A Novel Ear Impression-Taking Method Using Structured Light Imaging and Machine Learning: A Pilot Proof of Concept Study with Patients’ Feedback on Prototype
Abstract
1. Introduction
2. Materials and Methods
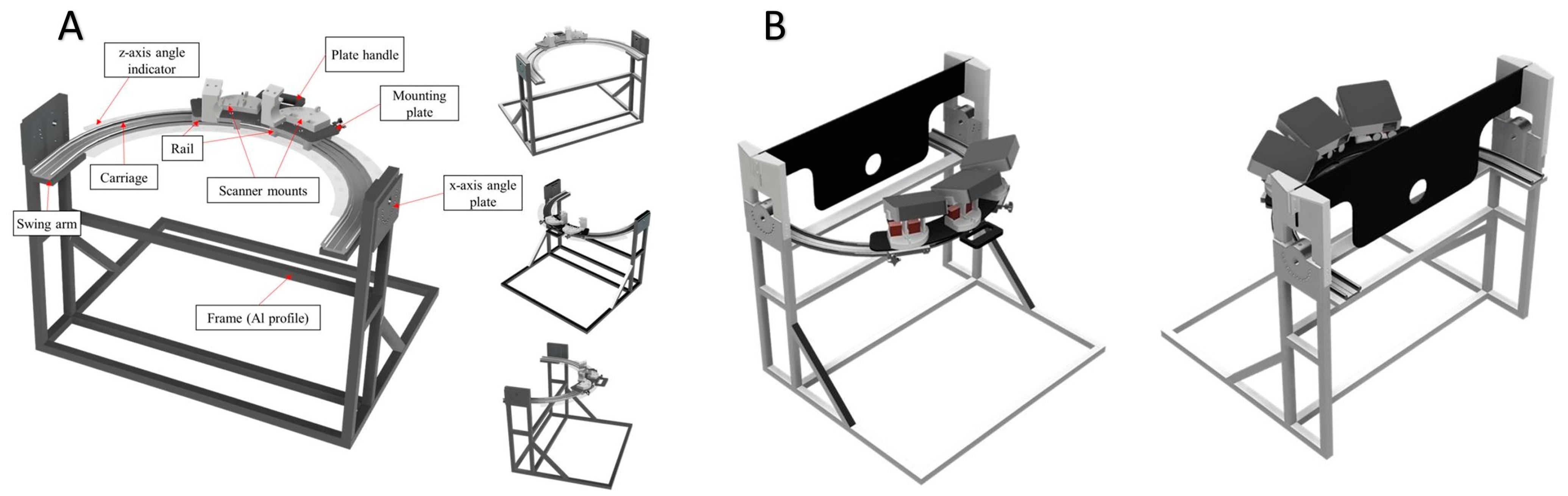
2.1. Prototype Development, Phase I
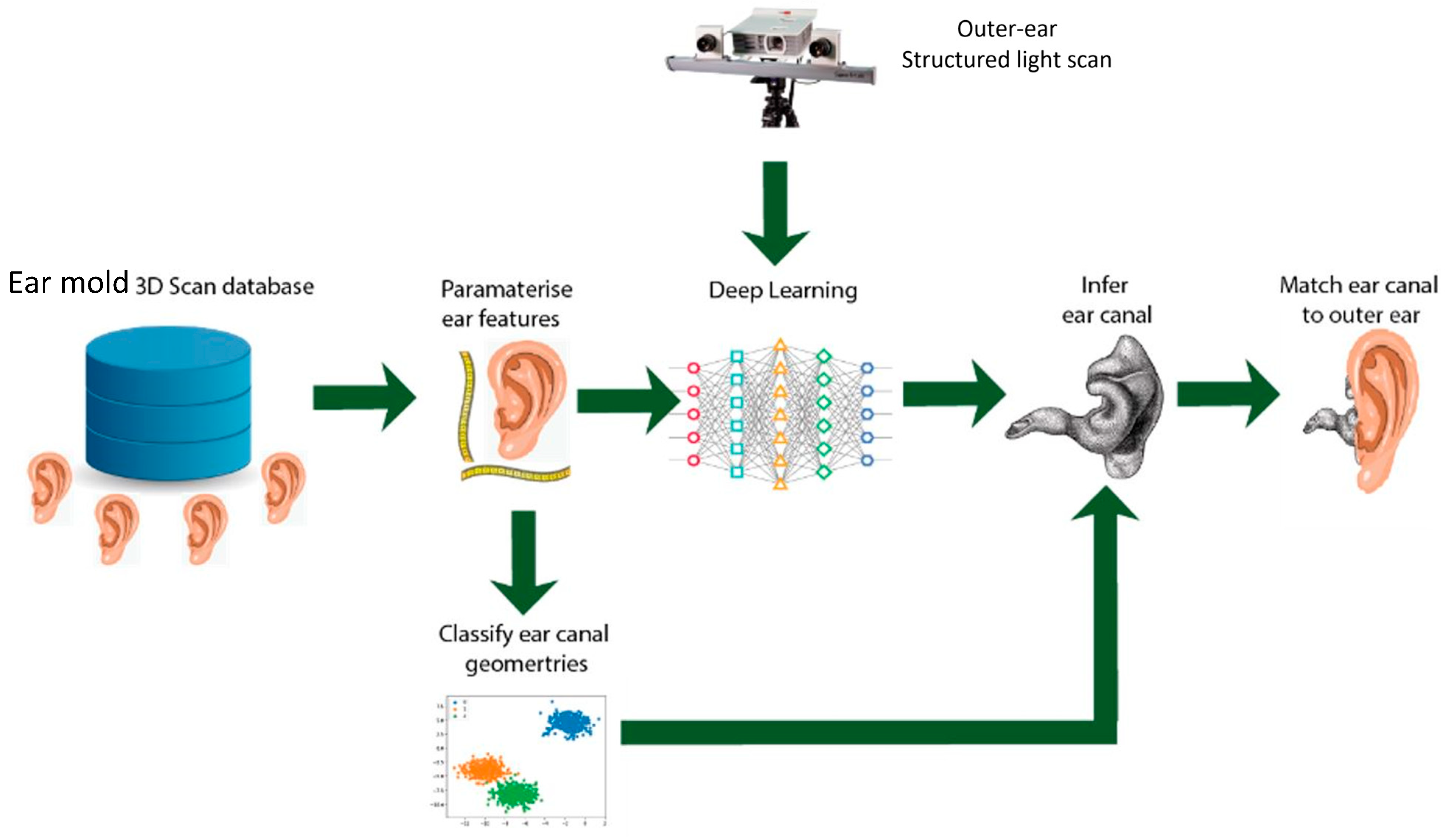
2.2. 6500 3D Ear Impressions Obtained for Machine Learning, Phase II
2.3. Deployment of Prototype for Clinical Trial, Phase III
2.4. Brief Description of Structured Light Technique Using Novel Prototype
3. Results
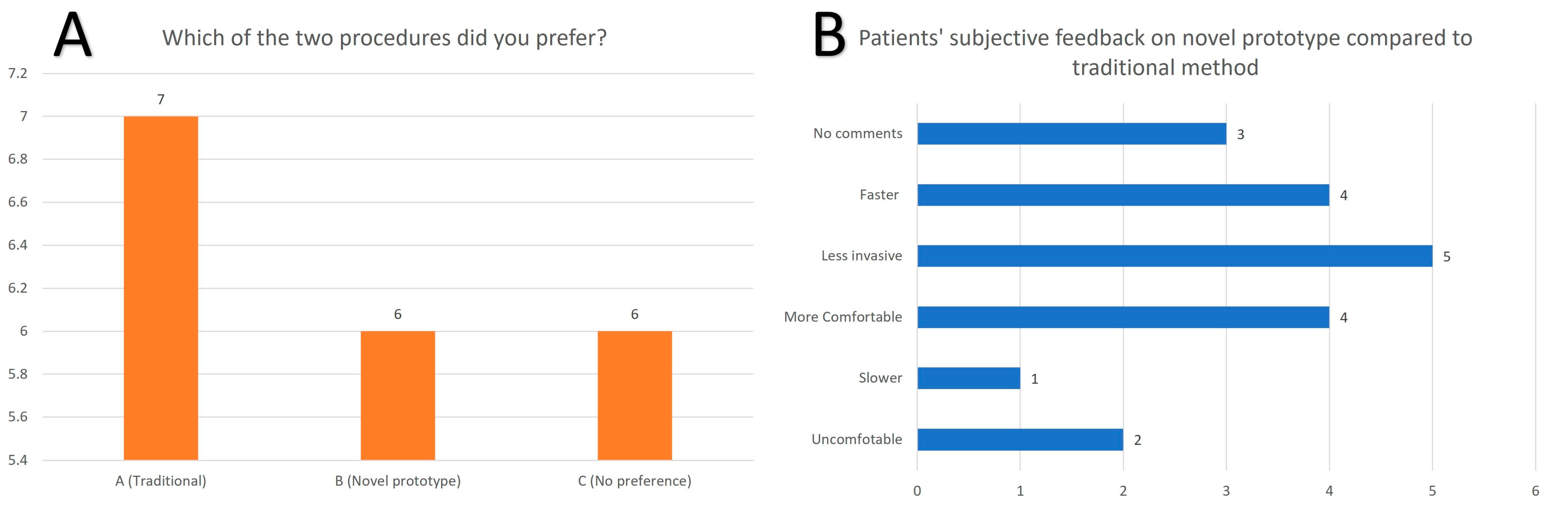
3.1. Patients’ Subjective Feedback
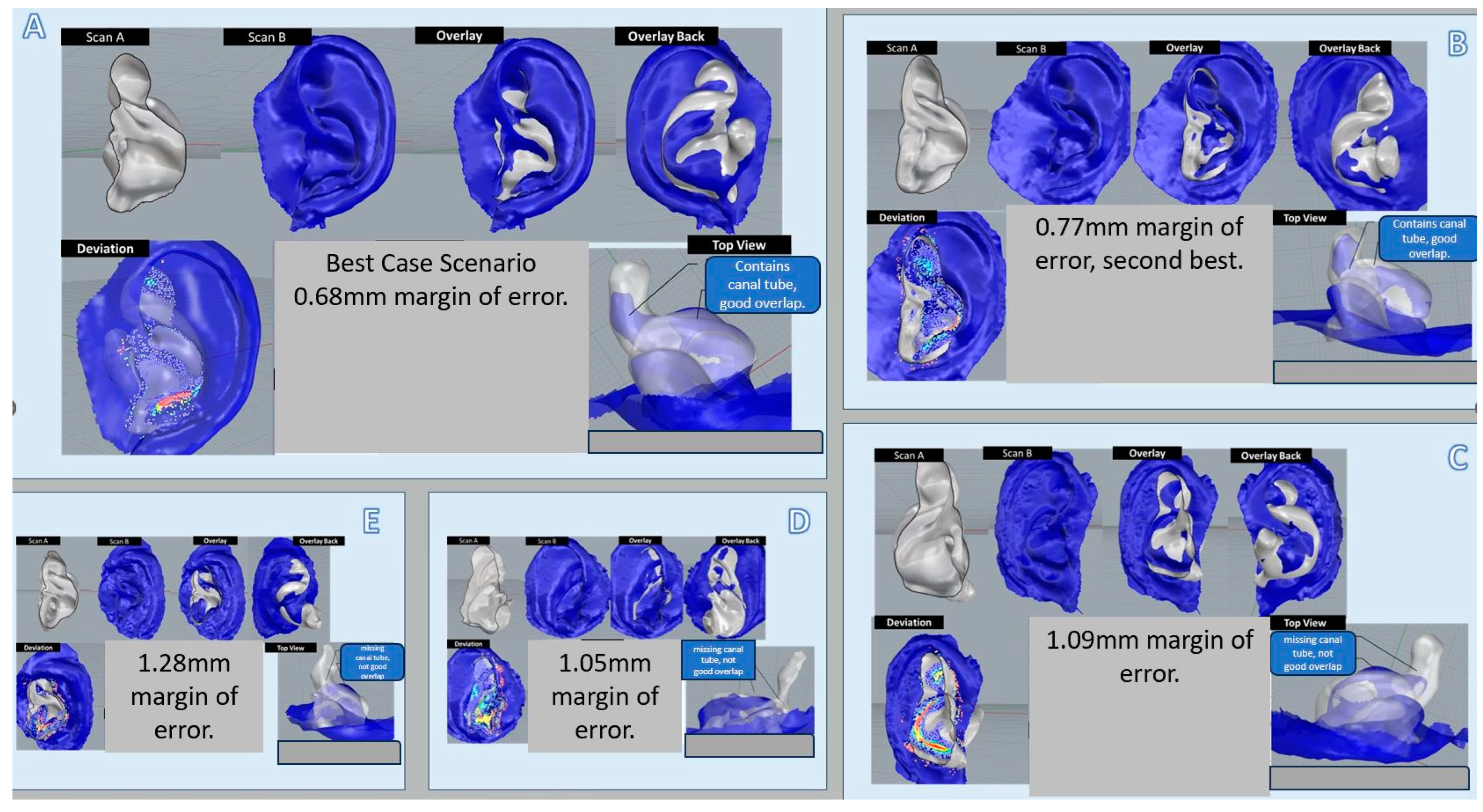
3.2. Objective Comparative Analyses of 8 Samples of Prototype Images with Actual Physical Ear Impressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, S.-D.; Jang, J.H.; Kim, H.; Cho, Y.-S.; Kim, Y.; Koo, J.-W.; Song, J.-J. Earmold foreign bodies in the middle ear necessitating surgical removal: Why otology specialists should screen candidates for hearing aids. Clin. Exp. Otorhinolaryngol. 