | 0.059 | |||
| Lymphocyte count after NACRT, <958/mm3 | 3.04 | 0.024 | |||
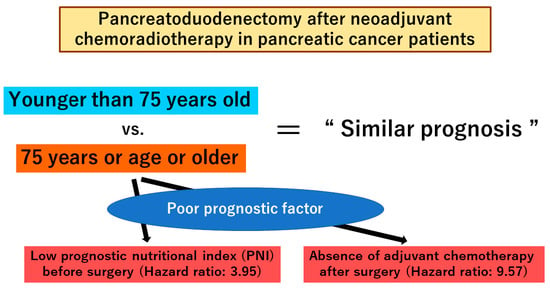
| Prognostic Nutritional Index after NACRT, ≤36.9 | 3.09 | 0.019 | 3.95 | 1.06–14.74 | 0.041 |
| Induction of postoperative adjuvant chemotherapy, no | 7.28 | <0.001 | 9.57 | 2.98–30.68 | <0.001 |
| Intraoperative transfusion, yes | 2.83 | 0.018 | |||
| Age, ≥80 years | 1.17 | 0.715 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suto, H.; Fuke, T.; Matsukawa, H.; Ando, Y.; Oshima, M.; Nagao, M.; Takahashi, S.; Shibata, T.; Yamana, H.; Kamada, H.; et al. Short- and Long-Term Outcomes of Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy in Elderly Patients with Resectable and Borderline Resectable Pancreatic Cancer: A Retrospective Study. J. Clin. Med. 2024, 13, 1216. https://doi.org/10.3390/jcm13051216
Suto H, Fuke T, Matsukawa H, Ando Y, Oshima M, Nagao M, Takahashi S, Shibata T, Yamana H, Kamada H, et al. Short- and Long-Term Outcomes of Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy in Elderly Patients with Resectable and Borderline Resectable Pancreatic Cancer: A Retrospective Study. Journal of Clinical Medicine. 2024; 13(5):1216. https://doi.org/10.3390/jcm13051216
Chicago/Turabian StyleSuto, Hironobu, Takuro Fuke, Hiroyuki Matsukawa, Yasuhisa Ando, Minoru Oshima, Mina Nagao, Shigeo Takahashi, Toru Shibata, Hiroki Yamana, Hideki Kamada, and et al. 2024. "Short- and Long-Term Outcomes of Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy in Elderly Patients with Resectable and Borderline Resectable Pancreatic Cancer: A Retrospective Study" Journal of Clinical Medicine 13, no. 5: 1216. https://doi.org/10.3390/jcm13051216
APA StyleSuto, H., Fuke, T., Matsukawa, H., Ando, Y., Oshima, M., Nagao, M., Takahashi, S., Shibata, T., Yamana, H., Kamada, H., Kobara, H., Okuyama, H., Kumamoto, K., & Okano, K. (2024). Short- and Long-Term Outcomes of Neoadjuvant Chemoradiotherapy Followed by Pancreatoduodenectomy in Elderly Patients with Resectable and Borderline Resectable Pancreatic Cancer: A Retrospective Study. Journal of Clinical Medicine, 13(5), 1216. https://doi.org/10.3390/jcm13051216