Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Sample Size
2.4. Intervention Protocol
2.5. Sample Collection
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Subjects’ Recruitment
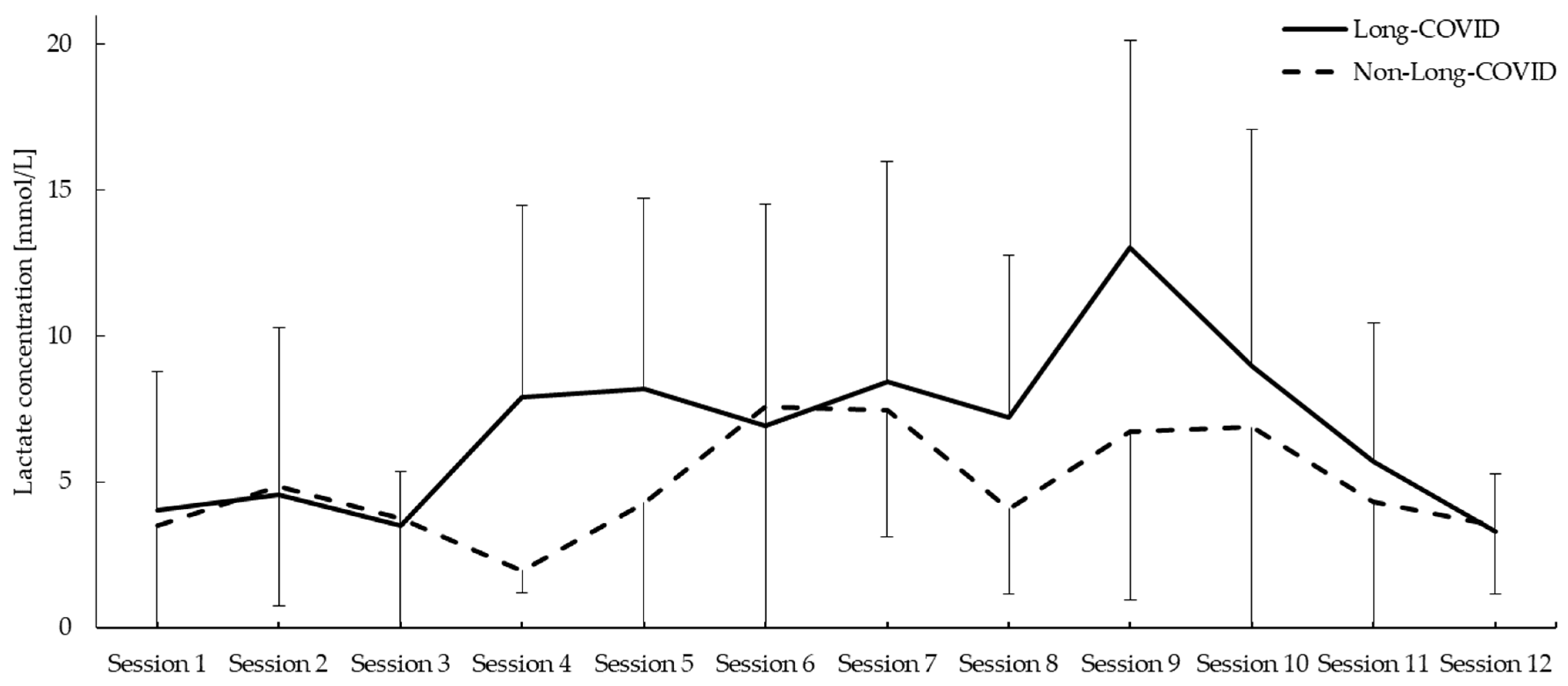
3.2. Lactate Concentration
3.3. Fatigue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-de-Las-Peñas, C. Long COVID: Current definition. Infection 2022, 50, 285–286. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Long-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Healey, Q.; Sheikh, A.; Daines, L.; Vasileiou, E. Symptoms and signs of long COVID: A rapid review and meta-analysis. J. Glob. Health 2022, 12, 05014. [Google Scholar] [CrossRef] [PubMed]
- Lledó, G.M.; Sellares, J.; Brotons, C.; Sans, M.; Antón, J.D.; Blanco, J.; Bassat, Q.; Sarukhan, A.; Miró, J.M.; de Sanjosé, S. Long-acute COVID-19 syndrome: A new tsunami requiring a universal case definition. Clin. Microbiol. Infect. 2022, 28, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ashton, R.; Ansdell, P.; Hume, E.; Maden-Wilkinson, T.; Ryan, D.; Tuttiett, E.; Faghy, M. COVID-19 and the long-term cardio-respiratory and metabolic health complications. Rev. Cardiovasc. Med. 2022, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after COVID-19: Qualitative study of 114 “long COVID” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef]
- Woodrow, M.; Carey, C.; Ziauddeen, N.; Thomas, R.; Akrami, A.; Lutje, V.; Greenwood, D.C.; Alwan, N.A. Systematic Review of the Prevalence of Long COVID. Open Forum Infect. Dis. 2023, 10, ofad233. [Google Scholar] [CrossRef]
- Lobo-Valbuena, B.; García-Arias, M.; Pérez, R.B.; Delgado, D.V.; Gordo, F. Characteristics of critical patients with COVID-19 in a Spanish second-level hospital. Med. Intensiva (Engl. Ed.) 2021, 45, 56–58. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Rao, M.; Bonilla, H.; Subramanian, A.; Hack, I.; Madrigal, M.; Singh, U.; Jagannathan, P.; Grant, P. Patients With Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale During a Global Pandemic. Clin. Infect. Dis. 2021, 73, e826–e829. [Google Scholar] [CrossRef]
- Osikomaiya, B.; Erinoso, O.; Wright, K.O.; Odusola, A.O.; Thomas, B.; Adeyemi, O.; Bowale, A.; Adejumo, O.; Falana, A.; Abdus-Salam, I.; et al. ‘Long COVID’: Persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect. Dis. 2021, 21, 304. [Google Scholar] [CrossRef]
- Gaber, T.A.K.; Ashish, A.; Unsworth, A. Persistent Long-COVID symptoms in healthcare workers. Occup. Med. (Lond.) 2021, 71, 144–146. [Google Scholar] [CrossRef]
