Moderate-to-Vigorous Physical Activity and Response Inhibition Predict Balance in Adults with Attention Deficit/Hyperactivity Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
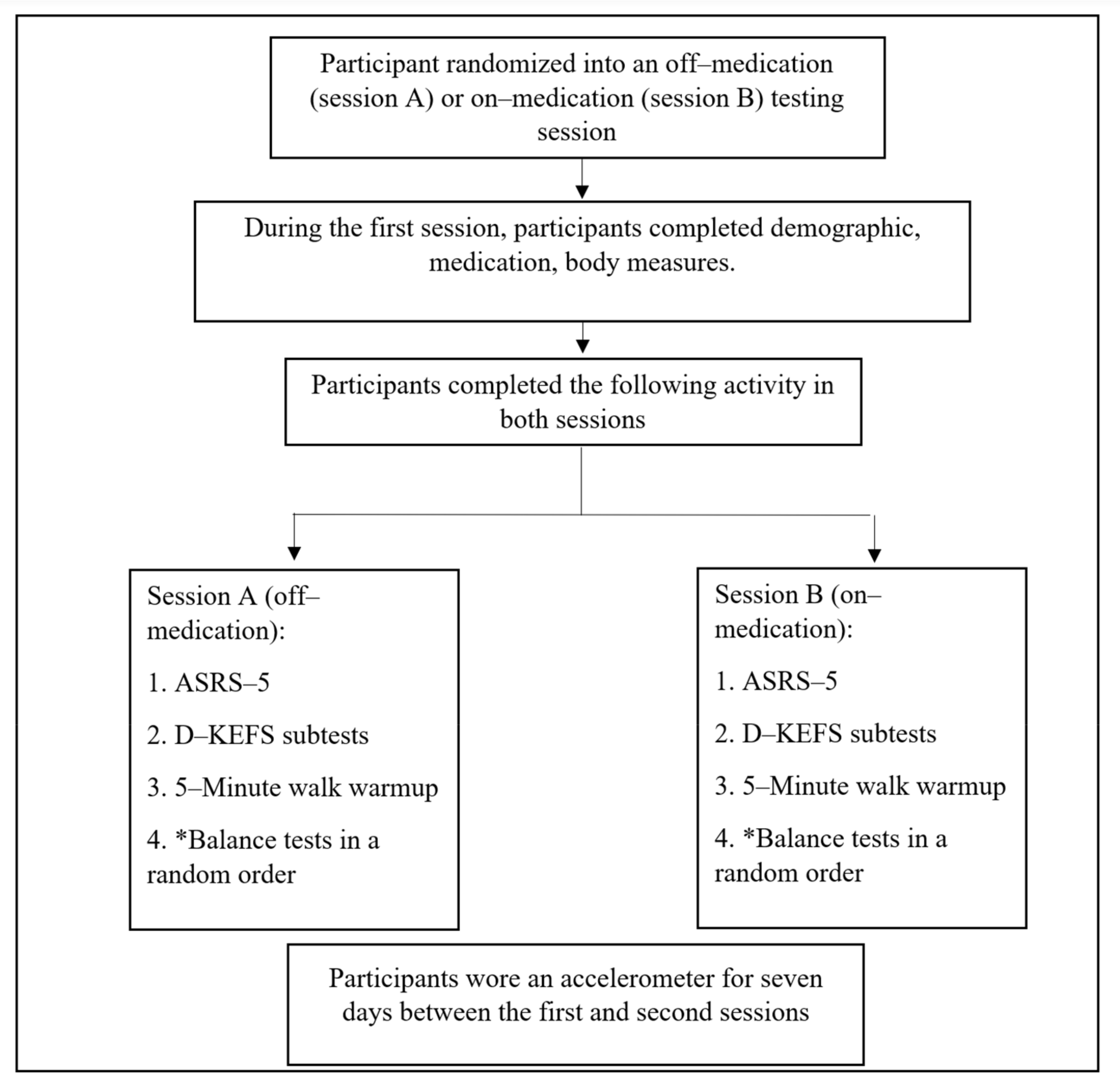
2.2. Procedures
2.3. Outcome Measures
2.3.1. Demographic and Anthropometrics
2.3.2. ADHD Symptoms
2.3.3. Physical Activity Accelerometry Measure
2.3.4. RI–Delis–Kaplan Executive Function System
2.3.5. Postural Sway Measurements and Single-Leg Standing Tests
2.4. Statistical Analyses
3. Results
3.1. Associations among the Outcomes: Off-Medication Status
3.2. Associations among the Outcomes: On-Medication Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Danielson, M.L.; Bitsko, R.H.; Ghandour, R.M.; Holbrook, J.R.; Kogan, M.D.; Blumberg, S.J. Prevalence of Parent-Reported ADHD Diagnosis and Associated Treatment among U.S. Children and Adolescents, 2016. J. Clin. Child Adolesc. Psychol. 2018, 47, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Adler, L.; Barkley, R.; Biederman, J.; Conners, C.K.; Demler, O.; Faraone, S.V.; Greenhill, L.L.; Howes, M.J.; Secnik, K.; et al. The prevalence and correlates of adult ADHD in the United States: Results from the National Comorbidity Survey Replication. Am. J. Psychiatry 2006, 163, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.; Jiang, S.F.; Paksarian, D.; Nikolaidis, A.; Castellanos, F.X.; Merikangas, K.R.; Milham, M.P. Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder among Adults and Children of Different Racial and Ethnic Groups. JAMA Network Open 2019, 2, e1914344. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Taner, Y.; Guclu, B.; Taner, E.; Kaya, Y.; Bahcivan, H.G.; Benli, I.T. Trauma and adult attention deficit hyperactivity disorder. J. Int. Med. Res. 2008, 36, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Jacobi-Polishook, T.; Shorer, Z.; Melzer, I. The effect of methylphenidate on postural stability under single and dual task conditions in children with attention deficit hyperactivity disorder—A double blind randomized control trial. J. Neurol. Sci. 2009, 280, 15–21. [Google Scholar] [CrossRef]
