Long-Term Outcomes Following Single-Stage Reamed Intramedullary Exchange Nailing in Apparently Aseptic Femoral Shaft Nonunion with Unsuspected Proof of Bacteria
Abstract
1. Introduction
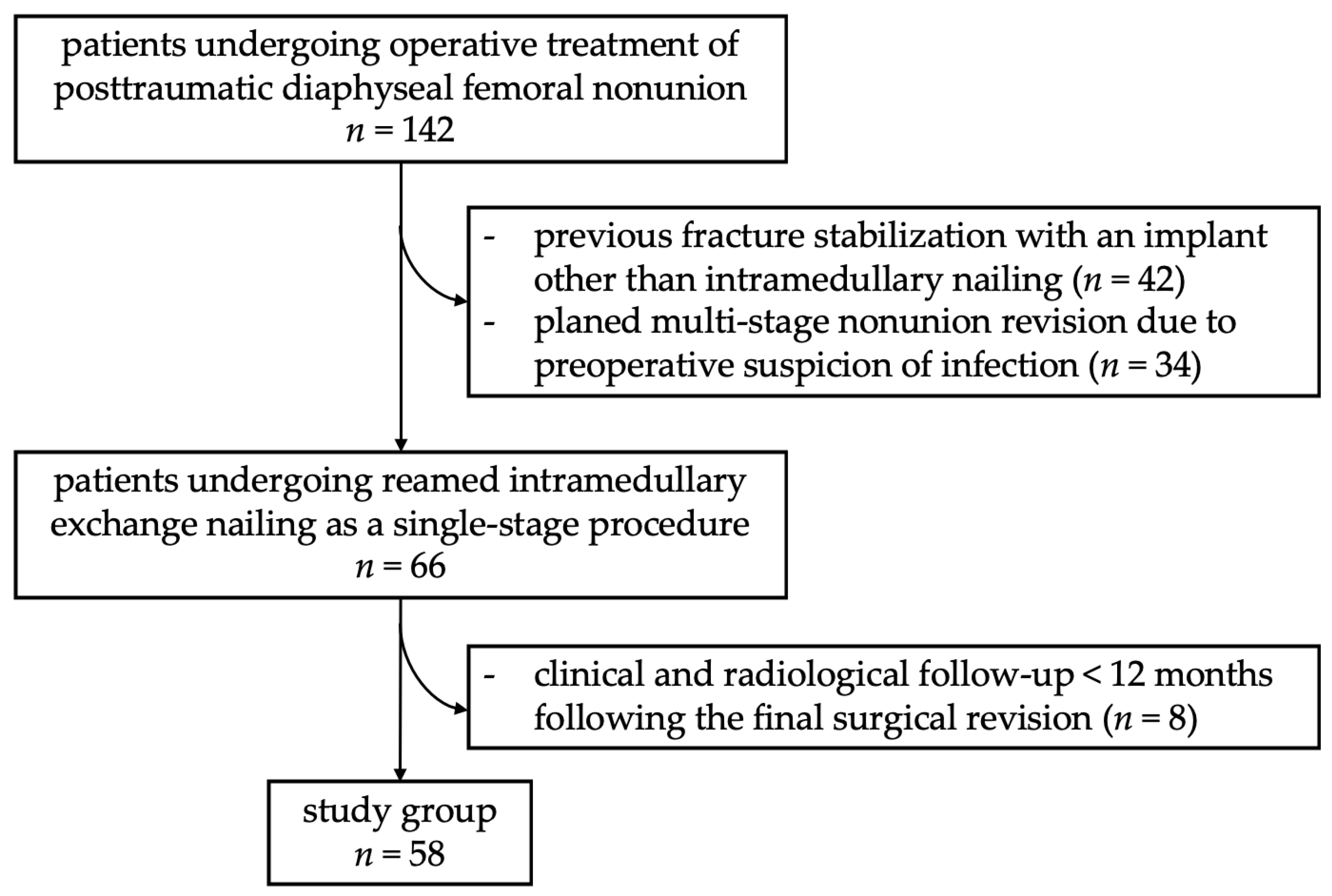
2. Materials and Methods
2.1. Surgical Procedure
2.2. Diagnostic Procedure
2.3. Follow-Up
2.4. Statistical Analysis and Ethical Standards
3. Results
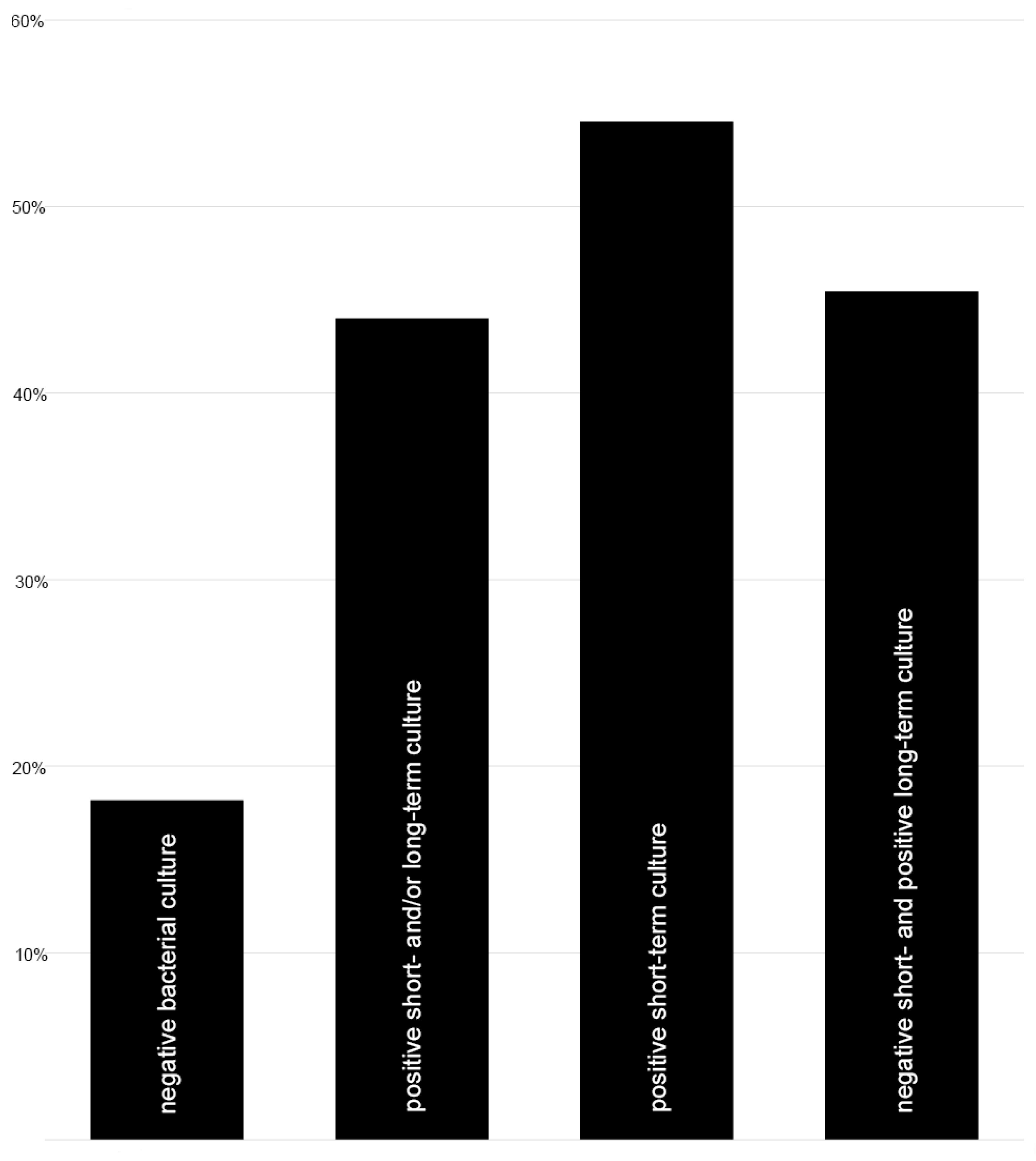
3.1. Rate of Low-Grade Infection in Femoral Shaft Nonunion
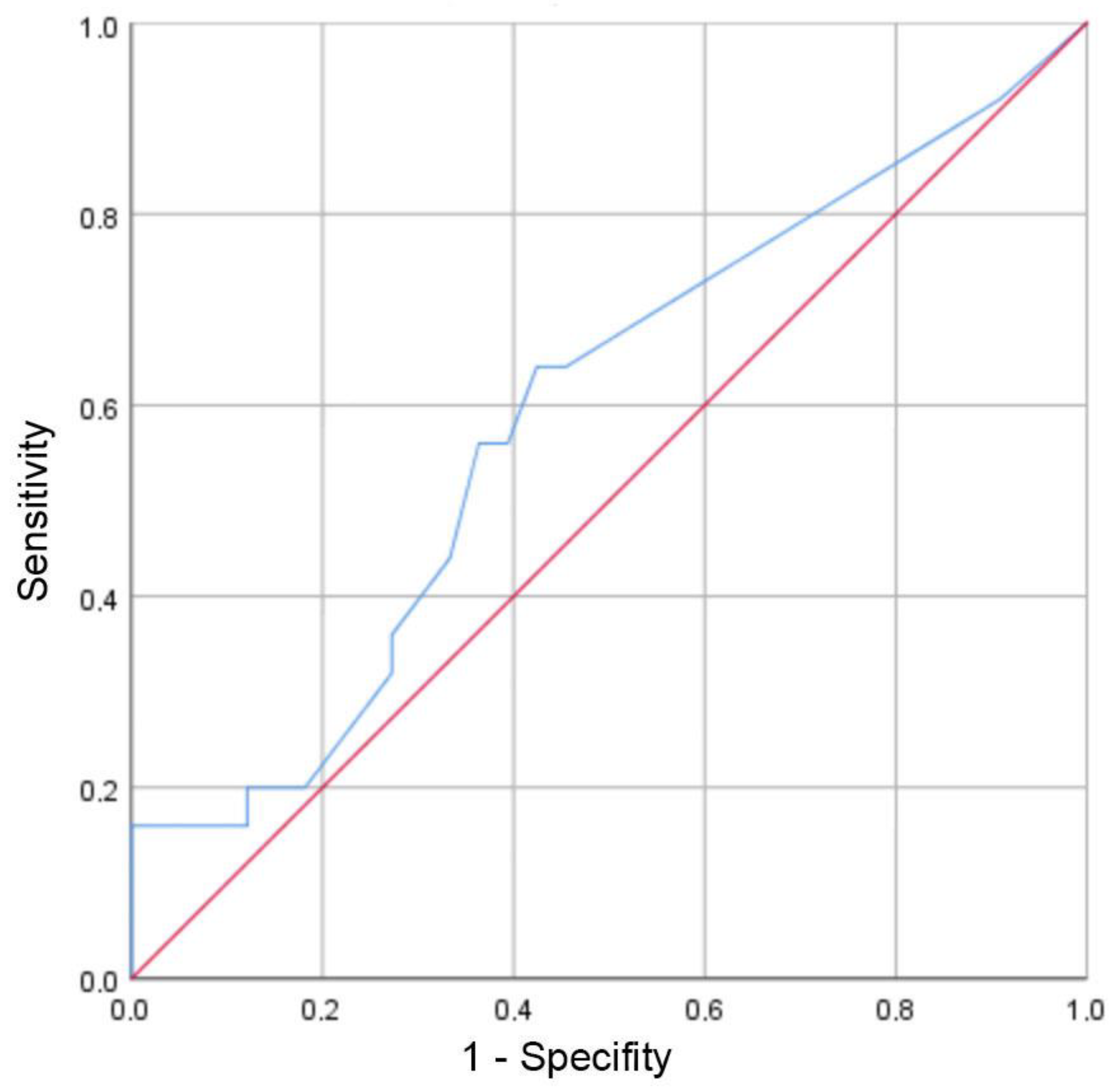
3.2. Evaluation of Risk Factors for the Occurrence of Positive Bacterial Cultures and/or Nonunion
3.3. Preoperative Systemic Inflammation Markers
3.4. Objective and Subjective Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vanderkarr, M.F.; Ruppenkamp, J.W.; Vanderkarr, M.; Holy, C.E.; Blauth, M. Risk factors and healthcare costs associated with long bone fracture non-union: A retrospective US claims database analysis. J. Orthop. Surg. Res. 2023, 18, 745. [Google Scholar] [CrossRef]
- Medlock, G.; Stevenson, I.M.; Johnstone, A.J. Uniting the un-united: Should established non-unions of femoral shaft fractures initially treated with IM nails be treated by plate augmentation instead of exchange IM nailing? A systematic review. Strategies Trauma Limb Reconstr. 2018, 13, 119–128. [Google Scholar] [CrossRef]
- Rupp, M.; Biehl, C.; Budak, M.; Thormann, U.; Heiss, C.; Alt, V. Diaphyseal long bone nonunions—Types, aetiology, economics, and treatment recommendations. Int. Orthop. 2018, 42, 247–258. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, Z.; Dimitriou, R.; Giannoudis, P.V. Health economics: A cost analysis of treatment of persistent fracture non-unions using bone morphogenetic protein-7. Injury 2007, 38, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38 (Suppl. 2), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pihlajamäki, H.K.; Salminen, S.T.; Böstman, O.M. The treatment of nonunions following intramedullary nailing of femoral shaft fractures. J. Orthop. Trauma 2002, 16, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Quan, K.; Xu, Q.; Zhu, M.; Liu, X.; Dai, M. Analysis of Risk Factors for Non-union After Surgery for Limb Fractures: A Case-Control Study of 669 Subjects. Front. Surg. 2021, 8, 754150. [Google Scholar] [CrossRef] [PubMed]
- Andrzejowski, P.; Giannoudis, P.V. The ‘diamond concept’ for long bone non-union management. J. Orthop. Traumatol. 2019, 20, 21. [Google Scholar] [CrossRef]
