Oral Manifestations in Pregnant Women: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Declaration and Protocol
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
- Sources of Information
2.3.1. Search Terms
2.3.2. Selection of Articles
2.3.3. Data Extraction
2.3.4. Quality Analysis—Risk of Bias
3. Results
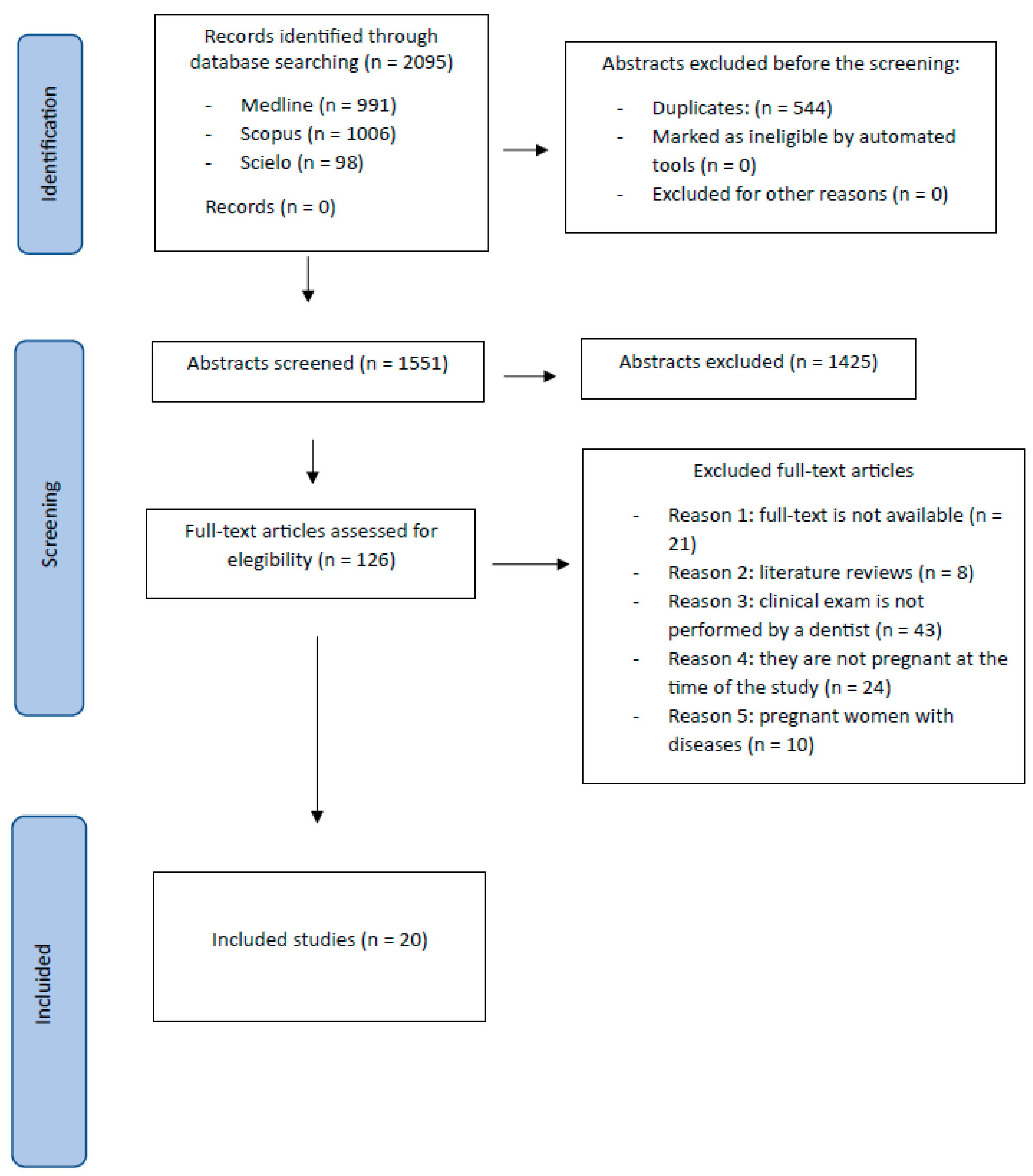
3.1. Study Selection and Flowchart
3.2. Types of Studies
3.3. Manifestations Studied
3.4. Prevalence of Manifestations
3.5. Comparison between Pregnant and Nonpregnant Women
3.6. Quality Evaluation—Risk of Bias
4. Discussion
5. Conclusions
- The oral manifestations that appear most frequently during pregnancy are caries, periodontitis, gingivitis, pyogenic granuloma, candidiasis, and dental mobility, but they are not the only ones that can appear.