- Debeaumont, D.; Boujibar, F.; Ferrand-Devouge, E.; Artaud-Macari, E.; Tamion, F.; Gravier, F.E.; Smondack, P.; Cuvelier, A.; Muir, J.F.; Alexandre, K.; et al. Cardiopulmonary Exercise Testing to Assess Persistent Symptoms at 6 Months in People With COVID-19 Who Survived Hospitalization: A Pilot Study. Phys. Ther. 2021, 101, pzab099. [Google Scholar] [CrossRef]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of Cardiopulmonary Stress Testing for Patients with Unexplained Dyspnea Long-Coronavirus Disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Silvapulle, E.; Johnson, D.; Darvall, J.N. Risk stratification of individuals undergoing surgery after COVID-19 recovery. Br. J. Anaesth. 2022, 128, e37–e39. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; D’Arminio Monforte, A.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef]
- Tansey, C.M.; Louie, M.; Loeb, M.; Gold, W.L.; Muller, M.P.; de Jager, J.; Cameron, J.I.; Tomlinson, G.; Mazzulli, T.; Walmsley, S.L.; et al. One-year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch. Intern. Med. 2007, 167, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Batawi, S.; Tarazan, N.; Al-Raddadi, R.; Al Qasim, E.; Sindi, A.; Al Johni, S.; Al-Hameed, F.M.; Arabi, Y.M.; Uyeki, T.M.; Alraddadi, B.M. Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS). Health Qual. Life Outcomes 2019, 17, 101. [Google Scholar] [CrossRef]
- Herridge, M.S.; Cheung, A.M.; Tansey, C.M.; Matte-Martyn, A.; Diaz-Granados, N.; Al-Saidi, F.; Cooper, A.B.; Guest, C.B.; Mazer, C.D.; Mehta, S.; et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003, 348, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Herridge, M.S.; Tansey, C.M.; Matté, A.; Tomlinson, G.; Diaz-Granados, N.; Cooper, A.; Guest, C.B.; Mazer, C.D.; Mehta, S.; Stewart, T.E.; et al. Functional disability 5 years after acute respiratory distress syndrome. N. Engl. J. Med. 2011, 364, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Long-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- de Boer, E.; Petrache, I.; Goldstein, N.M.; Olin, J.T.; Keith, R.C.; Modena, B.; Mohning, M.P.; Yunt, Z.X.; San-Millán, I.; Swigris, J.J. Decreased Fatty Acid Oxidation and Altered Lactate Production during Exercise in Patients with Long-acute COVID-19 Syndrome. Am. J. Respir. Crit. Care Med. 2022, 205, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Guo, D.; Lin, S.H.; Liang, J.; Yang, D.; Ma, C.; Shao, F.; Li, M.; Yu, Q.; Jiang, Y.; et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 2021, 81, 2303–2316.e8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhao, Z.; Lu, H.; Ke, B.; Ye, X.; Wu, B.; Ye, J. NF-κ B/HDAC1/SREBP1c pathway mediates the inflammation signal in progression of hepatic steatosis. Acta Pharm. Sin. B 2020, 10, 825–836. [Google Scholar] [CrossRef]
