Weak Points of Double-Plate Stabilization Used in the Treatment of Distal Humerus Fracture through Finite Element Analysis
Abstract
1. Introduction
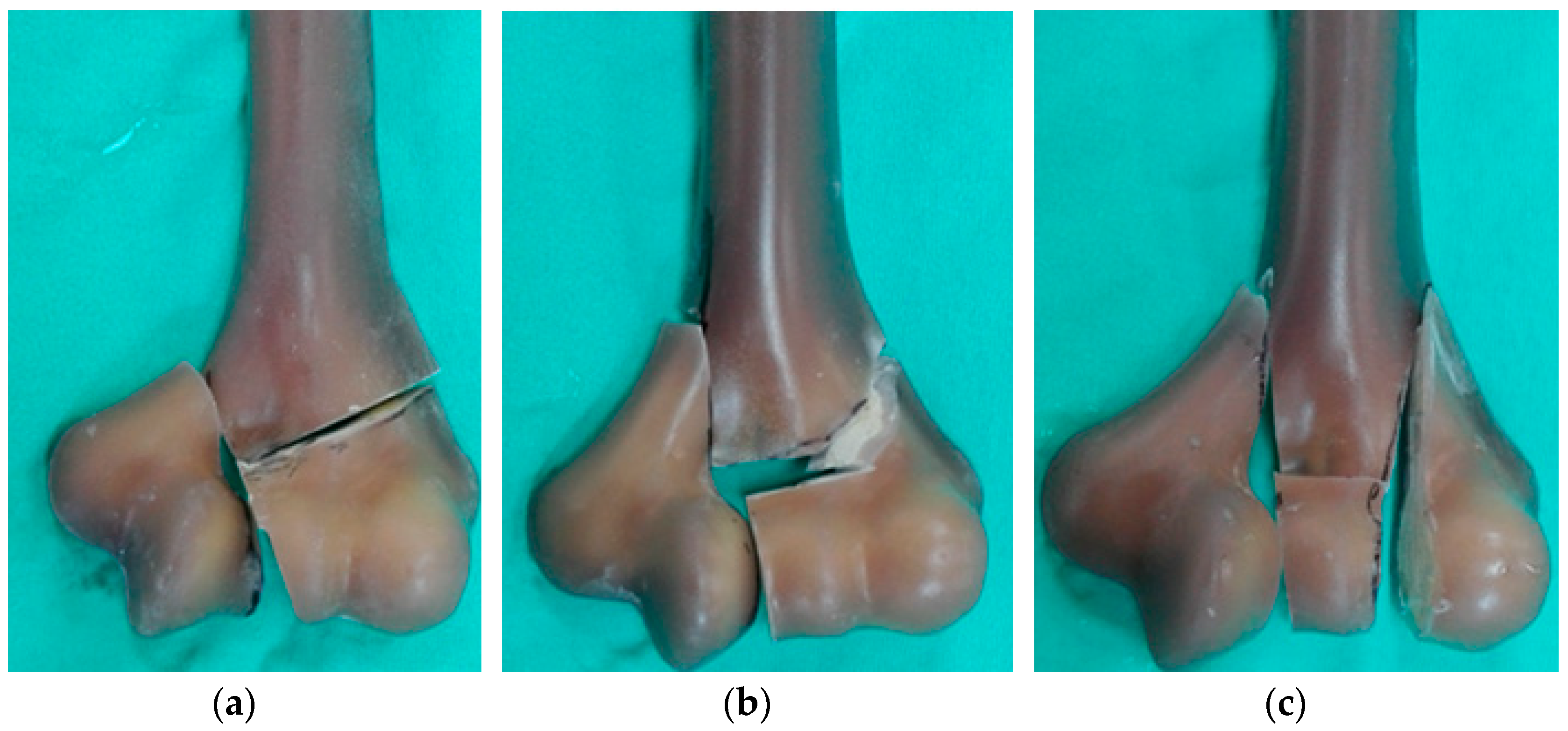
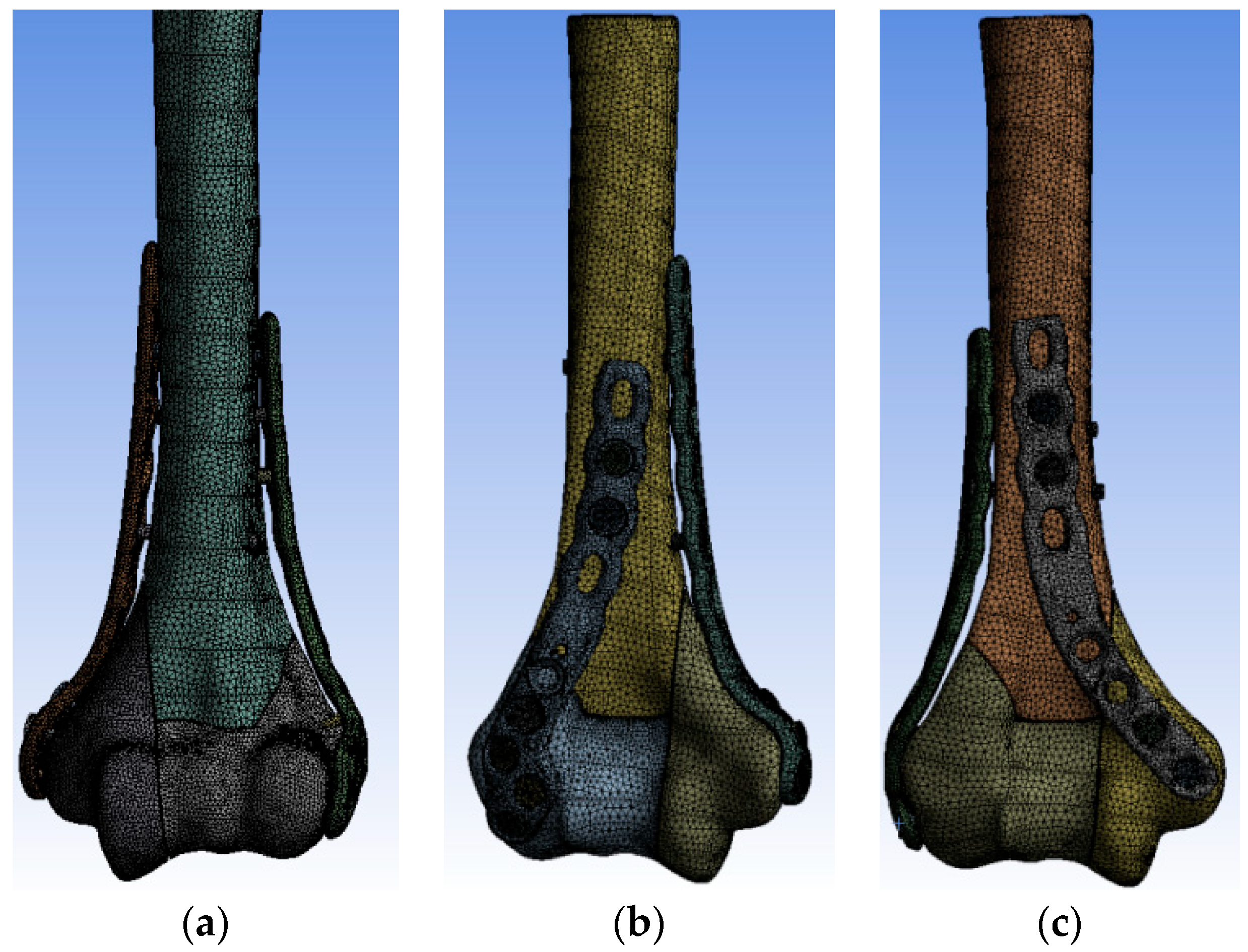
2. Materials and Methods
2.1. Loads and Boundary Conditions
2.2. Material Properties
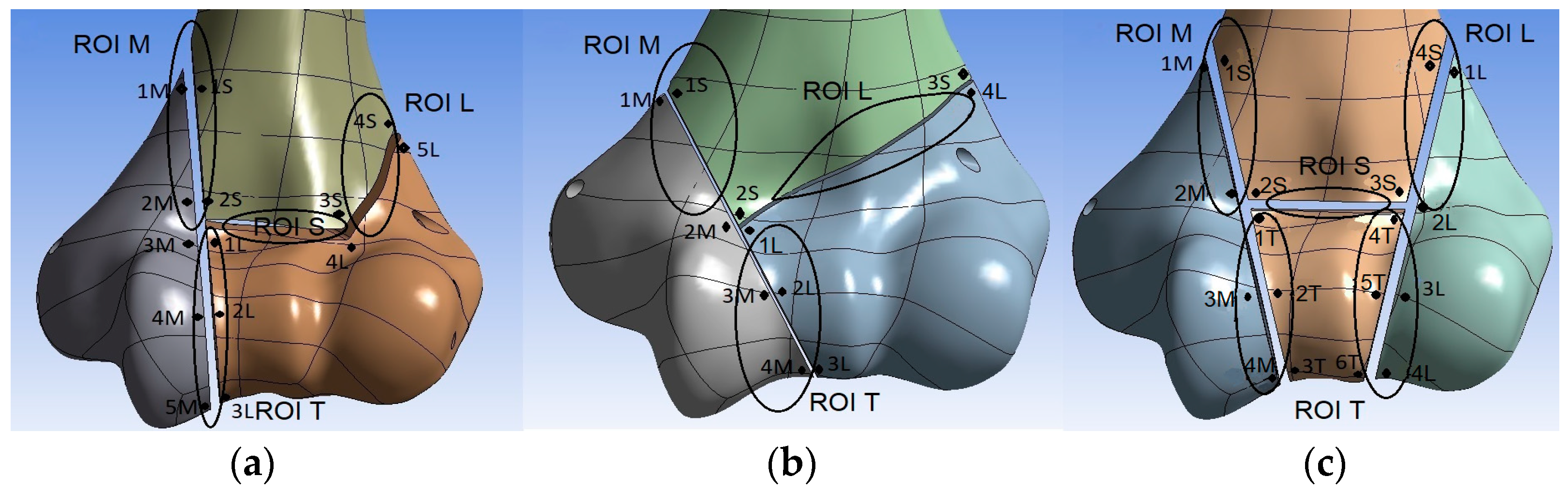