- Hackl, S.; Hierholzer, C.; Friederichs, J.; Woltmann, A.; Bühren, V.; von Rüden, C. Long-term outcome following additional rhBMP-7 application in revision surgery of aseptic humeral, femoral, and tibial shaft nonunion. BMC Musculoskelet. Disord. 2017, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Copuroglu, C.; Calori, G.M.; Giannoudis, P.V. Fracture non-union: Who is at risk? Injury 2013, 44, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Gelalis, I.D.; Politis, A.N.; Arnaoutoglou, C.M.; Korompilias, A.V.; Pakos, E.E.; Vekris, M.D.; Karageorgos, A.; Xenakis, T.A. Diagnostic and treatment modalities in nonunions of the femoral shaft: A review. Injury 2012, 43, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesántez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Primers 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Hackl, S.; Keppler, L.; von Rüden, C.; Friederichs, J.; Perl, M.; Hierholzer, C. The role of low-grade infection in the pathogenesis of apparently aseptic tibial shaft nonunion. Injury 2021, 52, 3498–3504. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R.; Macek, J.; Laughlin, M.; Dunn, W.R. Utility of Common Biomarkers for Diagnosing Infection in Nonunion. J. Orthop. Trauma 2021, 35, 121–127. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Soumya, K.R.; Philip, S.; Sugathan, S.; Mathew, J.; Radhakrishnan, E.K. Virulence factors associated with Coagulase Negative Staphylococci isolated from human infections. 3 Biotech 2017, 7, 140. [Google Scholar] [CrossRef]
- Steinhausen, E. Low-Grade-Infekt. Trauma Berufskrankh. 2017, 19, 267–271. [Google Scholar] [CrossRef][Green Version]
- Mouzopoulos, G.; Kanakaris, N.K.; Kontakis, G.; Obakponovwe, O.; Townsend, R.; Giannoudis, P.V. Management of bone infections in adults: The surgeon’s and microbiologist’s perspectives. Injury 2011, 42 (Suppl. 5), 18–23. [Google Scholar] [CrossRef]
- Vaughn, J.E.; Shah, R.V.; Samman, T.; Stirton, J.; Liu, J.; Ebraheim, N.A. Systematic review of dynamization vs exchange nailing for delayed/non-union femoral fractures. World J. Orthop. 2018, 9, 92–99. [Google Scholar] [CrossRef]
- Hierholzer, C.; Glowalla, C.; Herrler, M.; von Rüden, C.; Hungerer, S.; Bühren, V.; Friederichs, J. Reamed intramedullary exchange nailing: Treatment of choice of aseptic femoral shaft nonunion. J. Orthop. Surg. Res. 2014, 9, 88. [Google Scholar] [CrossRef]
- Shroeder, J.E.; Mosheiff, R.; Khoury, A.; Liebergall, M.; Weil, Y.A. The outcome of closed, intramedullary exchange nailing with reamed insertion in the treatment of femoral shaft nonunions. J. Orthop. Trauma 2009, 23, 653–657. [Google Scholar] [CrossRef]