- Hove, M.J.; Zeffiro, T.A.; Biederman, J.; Li, Z.; Schmahmann, J.; Valera, E.M. Postural sway and regional cerebellar volume in adults with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2015, 8, 422–428. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: Dealing with ADHD: What You Need to Know. Available online: https://www.fda.gov/consumers/consumer-updates/dealing-adhd-what-you-need-know (accessed on 26 April 2023).
- Alotaibi, M.M.; Stavrinos, D.; Motl, R.W.; Bell, M.; Snyder, S.W.; Hurt, C.P.; Singh, H.; Lein, D.H., Jr. Effect of psychostimulant medications on functional balance performance in persons with Attention-Deficit/Hyperactivity Disorder: A systematic review. Gait Posture 2023, 102, 146–158. [Google Scholar] [CrossRef]
- Stray, L.L.; Stray, T.; Iversen, S.; Ruud, A.; Ellertsen, B. Methylphenidate improves motor functions in children diagnosed with Hyperkinetic Disorder. Behav. Brain Funct. 2009, 5, 21. [Google Scholar] [CrossRef]
- Alotaibi, M.M.; Motl, R.W.; Stavrinos, D.; Snyder, S.W.; Singh, H.; Lein, D.H. Effect of psychostimulant medications on static balance performance in adults with attention deficit hyperactivity disorder: Within-subjects repeated-measure study. Hum. Mov. Sci. 2023, 88, 103067. [Google Scholar] [CrossRef]
- Aron, A.R.; Dowson, J.H.; Sahakian, B.J.; Robbins, T.W. A Randomized, Placebo-Controlled Trial of Three Fixed Dosages of Prolonged-Release OROS Methylphenidate in Adults with Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2008, 63, 981–989. [Google Scholar]
- Aron, A.R.; Dowson, J.H.; Sahakian, B.J.; Robbins, T.W. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2003, 54, 1465–1468. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Faraone, S.V.; Spencer, T.J.; Montano, C.B.; Biederman, J. Attention-deficit/hyperactivity disorder in adults: A survey of current practice in psychiatry and primary care. Arch. Intern. Med. 2004, 164, 1221–1226. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly: A systematic review. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Wang, B.; Zhao, C.; Wu, T.; Liu, T.; Sun, F. Objectively Measured Physical Activity Is Associated with Static Balance in Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 10787. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A. Differential diagnosis of adults with ADHD: The role of executive function and self-regulation. J. Clin. Psychiatry 2010, 71, e17. [Google Scholar] [CrossRef] [PubMed]