- Most injuries studied in pregnant women compared with the nonpregnant group showed a higher prevalence in the first group.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrera León, L.I.; Martínez Barreiro, A.; Barros Díaz, O. Peso, edad gestacional e historia genésica previa de la gestante. Rev. Cuba. Salud Pública 2007, 33, 1765–1773. [Google Scholar] [CrossRef]
- Longhi Rezende, C.; Grubits Freire, H.B.; Vera Noriega, J.Á.; Durazo Salas, F.F. Qualidade de Vida e Estratégias de Coping de Gestantes de Alto Risco e Risco Habitual. Divers. Perspect. Psicol. 2021, 17, 213–226. [Google Scholar] [CrossRef]
- Sá De Lira, A.D.L.; Silva Da, N.R.F.D.; Caetano, V.D.S.; Araújo Junior De, A.G.; Portela, I.J.Z. Prevalence and etiological factors of piogenic granuloma in gestants. Braz. Dent. Sci. 2019, 22, 443–449. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; Leon-Rios, X.; Rivero, T.; Expósito-Ruiz, M.; Perez-Castillo, I.; Aguilar-Cordero, M.J. Factors associated with oral health-related quality of life during pregnancy: A prospective observational study. Qual. Life Res. 2021, 30, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- Lieske, B.; Makarova, N.; Jagemann, B.; Walther, C.; Ebinghaus, M.; Zyriax, B.C.; Aarabi, G. Inflammatory Response in Oral Biofilm during Pregnancy: A Systematic Review. Nutrients 2022, 14, 4894. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Prevalence of temporomandibular disorders (TMD) in pregnancy: A systematic review with meta-analysis. J. Oral Rehabil. 2023, 50, 627–634. [Google Scholar] [CrossRef]
- Sari, E.Y.; Saddki, N.; Yuso, A. Association between perceived oral symptoms and presence of clinically diagnosed oral diseases in a sample of pregnant women in malaysia. Int. J. Environ. Res. Public Health 2020, 17, 7337. [Google Scholar] [CrossRef]
- Kateeb, E.; Momany, E. Dental caries experience and associated risk indicators among Palestinian pregnant women in the Jerusalem area: A cross-sectional study. BMC Oral Health 2018, 18, 170. [Google Scholar] [CrossRef]
- Alfaro Alfaro, A.; Castejón Navas, I.; Magán Sánchez, R.; Alfaro Alfaro, M.J. Embarazo y salud oral. Rev. Clínica Med. Fam. 2018, 11, 144–153. [Google Scholar]
- Bouza Vera, M.; Martínez Abreu, J.; Carmenate Rodríguez, Y.; Betancourt González, M.; García Nicieza, M. El embarazo y la salud bucal. Rev. Médica Electrónica 2016, 38, 628–634. [Google Scholar]
- Gil-Montoya, J.A.; Rivero-Blanco, T.; Leon-Rios, X.; Exposito-Ruiz, M.; Pérez-Castillo, I.; Aguilar-Cordero, M.J. Oral and general health conditions involved in periodontal status during pregnancy: A prospective cohort study. Arch. Gynecol. Obs. 2022, 308, 1765–1773. [Google Scholar] [CrossRef]
- AlRatroot, S.; Alotaibi, G.; AlBishi, F.; Khan, S.; Ashraf Nazir, M. Dental Anxiety Amongst Pregnant Women: Relationship with Dental Attendance and Sociodemographic Factors. Int. Dent. J. 2022, 72, 179–185. [Google Scholar] [CrossRef]
- Nasiri, K.; Dimitrova, A.; Wrbas, K.T. Managing halitosis during the SARS-CoV-2 pandemic. J. Dent. Sci. 2022, 17, 1418–1419. [Google Scholar] [CrossRef]