2.3. Interfragmentary Movement
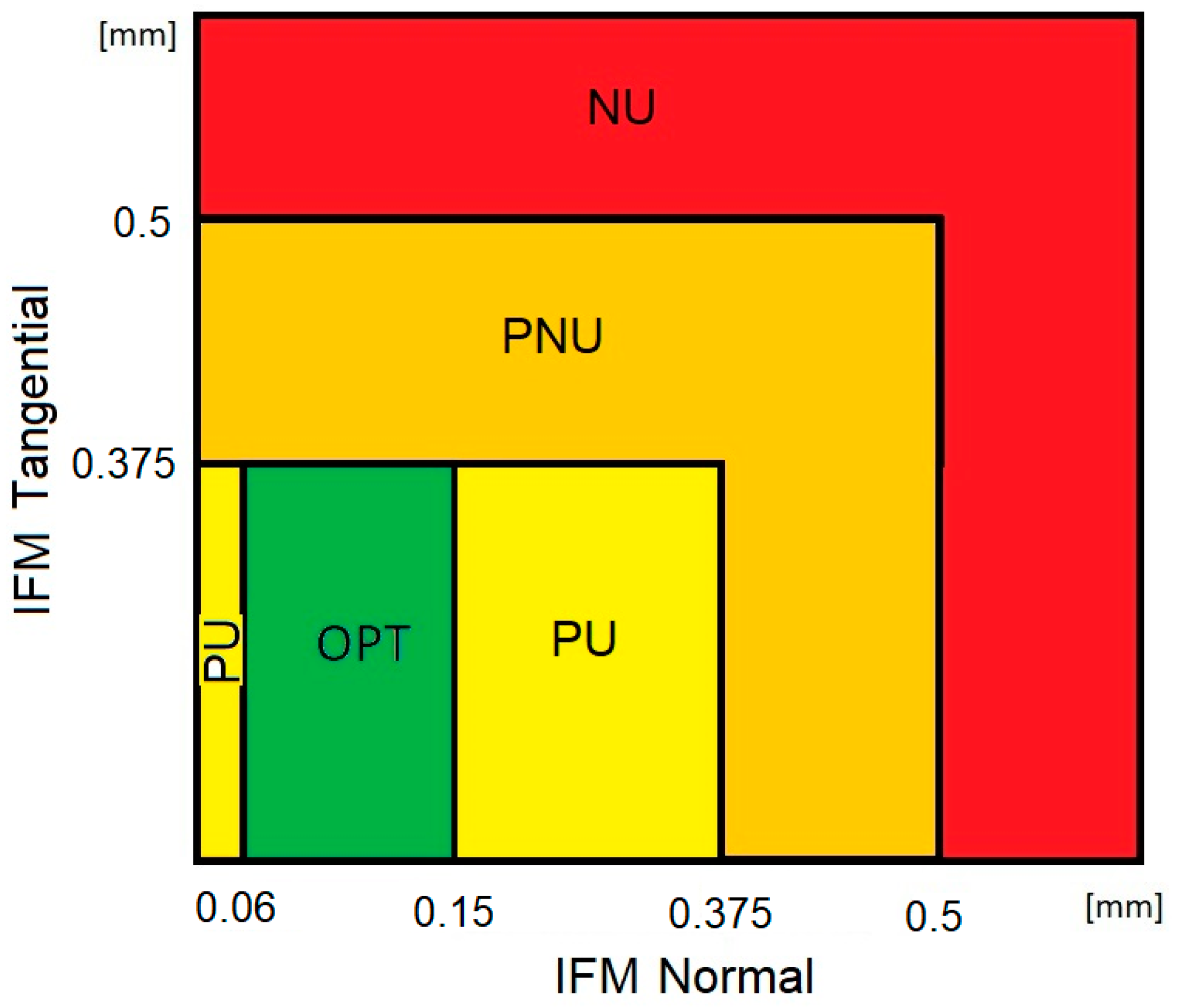
2.4. Assessment of the Biomechanical Conditions of Fracture Healing
- –
- Optimal bone union (OPT)—the value of the normal component of IFM within the range of 0.06–0.15 mm and the value of the tangential component of IFM below 0.375 mm;
- –
- Probable union (PU)—the value of normal displacements in the range of 0–0.06 mm or 0.15–0.375 mm and the value of tangential displacements below 0.375 mm;
- –
- Probable non-union (PNU)—the value of both tangential and normal displacements greater than 0.375 mm but below 0.5 mm;
- –
- Non-union (NU)—the value of normal or tangential displacements greater than 0.5 mm.