2021, 14, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hsieh, L.; Chiang, Y.; Cheng, W. Feasibility of High-Resolution Computed Tomography Imaging for Obtaining Ear Impressions for Hearing Aid Fitting. Otolaryngol. Neck Surg. 2019, 161, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Inuzuka, E.; Kato, H.; Naito, K.; Suzuki, Y.; Hattori, T. Surgical removal of hearing aid ear mold impression material from the middle ear: A report of two cases. Fujita Med. J. 2017, 3, 72–75. [Google Scholar]
- Boer, C.V.D.; van Spronsen, E.; Holland, C.T.Q.; Ebbens, F.A.; Waterval, J.J. Clinical Approach After Complicated Ear Mold Fitting: A Case Series of Six Patients and Evaluation of Literature. Ann. Otol. Rhinol. Laryngol. 2019, 128, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Yi, K.-I.; Jung, J.-H.; Lee, I.-W. Hearing aid silicone impression material as a foreign body in the middle ear. Am. J. Otolaryngol. 2017, 38, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.; Tange, R.; Thomeer, H. Impression material in the external and middle ear: An overview of the literature and a stepwise approach for removal. J. Int. Adv. Otol. 2016, 12, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.K.L.; Goh, Z.H.; De Chua, K.W.; Kamath, S.; Chung, C.K.R.; Teo, W.B.Y.; Martinez, J.C.; Dritsas, S.; Simpson, R.E. An improved hearing aid fitting journey; the role of 3D scanning, additive manufacturing, and sustainable practices. Mater. Today Proc. 2022, 70, 504–511. [Google Scholar] [CrossRef]
- Martinez, J.C.; Hwee, G.Z.; Yap, L.; De Chua, K.W.; Kamath, S.; Chung, C.K.R.; Teo, W.Y.B.; Tan, C.K.L.; Dritsas, S.; Simpson, R.E. Lexicon for classifying ear-canal shapes. Sci. Rep. 2023, 13, 11866. [Google Scholar] [CrossRef] [PubMed]
- Staab, W.J.; Sjursen, W.; Preves, D.; Squeglia, T. A one-size disposable hearing aid is introduced. Hear. J. 2000, 53, 36. [Google Scholar] [CrossRef]
- Demir, E.; Eyers, D.; Huang, Y. Competing through the last mile: Strategic 3D printing in a city logistics context. Comput. Oper. Res. 2021, 131, 105248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, K.W.D.; Yeo, H.K.H.; Tan, C.K.L.; Martinez, J.C.; Goh, Z.H.; Dritsas, S.; Simpson, R.E. A Novel Ear Impression-Taking Method Using Structured Light Imaging and Machine Learning: A Pilot Proof of Concept Study with Patients’ Feedback on Prototype. J. Clin. Med. 2024, 13, 1214. https://doi.org/10.3390/jcm13051214