- Galván-Peña, S.; Carroll, R.G.; Newman, C.; Hinchy, E.C.; Palsson-McDermott, E.; Robinson, E.K.; Covarrubias, S.; Nadin, A.; James, A.M.; Haneklaus, M.; et al. Malonylation of GAPDH is an inflammatory signal in macrophages. Nat. Commun. 2019, 10, 338. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Lien, K.; Johansen, B.; Veierød, M.B.; Haslestad, A.S.; Bøhn, S.K.; Melsom, M.N.; Kardel, K.R.; Iversen, P.O. Abnormal blood lactate accumulation during repeated exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Physiol. Rep. 2019, 7, e14138. [Google Scholar] [CrossRef]
- Miyazaki, A.; Okuyama, T.; Mori, H.; Sato, K.; Kumamoto, K.; Hiyama, A. Effects of Two Short-Term Aerobic Exercises on Cognitive Function in Healthy Older Adults during COVID-19 Confinement in Japan: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 6202. [Google Scholar] [CrossRef]
- Micielska, K.; Flis, M.; Kortas, J.A.; Rodziewicz-Flis, E.; Antosiewicz, J.; Wochna, K.; Lombardi, G.; Ziemann, E. Nordic Walking Rather Than High Intensity Interval Training Reduced Myostatin Concentration More Effectively in Elderly Subjects and the Range of This Drop Was Modified by Metabolites of Vitamin D. Nutrients 2021, 13, 4393. [Google Scholar] [CrossRef] [PubMed]
- Tschentscher, M.; Niederseer, D.; Niebauer, J. Health benefits of Nordic walking: A systematic review. Am. J. Prev. Med. 2013, 44, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Arbillaga-Etxarri, A.; Gimeno-Santos, E.; Rodríguez, D.A.; Torralba, Y.; Roca, J.; Vilaró, J. Nordic walking enhances oxygen uptake without increasing the rate of perceived exertion in patients with chronic obstructive pulmonary disease. Respiration 2015, 89, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, W.; Li, J.; Ossowski, Z. Effects of aerobic exercise on metabolic indicators and physical performance in adult NAFLD patients: A systematic review and network meta-analysis. Medicine (Baltimore) 2023, 102, e33147. [Google Scholar] [CrossRef] [PubMed]
- Kos, D.; Kerckhofs, E.; Carrea, I.; Verza, R.; Ramos, M.; Jansa, J. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Mult. Scler. 2005, 11, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. The Spanish version of the Short Form 36 Health Survey: A decade of experience and new developments. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, B.; Zhu, L.A.; Leng, L.; Bucala, R.; Lu, L.J. Construct and criterion validity of the Euro Qol-5D in patients with systemic lupus erythematosus. PLoS ONE 2014, 9, e98883. [Google Scholar] [CrossRef]
- Pennacchia, F.; Rusi, E.; Ruqa, W.A.; Zingaropoli, M.A.; Pasculli, P.; Talarico, G.; Bruno, G.; Barbato, C.; Minni, A.; Tarani, L.; et al. Blood Biomarkers from the Emergency Department Disclose Severe Omicron COVID-19-Associated Outcomes. Microorganisms 2023, 11, 925. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, S.; Feng, Y.; Wu, W.; Chang, C.; Chen, S.; Zhen, G.; Yi, L. Decreased eosinophil counts and elevated lactate dehydrogenase predict severe COVID-19 in patients with underlying chronic airway diseases. Longgrad Med. J. 2022, 98, 906–913. [Google Scholar] [CrossRef]