- Mannarelli, D.; Pauletti, C.; Currà, A.; Marinelli, L.; Corrado, A.; Delle Chiaie, R.; Fattapposta, F. The Cerebellum Modulates Attention Network Functioning: Evidence from a Cerebellar Transcranial Direct Current Stimulation and Attention Network Test Study. Cerebellum 2019, 18, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Rondi-Reig, L.; Paradis, A.L.; Lefort, J.M.; Babayan, B.M.; Tobin, C. How the cerebellum may monitor sensory information for spatial representation. Front. Syst. Neurosci. 2014, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- del Campo, N.; Fryer, T.D.; Hong, Y.T.; Smith, R.; Brichard, L.; Acosta-Cabronero, J.; Chamberlain, S.R.; Tait, R.; Izquierdo, D.; Regenthal, R.; et al. A positron emission tomography study of nigro-striatal dopaminergic mechanisms underlying attention: Implications for ADHD and its treatment. Brain 2013, 136, 3252–3270. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Palumbo, D.; Walters, F.; Belden, H.; Berry, S.A. Single-dose Pharmacokinetic Properties and Relative Bioavailability of a Novel Methylphenidate Extended-release Chewable Tablet Compared with Immediate-release Methylphenidate Chewable Tablet. Clin. Ther. 2016, 38, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Herman, B.K.; Bouhajib, M.; King, T.R.; Kando, J.C.; Pardo, A. Single-Dose Pharmacokinetics of Amphetamine Extended-Release Oral Suspension in Healthy Adults. J. Atten. Disord. 2021, 25, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Ustun, B.; Adler, L.A.; Rudin, C.; Faraone, S.V.; Spencer, T.J.; Berglund, P.; Gruber, M.J.; Kessler, R.C. The World Health Organization Adult Attention-Deficit/Hyperactivity Disorder Self-Report Screening Scale for DSM-5. JAMA Psychiatry 2017, 74, 520–526. [Google Scholar] [CrossRef]
- Rhudy, M.B.; Dreisbach, S.B.; Moran, M.D.; Ruggiero, M.J.; Veerabhadrappa, P. Cut points of the Actigraph GT9X for moderate and vigorous intensity physical activity at four different wear locations. J. Sports Sci. 2020, 38, 503–510. [Google Scholar] [CrossRef]