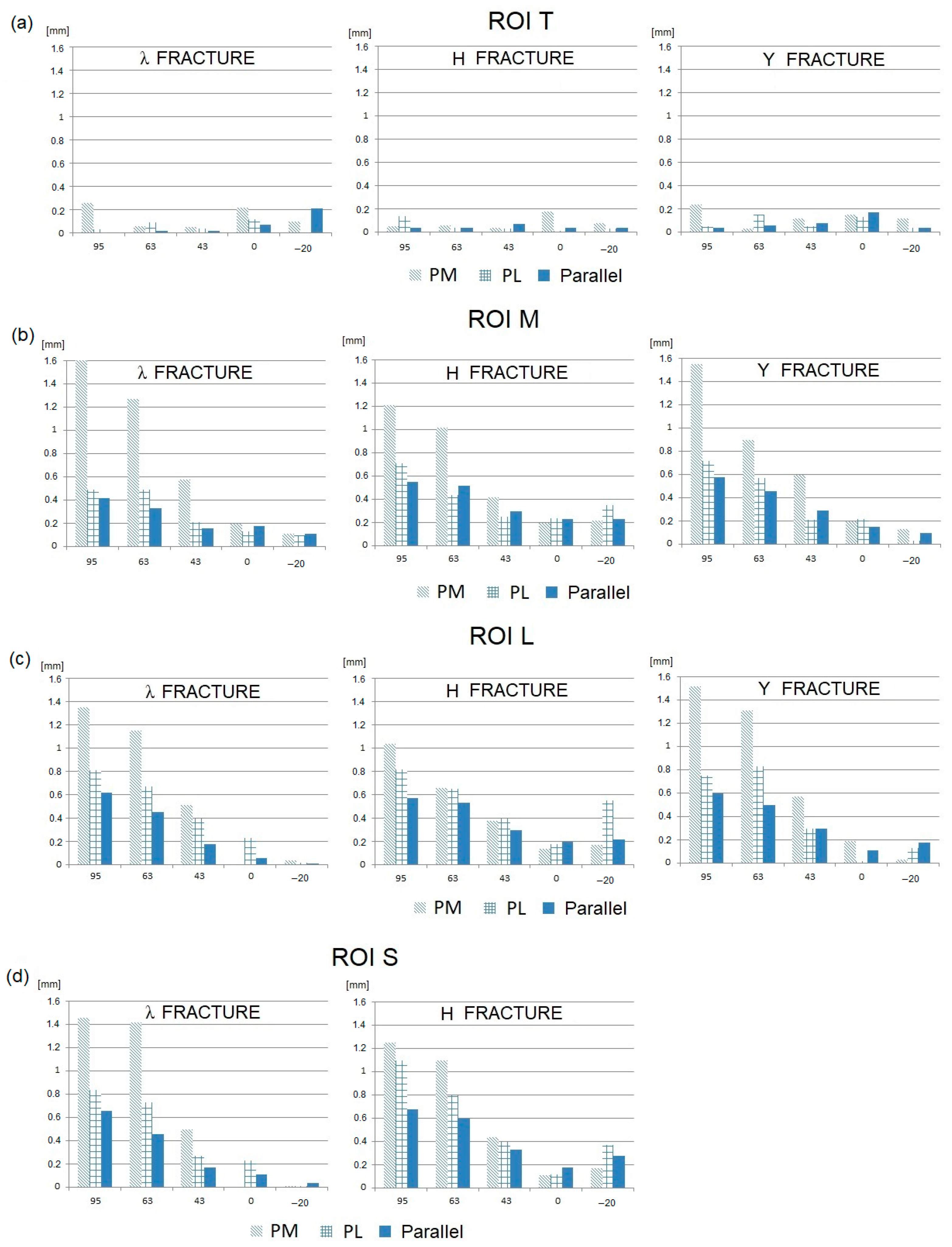
3. Results
- –
- In general, the tangential components of IFMs are significantly greater than the normal components.