- Liu, J.; Gong, S.; Lv, J.; Wang, G.; Guo, Y.; Gao, D.; Zhang, D.; Ma, S.; Luo, H.; Yang, H.; et al. Clinical Features and Blood Indicators for Severity and Prognosis in COVID-19 Patients. Clin. Lab. 2023, 69, 742. [Google Scholar] [CrossRef]
- Kumari, A.; Itagi, A.B.H.; Rukadikar, C.A.; D, A.; Naik, B.N.; Juhi, A.; Naik, S.; Dipankar, S.P. Effect of COVID-19 on Stress and Biomarkers: An Exploratory Cross-Sectional Study. Cureus 2023, 15, e35702. [Google Scholar] [CrossRef]
- Czerwińska-Ledwig, O.; Vesole, D.H.; Piotrowska, A.; Gradek, J.; Pilch, W.; Jurczyszyn, A. Effect of a 6-Week Cycle of Nordic Walking Training on Vitamin 25(OH)D(3), Calcium-Phosphate Metabolism and Muscle Damage in Multiple Myeloma Patients-Randomized Controlled Trial. J. Clin. Med. 2022, 11, 6534. [Google Scholar] [CrossRef]
- Smolander, J.; Kolari, P.; Korhonen, O.; Ilmarinen, R. Aerobic and anaerobic responses to incremental exercise in a thermoneutral and a hot dry environment. Acta Physiol. Scand. 1986, 128, 15–21. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Lambert, D.L.; Starkie, R.L.; Proietto, J.; Hargreaves, M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. J. Appl. Physiol. (1985) 1998, 84, 465–470. [Google Scholar] [CrossRef]
- González-Alonso, J.; Calbet, J.A.; Nielsen, B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. J. Physiol. 1999, 520 Pt 2, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodríguez, R.; González-Alonso, J.; Below, P.R.; Coyle, E.F. Plasma catecholamines and hyperglycaemia influence thermoregulation in man during prolonged exercise in the heat. J. Physiol. 1996, 491 Pt 2, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Terada, T.; Cotie, L.M.; Tulloch, H.E.; Leenen, F.H.; Mistura, M.; Hans, H.; Wang, H.W.; Vidal-Almela, S.; Reid, R.D.; et al. The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: A randomized controlled trial (CRX study). Prog. Cardiovasc. Dis. 2022, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Joli, J.; Buck, P.; Zipfel, S.; Stengel, A. Long-COVID-19 fatigue: A systematic review. Front. Psychiatry 2022, 13, 947973. [Google Scholar] [CrossRef] [PubMed]
- van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.; McCann, K.; O’Brien, C.; Savinelli, S.; Tinago, W.; Yousif, O.; Lambert, J.S.; O’Broin, C.; Feeney, E.R.; De Barra, E.; et al. Identification of Distinct Long COVID Clinical Phenotypes Through Cluster Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 2022, 9, ofac060. [Google Scholar] [CrossRef]