- Higgins, S.; Higgins, L.Q.; Vallabhajosula, S. Site-specific Concurrent Validity of the ActiGraph GT9X Link in the Estimation of Activity-related Skeletal Loading. Med. Sci. Sports Exerc. 2021, 53, 951–959. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Johnson, W.D.; Katzmarzyk, P.T. Accelerometer-determined steps per day in US adults. Med. Sci. Sports Exerc. 2009, 41, 1384–1391. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Noonan, R.J.; Fairclough, S.J.; Knowles, Z.R.; Boddy, L.M. Context matters! Sources of variability in weekend physical activity among families: A repeated measures study. BMC Public Health 2017, 17, 330. [Google Scholar] [CrossRef] [PubMed]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System: Technical Manual; The Psychological Corporation: San Antonio, TX, USA, 2001. [Google Scholar]
- Brown, T.E.; Landgraf, J.M. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: Evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad. Med. 2010, 122, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Allman, A.A.; Benkelfat, C.; Durand, F.; Sibon, I.; Dagher, A.; Leyton, M.; Baker, G.B.; O’Driscoll, G.A. Effect of D-amphetamine on inhibition and motor planning as a function of baseline performance. Psychopharmacology 2010, 211, 423–433. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Letz, R.; Gerr, F. Standing steadiness measurements: Empirical selection of testing protocol and outcome measures. Neurotoxicol. Teratol. 1995, 17, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Yamauchi, J. Altered postural sway following fatiguing foot muscle exercises. PLoS ONE 2017, 12, e0189184. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.J.; Hsiao-Wecksler, E.T.; Ragan, B.G.; Rosengren, K.S. Generalizability of center of pressure measures of quiet standing. Gait Posture 2007, 25, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.A.; Marin, R.; Cyhan, T.; Roberts, H.; Gill, N.W. Normative values for the unipedal stance test with eyes open and closed. J. Geriatr. Phys. Ther. 2007, 30, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Saville, D.J. Multiple Comparison Procedures: The Practical Solution. Am. Stat. 1990, 44, 174–180. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 1988. [Google Scholar]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Cho, J.; Lee, S. Short-Foot Exercise Promotes Quantitative Somatosensory Function in Ankle Instability: A Randomized Controlled Trial. Med. Sci. Monit. 2019, 25, 618–626. [Google Scholar] [CrossRef]