Chua KWD, Yeo HKH, Tan CKL, Martinez JC, Goh ZH, Dritsas S, Simpson RE. A Novel Ear Impression-Taking Method Using Structured Light Imaging and Machine Learning: A Pilot Proof of Concept Study with Patients’ Feedback on Prototype. Journal of Clinical Medicine. 2024; 13(5):1214. https://doi.org/10.3390/jcm13051214
Chicago/Turabian StyleChua, Kenneth Wei De, Hazel Kai Hui Yeo, Charmaine Kai Ling Tan, Jose C. Martinez, Zhi Hwee Goh, Stylianos Dritsas, and Robert E. Simpson. 2024. "A Novel Ear Impression-Taking Method Using Structured Light Imaging and Machine Learning: A Pilot Proof of Concept Study with Patients’ Feedback on Prototype" Journal of Clinical Medicine 13, no. 5: 1214. https://doi.org/10.3390/jcm13051214
APA StyleChua, K. W. D., Yeo, H. K. H., Tan, C. K. L., Martinez, J. C., Goh, Z. H., Dritsas, S., & Simpson, R. E. (2024). A Novel Ear Impression-Taking Method Using Structured Light Imaging and Machine Learning: A Pilot Proof of Concept Study with Patients’ Feedback on Prototype. Journal of Clinical Medicine, 13(5), 1214. https://doi.org/10.3390/jcm13051214