- –
- The smallest IFMs, both tangential and normal, are observed for the parallel plate configuration in the majority of fracture types and elbow joint flexion angles.
- –
- In most cases, the largest IFMs are observed for posteromedial (PM) stabilization.
- –
- The angular position of the elbow joint and the related direction of the joint force reaction has a very strong influence on the value of IFM. It can be observed that the maximum IFM values occur when the elbow joint is almost fully flexed (JRF direction 63–95°; joint angle 120–145°) in all plating configurations and all types of fractures.
- –
- For elbow joint angles in the range of 0–90° (JRF direction −20–43°), the IFM values are relatively low. In this angular range, the differences in the IFM values obtained for different plating configurations and different types of fractures are somewhat unclear.
- –
- All variants of the fracture gap except in the trochlear region (i.e., the S, M, and L regions) in all types of fractures (λ, Y, and H) stabilized in a perpendicular configuration, both posterolateral (PL) and posteromedial (PM), for an elbow flexion angle equal to or greater than 120°;
- –
- All variants of the fracture gap except in the trochlear region (i.e., the S, M, and L regions) in all types of fractures (λ, Y, and H) stabilized in a parallel configuration, for the maximum elbow joint flexion angle (145°),
- –
- The fracture gap between the lateral fragment and the shaft (region L) in Y- and H-type fractures when they are stabilized in a parallel configuration, and when the elbow joint is flexed to 120°;
- –
- The fracture gap between the medial fragment and the shaft (region M) in Y-type fractures with stabilization in a posteromedial (PM) configuration, for an elbow flexion angle of 60–90°.
- –
- In the case of a λ-type fracture stabilized in a posterolateral (PL) or parallel configuration, the chance of union is not eliminated in the entire angular range of the loading direction;
- –
- In the case of an H-type fracture stabilized in a posterolateral (PL) configuration, a high probability of non-union is obtained only for the maximum elbow flexion (145°).
- –
- In the case of stabilization in a posteromedial (PM) configuration for all types of fractures and all fracture gaps, for elbow joint flexed at 60° or 90°, with the exception of the gap between the lateral fragment and the shaft (ROI L) in a Y-type fracture;
- –
- In the case of stabilization in a parallel configuration, for the elbow joint flexed at 120° in following situations:
- –
- In the gap between the medial fragment and the shaft (ROI M) for Y- and H-type fractures;
- –
- In the gap between the lateral fragment and the shaft (ROI L) for λ-type fractures;
- –
- In the gap between the trochlea and the shaft (ROI S) for H-type fractures.
4. Discussion
5. Conclusions
- (1)
- No plating configuration is able to ensure DHF union when the full range of motion in the elbow (0–145°) is allowed while holding a weight of 1 kg in the hand.
- (2)
- Elbow flexion in the range of 0–90°, lifting a weight of 1 kg, is allowed when using parallel plating, which is a positive finding in view of early rehabilitation.