- Alcock, L.; O’Brien, T.D.; Vanicek, N. Association between somatosensory, visual and vestibular contributions to postural control, reactive balance capacity and healthy ageing in older women. Health Care Women Int. 2018, 39, 1366–1380. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Fleck, S.J.; Maresh, C.M.; Ratamess, N.A.; Gordon, S.E.; Goetz, K.L.; Harman, E.A.; Frykman, P.N.; Volek, J.S.; Mazzetti, S.A.; et al. Acute hormonal responses to a single bout of heavy resistance exercise in trained power lifters and untrained men. Can. J. Appl. Physiol. 1999, 24, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.L.; Sherrington, C.; Smith, K.; Carswell, P.; Bell, R.; Bell, M.; Nascimento, D.P.; Máximo Pereira, L.S.; Vardon, P. Physical activity improves strength, balance and endurance in adults aged 40–65 years: A systematic review. J. Physiother. 2012, 58, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.O.; Voos, M.C.; Barbosa, A.F.; Chen, J.; Francato, D.C.V.; Milosevic, M.; Popovic, M.; Fonoff, E.T.; Chien, H.F.; Barbosa, E.R. Relationship Between Posturography, Clinical Balance and Executive Function in Parkinson´s Disease. J. Mot. Behav. 2019, 51, 212–221. [Google Scholar] [CrossRef] [PubMed]
- UAB: UAB Udergraduate Student Demographics Fall 2021. Available online: https://www.uab.edu/institutionaleffectiveness/images/documents/student-demographics/Undergraduate-Fall-2021.pdf (accessed on 1 July 2021).
- Nimmo-Smith, V.; Merwood, A.; Hank, D.; Brandling, J.; Greenwood, R.; Skinner, L.; Law, S.; Patel, V.; Rai, D. Non-pharmacological interventions for adult ADHD: A systematic review. Psychol. Med. 2020, 50, 529–541. [Google Scholar] [CrossRef]
- Heijer, A.E.D.; Groen, Y.; Tucha, L.; Fuermaier, A.B.M.; Koerts, J.; Lange, K.W.; Thome, J.; Tucha, O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: A systematic literature review. J. Neural. Transm. 2017, 124, 3–26. [Google Scholar] [CrossRef]
| Characteristic | All Participants n = 40 | |
|---|---|---|
| Mean | SD/% | |
| Age (y) | 29.0 | 6.3 |
| Sex n (%) | ||
| Male | 10 | 25.0 |
| Female | 30 | 75.0 |
| Race n (%) | ||
| Caucasian | 30 | 75.0 |