- Rupp, M.; Walter, N.; Baertl, S.; Lang, S.; Lowenberg, D.W.; Alt, V. Terminology of bone and joint infection. Bone Joint Res. 2021, 10, 742–743. [Google Scholar] [CrossRef]
- Morgenstern, M.; Kuehl, R.; Zalavras, C.G.; McNally, M.; Zimmerli, W.; Burch, M.A.; Vandendriessche, T.; Obremskey, W.T.; Verhofstad, M.H.J.; Metsemakers, W.J. The influence of duration of infection on outcome of debridement and implant retention in fracture-related infection. Bone Joint J. 2021, 103-B, 213–221. [Google Scholar] [CrossRef]
- Foster, A.L.; Moriarty, T.F.; Trampuz, A.; Jaiprakash, A.; Burch, M.A.; Crawford, R.; Paterson, D.L.; Metsemakers, W.J.; Schuetz, M.; Richards, R.G. Fracture-related infection: Current methods for prevention and treatment. Expert Rev. Anti-Infect. Ther. 2020, 18, 307–321. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef]
- Simpson, A.H.; Tsang, J.S.T. Current treatment of infected non-union after intramedullary nailing. Injury 2017, 48 (Suppl. 1), 82–90. [Google Scholar] [CrossRef]
- Ueng, S.W.; Wei, F.C.; Shih, C.H. Management of femoral diaphyseal infected nonunion with antibiotic beads local therapy, external skeletal fixation, and staged bone grafting. J. Trauma 1999, 46, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Prasarn, M.L.; Ahn, J.; Achor, T.; Matuszewski, P.; Lorich, D.G.; Helfet, D.L. Management of infected femoral nonunions with a single-staged protocol utilizing internal fixation. Injury 2009, 40, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.H.; Leopold, S.S. In brief: Gustilo-Anderson classification. Clin. Orthop. Relat. Res. 2012, 470, 3270–3274. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Wittauer, M.; Burch, M.A.; McNally, M.; Vandendriessche, T.; Clauss, M.; Della Rocca, G.J.; Giannoudis, P.V.; Metsemakers, W.J.; Morgenstern, M. Definition of long-bone nonunion: A scoping review of prospective clinical trials to evaluate current practice. Injury 2021, 52, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, S.; Schwilk, M.; Haubruck, P.; Ferbert, T.; Helbig, L.; Miska, M.; Schmidmaier, G.; Tanner, M.C. Matched-Pair Analysis: Large-Sized Defects in Surgery of Lower Limb Nonunions. J. Clin. Med. 2023, 12, 4239. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Kazam, J.J.; Fufa, D.; Bartolotta, R.J. Radiologic evaluation of fracture healing. Skeletal. Radiol. 2019, 48, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38 (Suppl. 4), 3–6. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Pallay, J.; Depypere, M.; Moriarty, T.F.; Van Lieshout, E.M.M.; Obremskey, W.T.; Sermon, A.; Hoekstra, H.; Verhofstad, M.H.J.; Nijs, S.; et al. Intramedullary tissue cultures from the Reamer-Irrigator-Aspirator system for diagnosing fracture-related infection. J. Orthop. Res. 2021, 39, 281–290. [Google Scholar] [CrossRef]