- (3)
- Better conditions for fracture healing are ensured when parallel plating is used. Worse conditions occur when perpendicular plating is used, especially the posteromedial version. In this case, the active elbow flexion should be limited to about 30°.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Court-Brown, C.M.; Clement, N.D.; Duckworth, A.D.; Biant, L.C.; McQueen, M.M. The changing epidemiology of fall-related fractures in adults. Injury 2017, 48, 819–824. [Google Scholar] [CrossRef]
- Bergdahl, C.; Ekholm, C.; Wennergren, D.; Nilsson, F.; Möller, M. Epidemiology and patho-anatomical pattern of 2,011 humeral fractures: Data from the Swedish Fracture Register. BMC Musculoskelet. Disord. 2016, 17, 159. [Google Scholar] [CrossRef]
- Claessen, F.M.A.P.; Braun, Y.; van Leeuwen, W.F.; Dyer, G.S.; van den Bekerom, M.P.J.; Ring, D. What factors are associated with a surgical site infection after operative treatment of an elbow fracture? Clin. Orthop. Relat. Res. 2016, 474, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Goel, D.P.; Pike, J.M.; Athwal, G.S. Open reduction and internal fixation of distal humerus fractures. Oper. Tech. Orthop. 2010, 20, 24–33. [Google Scholar] [CrossRef]
- Steinitz, A.; Sailer, J.; Rikli, D. Distal humerus fractures: A review of current therapy concepts. Curr. Rev. Musculoskelet. Med. 2016, 9, 199–206. [Google Scholar]
- Rueadi, T.P.; Murphy, W.E. AO Principle of Fracture Management; AO Publishing Switzerland: Davos, Switzerland, 2009. [Google Scholar] [CrossRef]
- Yetter, T.R.; Weatherby, P.J.; Somerson, J.S. Complications of articular distal humeral fracture fixation: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2021, 30, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Park, J.S.; Baek, J.H.; Kim, S.; Ku, K.H. Complications associated with open reduction and internal fixation for adult distal humerus fractures: A multicenter retrospective study. J. Orthop. Surg. Res. 2022, 17, 399. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Mir, H.R.; Horowitz, E.; Smith, C.; Ahmed, A.S.; Downes, K.; Nydick, J.A. ORIF of distal humerus fractures with modern pre-contoured implants is still associated with a high rate of complications. Indian J. Orthop. 2022, 54, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Vauclair, F.; Goetti, P.; Nguyen, N.T.; Sanchez-Sotelo, J. Distal humerus nonunion: Evaluation and management. EFORT Open Rev. 2020, 5, 289–298. [Google Scholar] [CrossRef]
- Leung, B.; McKee, M.; Peach, C.; Matthews, T.; Arnander, M.; Moverley, R.; Murphy, R.; Phadnis, J. Elbow arthroplasty is safe for the management of simple open distal humeral fractures. J. Shoulder Elb. Surg. 2022, 31, 1005–1014. [Google Scholar] [CrossRef]
- Ku, K.H.; Baek, J.H.; Kim, M.S. Risk Factors for Non-Union after Open Reduction and Internal Fixation in Patients with Distal Humerus Fractures. J. Clin. Med. 2022, 11, 2679. [Google Scholar] [CrossRef]
- Savvidou, O.D.; Zampeli, F.; Koutsouradis, P.; Chloros, G.D.; Kaspiris, A.; Sourmelis, S.; Papagelopoulos, P.J. Complications of open reduction and internal fixation of distal humerus fractures. EFORT Open Rev. 2018, 3, 558–567. [Google Scholar] [CrossRef]
- O’Driscoll, S.W. Optimizing stability in distal humeral fracture fixation. J. Shoulder Elb. Surg. 2005, 14, S186–S194. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J.; Torchia, M.E.; O’Driscoll, S.W. Complex distal humeral fractures: Internal fixation with a principle-based parallel-plate technique. J. Bone Joint Surg. Am. 2008, 90, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Got, C.; Shuck, J.; Biercevicz, A.; Paller, D.; Mulcahey, M.; Zimmermann, M.; Blaine, T.; Green, A. Biomechanical comparison of parallel versus 90-90 plating of bicolumn distal humerus fractures with intra-articular comminution. J. Hand Surg. Am. 2012, 37, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Kang, S.H.; Jeong, M.; Lim, H.S. Triangular fixation technique for bicolumn restoration in treatment of distal humerus intercondylar fracture. Clin. Orthop. Surg. 2016, 8, 9–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Penzkofer, R.; Hungerer, S.; Wipf, F.; Oldenburg, G.; Augat, P. Anatomical plate configuration affects mechanical performance in distal humerus fracture. Clin. Biomech. 