| Black or African American | 5 | 12.5 |
| Asian | 2 | 5.0 |
| Mixed Race | 3 | 7.5 |
| Body Mass (kg) | 81.8 | 22.4 |
| Body Height (cm) | 170.5 | 9.4 |
| Body Mass Index (kg/m2) | 28.0 | 7.7 |
| Dominant Leg n (%) | ||
| Left | 5 | 12.5 |
| Right | 35 | 87.5 |
| Education Level n (%) | ||
| Some College Education | 5 | 12.5 |
| Undergraduate | 13 | 32.5 |
| Graduate Level | 22 | 55.0 |
| Psychostimulant Medication n (%) | ||
| MPH-based | 5 | 12.5 |
| AMP-based | 35 | 87.5 |
| Adult Self-Report ADHD Scale–5 | ||
| Off medication | 18.6 | 2.4 |
| On medication | 13.6 | 4.0 |
| Variable | N | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Off Medication | ||||||||||||
| 1. SLEC (touch time in seconds) | 40 | 11.3 | 10.2 | — | ||||||||
| 2. FAEO (sway area in cm2) | 40 | 1.5 | 2.2 | –0.18 | — | |||||||
| 3. FAEC (sway area in cm2) | 40 | 1.7 | 1.7 | –0.11 | 0.16 | — | ||||||
| 4. FTEO (sway area in cm2) | 40 | 6.4 | 5.5 | –0.04 | –0.02 | 0.40 * | — | |||||
| 5. FTEC (sway area in cm2) | 40 | 9.2 | 5.8 | –0.21 | 0.06 | 0.46 ** | 0.52 ** | — | ||||
| 6. MVPA (minutes/day) | 40 | 13.2 | 12.8 | 0.38 * | 0.01 | –0.16 | –0.37 * | –0.01 | — | |||
| 7. TMT Number–Letter Switching | 40 | 11.5 | 1.8 | 0.01 | –0.21 | –0.05 | –0.35 * | –0.16 | 0.11 | — | ||
| 8. CWIT Inhibition | 40 | 11.3 | 2.7 | 0.19 | –0.15 | 0.04 | –0.28 | –0.11 | 0.33 * | 0.44 ** | — | |
| 9. CWIT Inhibition/Switching | 40 | 10.5 | 2.8 | 0.03 | –0.27 | 0.03 | –0.28 | –0.04 | 0.19 | 0.34 * | 0.72 ** | — |
| On Medication | ||||||||||||
| 1. SLEC (touch time in seconds) | 40 | 13.7 | 10.8 | — | ||||||||
| 2. FAEO (sway area in cm2) | 40 | 1.4 | 1.4 | 0.08 | — | |||||||
| 3. FAEC (sway area in cm2) | 40 | 1.3 | 0.8 | 0.34 * | 0.45 ** | — | ||||||
| 4. FTEO (sway area in cm2) | 40 | 4.6 | 2.0 | –0.17 | 0.38 * | 0.45 ** | — | |||||
| 5. FTEC (sway area in cm2) | 40 | 7.6 | 4.8 | 0.34 * | 0.22 | 0.43 ** | 0.25 | — | ||||
| 6. MVPA (minutes/day) | 40 | 13.2 | 12.8 | 0.27 | –0.18 | –0.22 | –0.28 | –0.08 | — | |||
| 7. TMT Number–Letter Switching | 40 | 11.4 | 1.5 | –0.17 | –0.11 | 0.32 * | 0.14 | 0.13 | 0.04 | — | ||
| 8. CWIT Inhibition | 40 | 11.6 | 2.0 | –0.07 | 0.16 | 0.22 | 0.12 | –0.11 | 0.04 | 0.25 | — | |
| 9. CWIT Inhibition/Switching | 40 | 10.4 | 2.7 | –0.06 | 0.13 | 0.23 | 0.15 | –0.14 | 0.08 | 0.19 | 0.72 ** | — |