- Canadian Orthopaedic Trauma Society. Nonunion following intramedullary nailing of the femur with and without reaming. Results of a multicenter randomized clinical trial. J. Bone Joint Surg. Am. 2003, 85, 2093–2096. [Google Scholar] [CrossRef]
- Peel, T.N.; Spelman, T.; Dylla, B.L.; Hughes, J.G.; Greenwood-Quaintance, K.E.; Cheng, A.C.; Mandrekar, J.N.; Patel, R. Optimal Periprosthetic Tissue Specimen Number for Diagnosis of Prosthetic Joint Infection. J. Clin. Microbiol. 2016, 55, 234–243. [Google Scholar] [CrossRef]
- Friederichs, J.; von Rüden, C.; Hierholzer, C.; Bühren, V. Antegrade femoral intramedullary nailing in a lateral position. Unfallchirurg 2015, 118, 295–301. [Google Scholar] [CrossRef]
- Stucken, C.; Olszewski, D.C.; Creevy, W.R.; Murakami, A.M.; Tornetta, P. Preoperative diagnosis of infection in patients with nonunions. J. Bone Joint Surg. Am. 2013, 95, 1409–1412. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Binkley, J.M.; Stratford, P.W.; Lott, S.A.; Riddle, D.L. The Lower Extremity Functional Scale (LEFS): Scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys. Ther. 1999, 79, 371–383. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Brinker, M.R.; Trivedi, A.; O’Connor, D.P. Debilitating Effects of Femoral Nonunion on Health-Related Quality of Life. J. Orthop. Trauma 2017, 31, e37–e42. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Thaler, B.; Bruckner, T.; Tanner, M.; Schmidmaier, G. Treatment of atrophic femoral non-unions according to the diamond concept: Results of one- and two-step surgical procedure. J. Orthop. 2016, 14, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Zeckey, C.; Mommsen, P.; Andruszkow, H.; Macke, C.; Frink, M.; Stübig, T.; Hüfner, T.; Krettek, C.; Hildebrand, F. The aseptic femoral and tibial shaft non-union in healthy patients—An analysis of the health-related quality of life and the socioeconomic outcome. Open Orthop. J. 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M.; Fernandez, A.; Regazzoni, P. Understanding Fracture Healing Biomechanics Based on the “Strain” Concept and its Clinical Applications. Acta Chir. Orthop. Traumatol. Cech. 2015, 82, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Arsoy, D.; Donders, J.C.E.; Kleeblad, L.J.; Miller, A.O.; Henry, M.W.; Wellman, D.S.; Helfet, D.L. Outcomes of Presumed Aseptic Long-Bone Nonunions With Positive Intraoperative Cultures Through a Single-Stage Surgical Protocol. J. Orthop. Trauma 2018, 32 (Suppl. 1), 35–39. [Google Scholar] [CrossRef] [PubMed]