- Magdy, D.M.; Metwally, A.; Tawab, D.A.; Hassan, S.A.; Makboul, M.; Farghaly, S. Long-term COVID-19 effects on pulmonary function, exercise capacity, and health status. Ann. Thorac. Med. 2022, 17, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Kierkegaard, M.; Zeitelhofer, M. A Call to Use the Multicomponent Exercise Tai Chi to Improve Recovery From COVID-19 and Long COVID. Front. Public Health 2022, 10, 827645. [Google Scholar] [CrossRef]
- Nongpiur, A.; Barman, B.; Syiem, K.; Mawiong, A.M.; Anand, N.; Nune, A. A cross-sectional study of the mental health burden among COVID-19 survivors. Indian J. Psychiatry 2023, 65, 661–666. [Google Scholar] [CrossRef]
- Santana, K.; França, E.; Sato, J.; Silva, A.; Queiroz, M.; de Farias, J.; Rodrigues, D.; Souza, I.; Ribeiro, V.; Caparelli-Dáquer, E.; et al. Non-invasive brain stimulation for fatigue in Long-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 2023, 16, 100–107. [Google Scholar] [CrossRef] [PubMed]
- van Gassel, R.J.J.; Bels, J.; Remij, L.; van Bussel, B.C.T.; Longhuma, R.; Gietema, H.A.; Verbunt, J.; van der Horst, I.C.C.; Olde Damink, S.W.M.; van Santen, S.; et al. Functional Outcomes and Their Association with Physical Performance in Mechanically Ventilated Coronavirus Disease 2019 Survivors at 3 Months Following Hospital Discharge: A Cohort Study. Crit. Care Med. 2021, 49, 1726–1738. [Google Scholar] [CrossRef]
- Vaes, A.W.; Goërtz, Y.M.J.; Van Herck, M.; Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Houben-Wilke, S.; Gaffron, S.; Maier, D.; Burtin, C.; et al. Recovery from COVID-19: A sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021, 7, 00141-2021. [Google Scholar] [CrossRef] [PubMed]
- Moens, M.; Duarte, R.V.; De Smedt, A.; Putman, K.; Callens, J.; Billot, M.; Roulaud, M.; Rigoard, P.; Goudman, L. Health-related quality of life in persons Long-COVID-19 infection in comparison to normative controls and chronic pain patients. Front. Public Health 2022, 10, 991572. [Google Scholar] [CrossRef] [PubMed]
- Löfström, E.; Kunkel, S.; Kötz, A.; Lingman, M.; Undén, J.; Nygren, J.M. Health-related quality of life and long-term symptoms among patients with non-severe COVID-19—A prospective cohort study. Infect. Dis. (Lond.) 2023, 55, 272–281. [Google Scholar] [CrossRef]
- Lim, R.K.; Rosentreter, R.; Chen, Y.; Mehta, R.; McLeod, G.; Wan, M.; Krett, J.D.; Mahjoub, Y.; Lee, A.; Schwartz, I.; et al. Quality of life, respiratory symptoms, and health care utilization 1 year following outpatient management of COVID-19: A prospective cohort study. Sci. Rep. 2022, 12, 12988. [Google Scholar] [CrossRef]