| Effect | Estimate | SE | Standardized Beta | 95% CI | p |
|---|---|---|---|---|---|
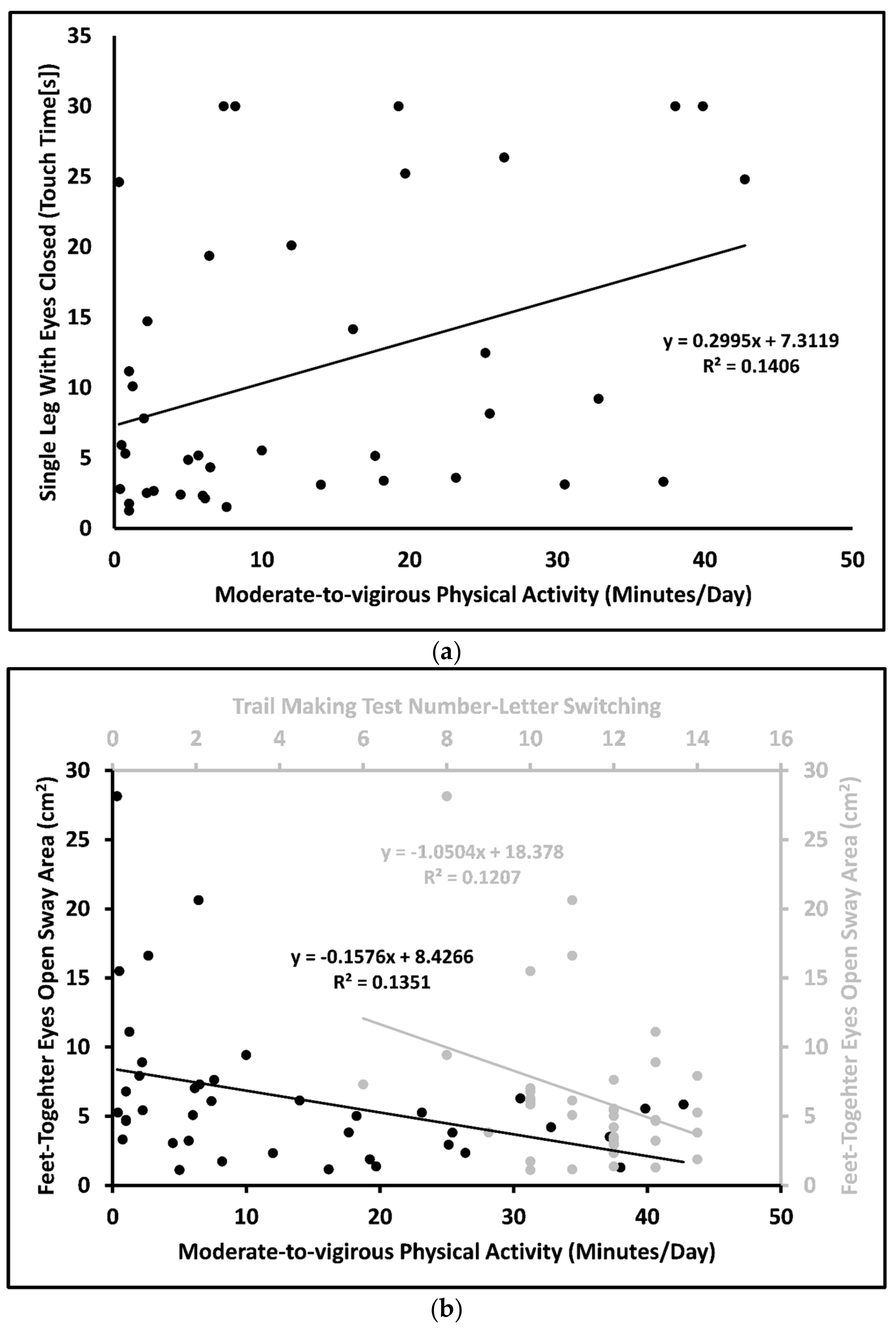
| A. Outcome SLEC touch time [F(1,38) = 6.218, adjusted R2 = 0.118, p = 0.017] | |||||
| Intercept | 7.312 | 2.191 | 2.877, 11.747 | 0.002 | |
| MVPA | 0.300 | 0.120 | 0.375 | 0.056, 0.543 | 0.017 |
| B. Outcome FTEO sway area [F(1,38) = 5.550, adjusted R2 = 0.189, p = 0.008] | |||||
| Intercept | 19.005 | 5.059 | 8.754, 29.255 | <0.001 | |
| MVPA | –0.143 | 0.062 | –0.334 | –0.269, –0.017 | 0.027 |
| TMT | –0.941 | 0.438 | –0.311 | –1.829, –0.052 | 0.039 |
| Effect | Estimate | SE | Standardized Beta | 95% CI | p |
|---|---|---|---|---|---|
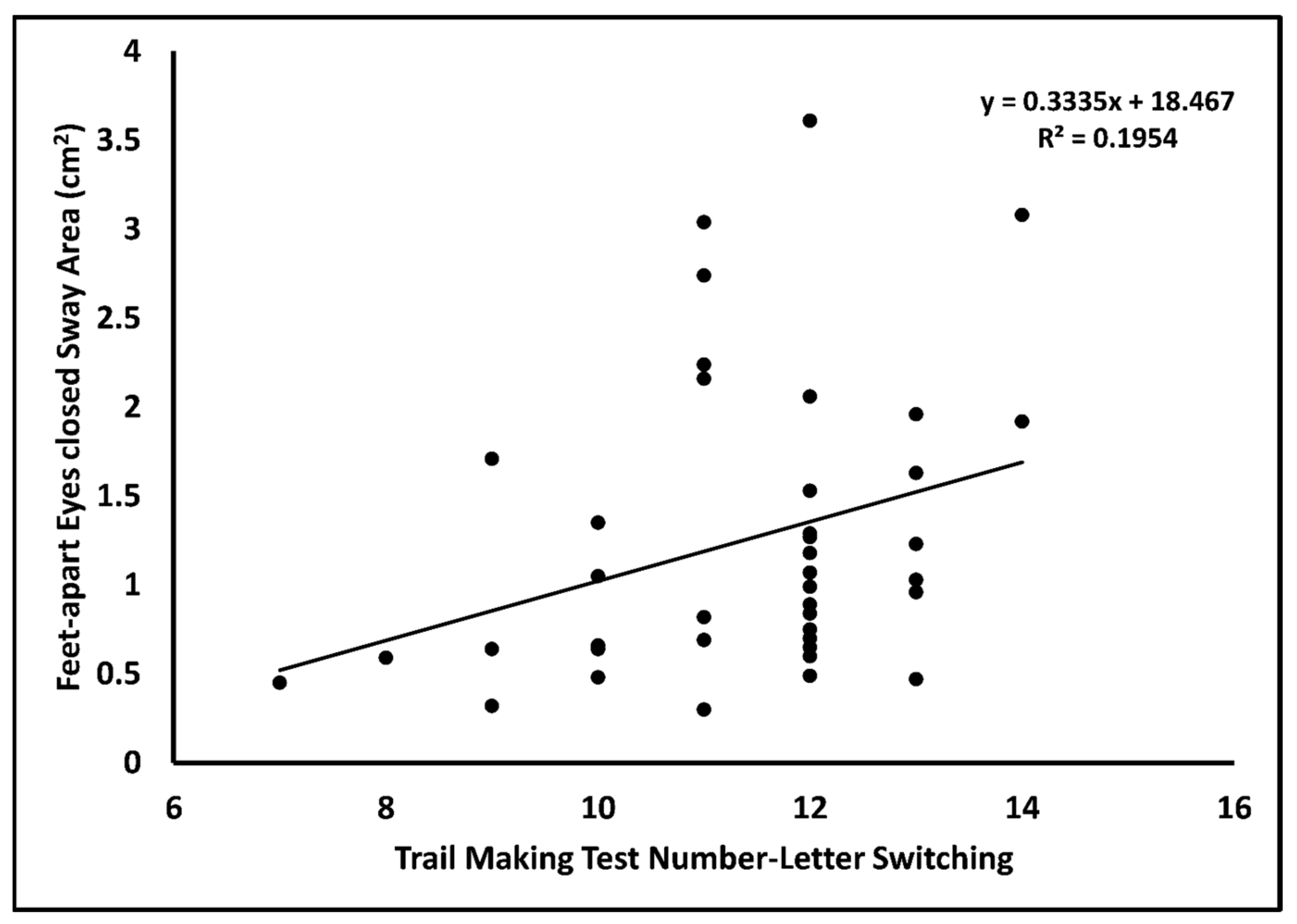
| Outcome FAEC sway area [F(1,38) = 4.213, adjusted R2 = 0.076, p = 0.047] | |||||
| Intercept | –0.657 | 0.938 | –2.557, 1.243 | <0.001 | |
| TMT | 0.168 | 0.082 | 0.316 | 0.002, 0.333 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, M.M.; Motl, R.W.; Stavrinos, D.; Snyder, S.W.; Singh, H.; Lein, D.H., Jr. Moderate-to-Vigorous Physical Activity and Response Inhibition Predict Balance in Adults with Attention Deficit/Hyperactivity Disorder. J. Clin. Med. 2024, 13, 968. https://doi.org/10.3390/jcm13040968
Alotaibi MM, Motl RW, Stavrinos D, Snyder SW, Singh H, Lein DH Jr. Moderate-to-Vigorous Physical Activity and Response Inhibition Predict Balance in Adults with Attention Deficit/Hyperactivity Disorder. Journal of Clinical Medicine. 2024; 13(4):968. https://doi.org/10.3390/jcm13040968
Chicago/Turabian StyleAlotaibi, Mansour M., Robert W. Motl, Despina Stavrinos, Scott W. Snyder, Harshvardhan Singh, and Donald H. Lein, Jr. 2024. "Moderate-to-Vigorous Physical Activity and Response Inhibition Predict Balance in Adults with Attention Deficit/Hyperactivity Disorder" Journal of Clinical Medicine 13, no. 4: 968. https://doi.org/10.3390/jcm13040968
APA StyleAlotaibi, M. M., Motl, R. W., Stavrinos, D., Snyder, S. W., Singh, H., & Lein, D. H., Jr. (2024). Moderate-to-Vigorous Physical Activity and Response Inhibition Predict Balance in Adults with Attention Deficit/Hyperactivity Disorder. Journal of Clinical Medicine, 13(4), 968. https://doi.org/10.3390/jcm13040968