- Amorosa, L.F.; Buirs, L.D.; Bexkens, R.; Wellman, D.S.; Kloen, P.; Lorich, D.G.; Helfet, D.L. A single-stage treatment protocol for presumptive aseptic diaphyseal nonunions: A review of outcomes. J. Orthop. Trauma 2013, 27, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Hackl, S.; Trenkwalder, K.; Militz, M.; Augat, P.; Stuby, F.M.; von Rüden, C. Infected nonunion: Diagnostic and therapeutic work-up. Unfallchirurgie 2022, 125, 602–610. [Google Scholar] [CrossRef]
- Bonicoli, E.; Piolanti, N.; Giuntoli, M.; Polloni, S.; Scaglione, M. Septic femoral shaft non-union treated by one-step surgery using a custom-made intramedullary antibiotic cement-coated carbon nail: Case report and focus on surgical technique. Acta Biomed. 2020, 91, e2020176. [Google Scholar]
- Wu, C.C. Aseptic femoral nonunion treated with exchange locked nailing with intramedullary augmentation cancellous bone graft. J. Orthop. Surg. Res. 2022, 17, 339. [Google Scholar] [CrossRef]
- Ding, P.; Chen, Q.; Zhang, C.; Yao, C. Revision with Locking Compression Plate by Compression Technique for Diaphyseal Nonunions of the Femur and the Tibia: A Retrospective Study of 54 Cases. Biomed Res. Int. 2021, 2021, 9905067. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Kugan, R.; Stubbs, D.; McNally, M. Management of infected nonunion of the long bones by a multidisciplinary team. Bone Joint J. 2015, 97-B, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.K.; van Trikt, C.H.; Visser, C.E.; Janssen, S.J.; Kloen, P. Surprise positive culture rate in the treatment of presumed aseptic long-bone nonunion: A systematic review with meta-analysis of 2397 patients. Arch. Orthop. Trauma Surg. 2024, 144, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wong, T.M.; Lau, T.W.; To, K.K.; Wong, S.S.; Leung, F. Infection after fracture osteosynthesis—Part I. J. Orthop. Surg. 2017, 25, 2309499017692712. [Google Scholar] [CrossRef] [PubMed]
- Zalavras, C.G.; Marcus, R.E.; Levin, L.S.; Patzakis, M.J. Management of open fractures and subsequent complications. J. Bone Joint Surg. Am. 2007, 89, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, D.; Streubel, P.N.; Stucken, C.; Ricci, W.M.; Hoffmann, M.F.; Jones, C.B.; Sietsema, D.L.; Tornetta, P. Fate of Patients With a “Surprise” Positive Culture After Nonunion Surgery. J. Orthop. Trauma 2016, 30, e19–e23. [Google Scholar] [CrossRef] [PubMed]