2010, 25, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.; Oka, R.; Odell, T.; Mahar, A. Biomechanical comparison of two different periarticular plating systems for stabilization of complex distal humerus fracture. Clin. Biomech. 2006, 21, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Arnander, M.W.; Reeves, A.; MacLeod, I.A.; Pinto, T.M.; Khaleel, A.A. Biomechanical comparison of plate configuration in distal humerus fractures. J. Orthop. Trauma 2008, 22, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zalavras, C.G.; Papasoulis, E. Intra-articular fractures of the distal humerus-a review of the current practice. Int. Orthop. 2018, 42, 2653–2662. [Google Scholar] [CrossRef]
- Koonce, R.C.; Baldini, T.H.; Morgan, S.J. Are conventional reconstruction plates equivalent to precontoured locking plates for distal humerus fracture fixation? A biomechanics cadaver study. Clin. Biomech. 2012, 27, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Sohn, H.S.; Do, N.H. A clinical comparison of two different double plating methods for intraarticular distal humerus fracture. J. Shoulder Elb. Surg. 2010, 19, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Puchwein, P.; Wildburger, R.; Archan, S.; Guschla, M.; Tanzer, K.; Gumpert, R. Outcome of type C (AO) distal humeral fractures: Follow-up of 22 patients with bicolumnar plating osteosynthesis. J. Shoulder Elb. Surg. 2011, 20, 631–636. [Google Scholar] [CrossRef]
- Reising, K.; Hauschild, O.; Strohm, P.C.; Suedkamp, N.P. Stabilization of articular fractures of the distal humerus: Early experience with a novel perpendicular plate system. Injury 2009, 40, 611–617. [Google Scholar] [CrossRef]
- Schmidt-Horlohe, K.H.; Bonk, A.; Wilde, P.; Becker, L.; Hoffmann, R. Promising results after a treatment of simple and complex distal humerus type C fractures by angular-stable double-plate osteosynthesis. J. Orthop. Trauma 2013, 99, 531–541. [Google Scholar] [CrossRef]
- Varady, P.; Rüden, C.; Greinwald, M.; Hungerer, S.; Pätzold, R.; Augat, P. Biomechanical comparison of anatomical plating systems for comminuted distal humeral fractures. Int. Orthop. 2017, 41, 1709–1714. [Google Scholar] [CrossRef][Green Version]
- Zha, Y.; Hua, K.; Huan, Y.; Chen, C.; Sun, W.; Ji, S.; Xiao, D.; Gong, M.; Jiang, X. Biomechanical comparison of three internal fixation configurations for low transcondylar fractures of the distal humerus. Injury 2023, 54, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Claes, L.; Ignatius, A.; Simon, U.; Wehner, T. Numerical Simulation of Callus Healing for Optimization of Fracture Fixation Stiffness. PLoS ONE 2014, 9, e101370. [Google Scholar] [CrossRef]
- Jupiter, J.B.; Mehne, D.K. Fractures of the distal humerus. Orthopedics 1992, 15, 825–833. [Google Scholar] [CrossRef]
- Kincaid, B.L.; An, K.N. Elbow joint biomechanics for preclinical evaluation of total elbow prosthese. J. Biomech. 2013, 46, 2331–2341. [Google Scholar] [CrossRef]
- Sawbones. Available online: https://www.sawbones.com/catalog/biomechanical.html?cat=72_composite-bones (accessed on 1 May 2023).
- Claes, L.; Meyers, N.; Schülke, J.; Reitmaier, S.; Klose, S.; Ignatius, A. The mode of interfragmentary movement affects bone formation and revascularization after callus distraction. PLoS ONE 2018, 13, e0202702. [Google Scholar] [CrossRef] [PubMed]
- Augat, P.; Hollensteiner, M.; von Rüdenb, C. The role of mechanical stimulation in the enhancement of bone healing. Injury 2021, 52, S78–S83. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.M.; Baylor, J.; Akoon, A.; Grandizio, L.C. Controversies in the Management of Bicolumnar Fractures of the Distal Humerus. J. Hand Surg. 2023, 48, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Helfet, D.L.; Kloen, P.; Anand, N.; Rosen, H.S. Open reduction and internal fixation of delayed unions and nonunions of fractures of the distal part of the humerus. J. Bone Joint Surg. 2003, 85, 33–40. [Google Scholar] [CrossRef]