- Swathi, K.; Koothati, R.K.; Motor, R.R.; Priyadarshini; RajaShekar, C.H.; Vallakonda, S. Knowledge and Experience of Women about Dental Services Utilization during Pregnancy: A Cross-Sectional Questionnaire Study. J. Pharm. Bioallied Sci. 2021, 13, S1042–S1046. [Google Scholar] [CrossRef] [PubMed]
- Vamos, C.A.; Thompson, E.L.; Avendano, M.; Daley, E.M.; Quinonez, R.B.; Boggess, K. Oral health promotion interventions during pregnancy: A systematic review. Community Dent. Oral Epidemiol. 2015, 43, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Fayans, E.P.; Stuart, H.R.; Carsten, D.; Ly, Q.; Kim, H. Local anesthetic use in the pregnant and postpartum patient. Dent. Clin. N. Am. 2010, 54, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Favero, V.; Bacci, C.; Volpato, A.; Bandiera, M.; Favero, L.; Zanette, G. Pregnancy and Dentistry: A Literature Review on Risk Management during Dental Surgical Procedures. Dent. J. 2021, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- da Silva, K.D.; Vargas-Ferreira, F.; Bertoldi, A.D.; de Barros, F.; Demarco, F.F.; Correa, M.B.; Tarquinio, S.B.C. Oral mucosal lesions in pregnant women: A population-based study. Oral Dis. 2022, 28, 1891–1900. [Google Scholar] [CrossRef]
- Cademartori, M.G.; Demarco, F.F.; Freitas da Silveira, M.; Barros, F.C.; Corrêa, M.B. Dental caries and depression in pregnant women: The role of oral health self-perception as mediator. Oral Dis. 2022, 28, 1733–1740. [Google Scholar] [CrossRef]
- Caneiro, L.; Lopez-Carral, J.M.; Martin-Lancharro, P.; Linares, A.; Batalla, P.; Blanco-Carrion, J. Periodontitis as a Preterm Birth Risk Factor in Caucasian Women: A Cohort Study. Oral Health Prev. Dent. 2020, 18, 77–84. [Google Scholar] [CrossRef]
- Nejad, E.S.; BigomTaheri, J.; Azimi, S. Frequency of gingival pregnancy tumor in iran (confirmed by biopsy). J. Int. Oral Health 2014, 6, 72–76. [Google Scholar]
- Onigbinde, O.; Sorunke, M.; Braimoh, M.; Adeniyi, A. Periodontal Status and Some Variables among Pregnant Women in a Nigeria Tertiary Institution. Ann. Med. Health Sci. Res. 2014, 4, 852–857. [Google Scholar] [CrossRef]
- Africa, C.W.J.; Turton, M. Oral Health Status and Treatment Needs of Pregnant Women Attending Antenatal Clinics in KwaZulu-Natal, South Africa. Int. J. Dent. 2019, 2019, 5475973. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fogarty, C.; Wu, T.T.; Alkhers, N.; Zeng, Y.; Thomas, M.; Youssef, M.; Wang, L.; Cowen, L.; Abdelsalam, H.; et al. Oral health and Candida carriage in socioeconomically disadvantaged US pregnant women. BMC Pregnancy Childbirth 2019, 19, 480. [Google Scholar] [CrossRef] [PubMed]