- Dapunt, U.; Spranger, O.; Gantz, S.; Burckhardt, I.; Zimmermann, S.; Schmidmaier, G.; Moghaddam, A. Are atrophic long-bone nonunions associated with low-grade infections? Ther. Clin. Risk Manag. 2015, 11, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.P.; Altman, D.T.; Altman, G.T.; Sewecke, J.J.; Ehrlich, G.D.; Hu, F.Z.; Nistico, L.; Melton-Kreft, R.; Gause, T.M.; Costerton, J.W. Can we trust intraoperative culture results in nonunions? J. Orthop. Trauma 2014, 28, 384–390. [Google Scholar] [CrossRef]
- Gille, J.; Wallstabe, S.; Schulz, A.P.; Paech, A.; Gerlach, U. Is non-union of tibial shaft fractures due to nonculturable bacterial pathogens? A clinical investigation using PCR and culture techniques. J. Orthop. Surg. Res. 2012, 7, 20. [Google Scholar] [CrossRef]
- Tiemann, A.; Hofmann, G.O.; Krukemeyer, M.G.; Krenn, V.; Langwald, S. Histopathological Osteomyelitis Evaluation Score (HOES)—An innovative approach to histopathological diagnostics and scoring of osteomyelitis. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2014, 3, Doc08. [Google Scholar] [CrossRef]
- Wang, S.; Yin, P.; Quan, C.; Khan, K.; Wang, G.; Wang, L.; Cui, L.; Zhang, L.; Zhang, L.; Tang, P. Evaluating the use of serum inflammatory markers for preoperative diagnosis of infection in patients with nonunions. Biomed Res. Int. 2017, 2017, 9146317. [Google Scholar] [CrossRef]
- Wu, C.C. Exchange nailing for aseptic nonunion of femoral shaft: A retrospective cohort study for effect of reaming size. J. Trauma 2007, 63, 859–865. [Google Scholar] [CrossRef]
- Hak, D.J.; Lee, S.S.; Goulet, J.A. Success of exchange reamed intramedullary nailing for femoral shaft nonunion or delayed union. J. Orthop. Trauma 2000, 14, 178–182. [Google Scholar] [CrossRef]
- Johnson, L.; Igoe, E.; Kleftouris, G.; Papachristos, I.V.; Papakostidis, C.; Giannoudis, P.V. Physical Health and Psychological Outcomes in Adult Patients with Long-bone Fracture Non-unions: Evidence Today. J. Clin. Med. 2019, 8, 1998. [Google Scholar] [CrossRef]
- Maurer, E.; Walter, N.; Baumgartner, H.; Histing, T.; Alt, V.; Rupp, M. Quality of life after fracture-related infection of the foot. Foot Ankle Surg. 2022, 28, 1421–1426. [Google Scholar] [CrossRef]
- Iliaens, J.; Onsea, J.; Hoekstra, H.; Nijs, S.; Peetermans, W.E.; Metsemakers, W.J. Fracture-related infection in long bone fractures: A comprehensive analysis of the economic impact and influence on quality of life. Injury 2021, 52, 3344–3349. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Bärtl, S.; Lang, S.; Walter, N.; Alt, V. Fracture-related infections after intramedullary nailing: Diagnostics and treatment. Unfallchirurgie 2022, 125, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bernard, L.; Arvieux, C.; Brunschweiler, B.; Touchais, S.; Ansart, S.; Bru, J.P.; Oziol, E.; Boeri, C.; Gras, G.; Druon, J.; et al. Antibiotic Therapy for 6 or 12 Weeks for Prosthetic Joint Infection. N. Engl. J. Med. 2021, 384, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Kuehl, R.; Metsemakers, W.J.; Senneville, E.; McNally, M.; Obremskey, W.T.; Zimmerli, W.; Atkins, B.L.; Trampuz, A.; Fracture-Related Infection Consensus Group. Recommendations for Systemic Antimicrobial Therapy in Fracture-Related Infection: A Consensus From an International Expert Group. J. Orthop. Trauma 2020, 34, 30–41. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Number |
|---|---|
| Gender | |
| Male | 45 |
| Female | 13 |
| Age | 46.3 ± 2.1 (range 18–81) years |
| Fracture location | |
| Proximal part of the femoral shaft | 19 |
| Middle part of the femoral shaft | 27 |
| Distal part of the femoral shaft | 12 |
| Fracture pattern according to the AO/OTA classification 1 | |
| Type A1 | 16 |
| Type A2 | 17 |
| Type A3 | 7 |
| Type B1 | 3 |
| Type B2 | 5 |
| Type B3 | 3 |
| Type C1 | 1 |
| Type C2 | 3 |
| Type C3 | 3 |
| Initial soft tissue injury | |
| Closed fracture | 50 |
| Gustilo–Anderson open fracture classification I–III | 8 |
| Nonunion type | |
| Hypertrophic | 40 |
| Atrophic/Oligotrophic | 18 |
| Comorbidities | |
| Charlson comorbidity index | 0.3 ± 0.1 (range 0–3) points |
| Nicotine abuse | 12 |
| Diabetes mellitus | 6 |
| Period of time between initial fracture fixation and nonunion revision | 11.2 ± 1.0 (range 4–32) months |
| Organism | Number of Isolates (Total n = 29) |
|---|---|
| Coagulase-negative Staphylococcus spp. | |
| Staphylococcus epidermidis | 10 |
| Staphylococcus capitis | 3 |
| Staphylococcus lugdunensis | 3 |
| Staphylococcus haemolyticus | 2 |
| Staphylococcus warneri | 2 |
| Staphylococcus hominis | 1 |
| Staphylococcus aureus | 1 |
| Streptococcus alactolyticus | 1 |
| Enterococcus faecalis | 2 |
| Pseudomonas aeruginosa | 1 |
| Pseudomonas fluorescenses | 1 |
| Cutibacterium acnes | 2 |
| Parameter | Group P (Positive Cultures) | Group N (Negative Cultures) | p-Value |
|---|---|---|---|
| Fracture location | |||
| Proximal part of the femoral shaft | 7 | 12 | |
| Middle part of the femoral shaft | 11 | 16 | |
| Distal part of the femoral shaft | 7 | 5 | 0.472 |
| Fracture pattern according to the AO/OTA classification | |||
| Type A | 19 | 21 | |
| Type B | 1 | 10 | |
| Type C | 5 | 2 | 0.068 |