- Ring, D.; Jupiter, J.B. Fracture–dislocation of the elbow. Hand Clin. 2002, 18, 55–63. [Google Scholar] [CrossRef]
- Ferrara, F.; Biancardi, E.; Touloupakis, G.; Bibiano, L.; Ghirardelli, S.; Antonini, G.; Crippa, C. Residual interfragmentary gap after intramedullary nailing of fragility fractures of the humeral diaphysis: Short and midterm term results. Acta Biomed. 2019, 90, 432–438. [Google Scholar]
- Baltov, A.; Mihail, R.; Dian, E. Complications after interlocking intramedullary nailing of humeral shaft fractures. Injury 2014, 45 (Suppl. S1), S9–S15. [Google Scholar] [CrossRef]



| Angle of the Elbow Flexion | 0° | * 1 | 30° | 60–90° | 120° | 145° |
|---|---|---|---|---|---|---|
| JRF direction | −20° | 0° | 10° | 43° | 63° | 95° |
| Plating Configuration | |||||
|---|---|---|---|---|---|
| Region (ROI) | Joint Reaction | Joint Angle | PM | PL | Parallel |
| λ Fracture | |||||
| T | −20 | 0° | OPT | PU | PU |
| 10 | 30° | PU | PU | PU | |
| 43 | 60–90° | PU | PU | PU | |
| 63 | 120° | PU | PU | PU | |
| 95 | 145° | PU | OPT | PU | |
| S | −20 | 0° | PU | OPT | OPT |
| 10 | 30° | OPT | OPT | OPT | |
| 43 | 60–90° | PNU | OPT | PU | |
| 63 | 120° | NU | NU | PU | |
| 95 | 145° | NU | NU | NU | |
| M | −20 | 0° | PU | PU | PU |
| 10 | 30° | PU | PU | PU | |
| 43 | 60–90° | PNU | PU | OPT | |
| 63 | 120° | NU | PNU | OPT | |
| 95 | 145° | NU | PNU | PNU | |
| L | −20 | 0° | PU | PU | PU |
| 10 | 30° | PU | OPT | PU | |
| 43 | 60–90° | PNU | OPT | PU | |
| 63 | 120° | NU | NU | PNU | |
| 95 | 145° | NU | NU | NU | |
| H Fracture | |||||
| T | −20 | 0° | PU | OPT | OPT |
| 10 | 30° | PU | OPT | PU | |
| 43 | 60–90° | PU | PU | PU | |
| 63 | 120° | OPT | PU | PU | |
| 95 | 145° | OPT | PU | PU | |
| M | −20 | 0° | PU | OPT | PU |
| 10 | 30° | PU | OPT | PU | |
| 43 | 60–90° | PNU | OPT | PU | |
| 63 | 120° | NU | PNU | PNU | |
| 95 | 145° | NU | NU | NU | |
| L | −20 | 0° | PU | PU | PU |
| 10 | 30° | PU | PU | OPT | |
| 43 | 60–90° | PNU | OPT | OPT | |
| 63 | 120° | NU | NU | NU | |
| 95 | 145° | NU | NU | NU | |
| S | −20 | 0° | PU | PU | PU |
| 10 | 30° | PU | PU | PU | |
| 43 | 60–90° | PNU | PU | PU | |
| 63 | 120° | NU | NU | PNU | |
| 95 | 145° | NU | NU | NU | |
| Y Fracture | |||||
| T | −20 | 0° | PU | PU | OPT |
| 10 | 30° | PU | PU | OPT | |
| 43 | 60–90° | PU | OPT | PU | |
| 63 | 120° | PU | PU | PU | |
| 95 | 145° | OPT | PU | PU | |
| M | −20 | 0° | PU | PU | PU |
| 10 | 30° | PU | PU | PU | |
| 43 | 60–90° | NU | PU | PU | |
| 63 | 120° | NU | NU | PNU | |
| 95 | 145° | NU | NU | NU | |
| L | −20 | 0° | PU | PU | PU |
| 10 | 30° | OPT | PU | PU | |
| 43 | 60–90° | OPT | PU | PU | |
| 63 | 120° | NU | NU | NU | |
| 95 | 145° | NU | NU | NU | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewski, A.; Piszczatowski, S.; Piekarczyk, P.; Cieślik, P.; Kwiatkowski, K. Weak Points of Double-Plate Stabilization Used in the Treatment of Distal Humerus Fracture through Finite Element Analysis. J. Clin. Med. 2024, 13, 1034. https://doi.org/10.3390/jcm13041034
Kruszewski A, Piszczatowski S, Piekarczyk P, Cieślik P, Kwiatkowski K. Weak Points of Double-Plate Stabilization Used in the Treatment of Distal Humerus Fracture through Finite Element Analysis. Journal of Clinical Medicine. 2024; 13(4):1034. https://doi.org/10.3390/jcm13041034
Chicago/Turabian StyleKruszewski, Artur, Szczepan Piszczatowski, Piotr Piekarczyk, Piotr Cieślik, and Krzysztof Kwiatkowski. 2024. "Weak Points of Double-Plate Stabilization Used in the Treatment of Distal Humerus Fracture through Finite Element Analysis" Journal of Clinical Medicine 13, no. 4: 1034. https://doi.org/10.3390/jcm13041034
APA StyleKruszewski, A., Piszczatowski, S., Piekarczyk, P., Cieślik, P., & Kwiatkowski, K. (2024). Weak Points of Double-Plate Stabilization Used in the Treatment of Distal Humerus Fracture through Finite Element Analysis. Journal of Clinical Medicine, 13(4), 1034. https://doi.org/10.3390/jcm13041034