- Scurati, R.; Papini, N.; Giussani, P.; Alberti, G.; Tringali, C. The Challenge of Long COVID-19 Management: From Disease Molecular Hallmarks to the Proposal of Exercise as Therapy. Int. J. Mol. Sci. 2022, 23, 12311. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Ascaso-Del-Río, A.; Mielgo-Ayuso, J. Physical Exercise as a Multimodal Tool for COVID-19: Could It Be Used as a Preventive Strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.L.; Smith, K.A.; Zúñiga, T.M.; Batatinha, H.; Niemiro, G.M.; Pedlar, C.R.; Burgess, S.C.; Katsanis, E.; Simpson, R.J. Acute exercise increases immune responses to SARS-CoV-2 in a previously infected man. Brain Behav. Immun. Health 2021, 18, 100343. [Google Scholar] [CrossRef] [PubMed]
- Tartibian, B.; Khayat, S.M.A.; Maleki, B.H.; Chehrazi, M. The Effects of Exercise Training on Recovery of Biochemical and Hematological Outcomes in Patients Surviving COVID-19: A Randomized Controlled Assessor-Blinded Trial. Sports Med. Open 2022, 8, 152. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Long-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Webster, A.; Paul, L. Systematic Review of Changes and Recovery in Physical Function and Fitness After Severe Acute Respiratory Syndrome-Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys. Ther. 2020, 100, 1717–1729. [Google Scholar] [CrossRef]
- Heerdt, P.M.; Shelley, B.; Singh, I. Impaired systemic oxygen extraction long after mild COVID-19: Potential perioperative implications. Br. J. Anaesth. 2022, 128, e246–e249. [Google Scholar] [CrossRef]
- Green, H.J. Mechanisms of muscle fatigue in intense exercise. J. Sports Sci. 1997, 15, 247–256. [Google Scholar] [CrossRef]

| Long-COVID (n = 16) | Non-Long-COVID (n = 13) | p-Value | ||
|---|---|---|---|---|
| Age, mean (SD) | 46.13 (7.91) | 46.92 (6.00) | 0.843 | |
| Height, mean (SD) | 65.52 (12.52) | 63.14 (13.39) | 0.417 | |
| Weight, mean (SD) | 166.23 (8.03) | 167.25 (7.99) | 0.810 | |
| Sex | Male, n (%) | 1 (6.2) | 4 (30.8) | 0.114 |
| Female, n (%) | 15 (93.8) | 9 (69.2) | ||
| Hospital admission | No, n (%) | 15 (93.8) | 13 (100.0) | 0.424 |
| Yes, n (%) | 1 (6.2) | 0 (0.0) | ||
| Reinfection | No, n (%) | 12 (75.0) | 13 (100.0) | 0.069 |
| Yes, n (%) | 4 (25.0) | 0 (0.0) | ||
| Pneumonia | No, n (%) | 13 (81.2) | 13 (100.0) | 0.125 |
| Yes, n (%) | 3 (18.8) | 0 (0.0) |
| Pre | Long | Group Main Effect | Time Main Effect | Time × Group Interaction | |||
|---|---|---|---|---|---|---|---|
| Long-COVID | Non-Long-COVID | Long-COVID | Non-Long-COVID | F; p-Value | F; p-Value | F; p-Value | |
| Physical MFIS Score | 28.62 (5.31) | 7.58 (2.74) | 23.63 (7.71) | 7.67 (3.09) | 28.698; p < 0.001 | 3.484; p = 0.089 | 2.708; p = 0.128 |
| Cognitive MFIS Score | 25.43 (9.89) | 8.75 (3.14) | 20.50 (10.89) | 8.58 (3.66) | 14.818; p = 0.003 | 0.852; p = 0.376 | 0.437; p = 0.522 |
| Psychosocial MFIS Score | 5.50 (1.89) | 1.21 (0.73) | 4.50 (1.59) | 1.33 (0.68) | 43.639; p < 0.001 | 2.200; p = 0.166 | 1.501; p = 0.246 |
| TOTAL MFIS Score | 59.56 (14.49) | 17.58 (6.73) | 48.62 (16.81) | 17.58 (7.97) | 26.235; p < 0.001 | 2.265; p = 0.160 | 1.584; p = 0.234 |