| Initial soft tissue injury | |||
| Closed fracture | 19 | 31 | |
| Gustilo–Anderson open fracture I–III | 6 | 2 | 0.045 |
| Nonunion type | |||
| Hypertrophic | 15 | 25 | |
| Atrophic/Oligotrophic | 10 | 8 | 0.199 |
| Test Procedure | Group P (Positive Cultures) | Group N (Negative Cultures) | p-Value |
|---|---|---|---|
| LEFS | 46.0 ± 5.1 points | 51.6 ± 5.7 points | 0.479 |
| PCS of SF-12 | 35.6 ± 3.1 points | 44.4 ± 2.6 points | 0.040 |
| MCS of SF-12 | 49.5 ± 3.2 points | 50.1 ± 2.4 points | 0.875 |
| Study | Inclusion Criteria | Number of Patients | Bacterial Detection Rate | |
|---|---|---|---|---|
| Gille et al. [62] | preoperatively aseptic classified tibial shaft nonunion | 23 | culturing for 14 days: | 0% |
| Olszewski et al. [59] | nonunion without signs of infection but with risk factors for infection | 453 | culturing for 5 days: | 20% |
| Dapunt et al. [60] | atrophic nonunion of long bones (32.7% with clinical signs of infection) | 49 | culturing for 2 days: | 6.8% |
| culturing for 5 days: | 10.2% | |||
| sonication and culturing for 14 days: | 57.1% | |||
| Palmer et al. [61] | nonunion of long bones | 34 | culturing for 5 days: | 23.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hackl, S.; von Rüden, C.; Trenkwalder, K.; Keppler, L.; Hierholzer, C.; Perl, M. Long-Term Outcomes Following Single-Stage Reamed Intramedullary Exchange Nailing in Apparently Aseptic Femoral Shaft Nonunion with Unsuspected Proof of Bacteria. J. Clin. Med. 2024, 13, 1414. https://doi.org/10.3390/jcm13051414
Hackl S, von Rüden C, Trenkwalder K, Keppler L, Hierholzer C, Perl M. Long-Term Outcomes Following Single-Stage Reamed Intramedullary Exchange Nailing in Apparently Aseptic Femoral Shaft Nonunion with Unsuspected Proof of Bacteria. Journal of Clinical Medicine. 2024; 13(5):1414. https://doi.org/10.3390/jcm13051414
Chicago/Turabian StyleHackl, Simon, Christian von Rüden, Katharina Trenkwalder, Lena Keppler, Christian Hierholzer, and Mario Perl. 2024. "Long-Term Outcomes Following Single-Stage Reamed Intramedullary Exchange Nailing in Apparently Aseptic Femoral Shaft Nonunion with Unsuspected Proof of Bacteria" Journal of Clinical Medicine 13, no. 5: 1414. https://doi.org/10.3390/jcm13051414
APA StyleHackl, S., von Rüden, C., Trenkwalder, K., Keppler, L., Hierholzer, C., & Perl, M. (2024). Long-Term Outcomes Following Single-Stage Reamed Intramedullary Exchange Nailing in Apparently Aseptic Femoral Shaft Nonunion with Unsuspected Proof of Bacteria. Journal of Clinical Medicine, 13(5), 1414. https://doi.org/10.3390/jcm13051414