| SF-36 Physical functioning | 55.00 (19.23) | 92.08 (9.64) | 64.06 (19.59) | 91.66 (15.71) | 14.243; p = 0.003 | 8.587; p = 0.014 | 6.947; p = 0.023 |
| SF-36 Role physical | 16.25 (5.84) | 89.58 (19.11) | 14.06 (7.58) | 81.25 (24.23) | 24.484; p < 0.001 | 0.048; p = 0.830 | 1.539; p = 0.123 |
| SF-36 Vitality | 30.00 (10.32) | 59.58 (15.44) | 38.43 (17.48) | 48.75 (16.25) | 13.653; p = 0.004 | 0.032; p = 0.861 | 14.207; p = 0.003 |
| SF-36 Role emotional | 54.15 (13.65) | 88.86 (16.44) | 66.65 (17.78) | 97.21 (9.63) | 9.521; p = 0.010 | 2.382; p = 0.151 | 0.048; p = 0.830 |
| SF-36 Social functioning | 31.56 (10.28) | 89.58 (15.84) | 51.41 (14.27) | 89.58 (15.84) | 34.015; p < 0.001 | 6.892; p = 0.024 | 4.577; p = 0.042 |
| SF-36 Bodily pain | 30.78 (10.05) | 78.75 (20.68) | 50.78 (12.98) | 76.04 (15.92) | 9.414; p = 0.011 | 4.799; p = 0.041 | 7.676; p = 0.018 |
| SF-36 General health | 34.06 (14.51) | 65.83 (21.58) | 39.06 (15.93) | 65.40 (13.63) | 21.319; p < 0.001 | 0.059; p = 0.813 | 1.483; p = 0.249 |
| SF-36 Mental health | 56.00 (16.39) | 68.33 (8.60) | 63.00 (19.67) | 74.66 (8.91) | 5.275; p = 0.042 | 4.969; p = 0.048 | 0.325; p = 0.580 |
| TOTAL SF-36 | 37.22 (12.61) | 75.39 (15.74) | 48.43 (14.97) | 76.78 (14.73) | 23.088; p < 0.001 | 6.625; p = 0.026 | 4.632; p = 0.002 |
| TOTAL EURO QoL-5D | 41.25 (9.57) | 77.50 (10.76) | 58.75 (18.64) | 81.25 (11.30) | 38.372; p < 0.001 | 12.424; p = 0.005 | 4.340; p = 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laguarta-Val, S.; Varillas-Delgado, D.; Lizcano-Álvarez, Á.; Molero-Sánchez, A.; Melian-Ortiz, A.; Cano-de-la-Cuerda, R.; Jiménez-Antona, C. Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial. J. Clin. Med. 2024, 13, 1035. https://doi.org/10.3390/jcm13041035
Laguarta-Val S, Varillas-Delgado D, Lizcano-Álvarez Á, Molero-Sánchez A, Melian-Ortiz A, Cano-de-la-Cuerda R, Jiménez-Antona C. Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial. Journal of Clinical Medicine. 2024; 13(4):1035. https://doi.org/10.3390/jcm13041035
Chicago/Turabian StyleLaguarta-Val, Sofía, David Varillas-Delgado, Ángel Lizcano-Álvarez, Alberto Molero-Sánchez, Alberto Melian-Ortiz, Roberto Cano-de-la-Cuerda, and Carmen Jiménez-Antona. 2024. "Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial" Journal of Clinical Medicine 13, no. 4: 1035. https://doi.org/10.3390/jcm13041035
APA StyleLaguarta-Val, S., Varillas-Delgado, D., Lizcano-Álvarez, Á., Molero-Sánchez, A., Melian-Ortiz, A., Cano-de-la-Cuerda, R., & Jiménez-Antona, C. (2024). Effects of Aerobic Exercise Therapy through Nordic Walking Program in Lactate Concentrations, Fatigue and Quality-of-Life in Patients with Long-COVID Syndrome: A Non-Randomized Parallel Controlled Trial. Journal of Clinical Medicine, 13(4), 1035. https://doi.org/10.3390/jcm13041035