- Deghatipour, M.; Ghorbani, Z.; Ghanbari, S.; Arshi, S.; Ehdayivand, F.; Namdari, M.; Pakkhesal, M. Oral health status in relation to socioeconomic and behavioral factors among pregnant women: A community-based cross-sectional study. BMC Oral Health 2019, 19, 117. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Liu, J.; Sun, W.L.; Chen, L.L.; Chai, L.G.; Xiao, X.; Cao, Z. Periodontal status and associated risk factors among childbearing age women in Cixi City of China. J. Zhejiang Univ. Sci. B 2013, 14, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, C.; Rossi, G.; Rama, A.; Gomez-Gutierrez, N.; Alvaredo, G.; Squassi, A.; Klemonskis, G. Oral health status and oral health-related quality of life in pregnant women from socially deprived populations. Acta Odontol. Latinoam. 2013, 26, 68–74. [Google Scholar] [PubMed]
- Soroye, M.O.; Ayanbadejo, P.O. Prevalence of gingivitis and perception of gingival colour among pregnant women attending the antenatal clinic of Lagos University Teaching Hospital, Idi-Araba. J. Orofac. Sci. 2016, 8, 53–58. [Google Scholar] [CrossRef]
- Ibrahim, H.M.E.M.; Mudawi, A.M.; Ghandour, I.A. Oral health status, knowledge and practice among pregnant women attending omdurman maternity hospital, Sudan. East. Mediterr. Health J. 2016, 22, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, A.; Baskaradoss, J.K.; Sarma, P.S. Oral Health-Related Quality of Life and Periodontal Status of Pregnant Women. Matern. Child. Health J. 2017, 21, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Corchuelo-Ojeda, J.; Soto-Llanos, L.; Villavicencio, J. Situación de caries, gingivitis e higiene oral en gestantes y no gestantes en hospitales del Valle del Cauca, Colombia. Univ. Y Salud 2017, 19, 67–74. [Google Scholar] [CrossRef]
- Adesina, K.T.; Ernest, M.A.; Tobin, A.O.; Isiaka-Lawal, S.A.; Adeyemi, M.F.; Olarinoye, A.O.; Ezeoke, G.G. Oral health status of pregnant women in Ilorin, Nigeria. J. Obs. Gynaecol. 2018, 38, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, P.; Korečko, V.; Turek, J.; Merglová, V. Oral health status of women with normal and high-risk pregnancies. Ceska Gynekol. 2014, 79, 29–33. [Google Scholar] [PubMed]
- Pérez Oviedo, A.C.; Betancourt Valladares, M.; Espeso Nápoles, N.; Miranda Naranjo, M.; González Barreras, B. Caries dental asociada a factores de riesgo durante el embarazo. Rev. Cuba. Estomatol. 2011, 48, 104–112. [Google Scholar]
- Foratori-Junior, G.A.; Pereira, P.R.; Gasparoto, I.A.; de Carvalho Sales-Peres, S.H.; Storniolo de Souza, J.M.; Khan, S. Is overweight associated with periodontitis in pregnant women? Systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2022, 58, 41–51. [Google Scholar] [CrossRef]
- Kashetty, M.; Kumbhar, S.; Patil, S.; Patil, P. Oral hygiene status, gingival status, periodontal status, and treatment needs among pregnant and nonpregnant women: A comparative study. J. Indian Soc. Periodontol. 2018, 22, 164–170. [Google Scholar] [CrossRef]
- Chowdhury, S.F.; Islam, M.N. Periodontal diseases among pregnant women attending an antenatal clinic at Dhaka, Bangladesh. J. Oral Res. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Wijaya, D.; Hanum, N.A.; Handayani, A. Relationship Between Gestational Age and Severity of Gingivitis. J. Kesehat. Gigi 2019, 6, 126–129. [Google Scholar] [CrossRef]
- Soroye, M.; Ayanbadejo, P.; Savage, K.; Oluwole, A. Association between periodontal disease and pregnancy outcomes. Odontostomatol. Trop. 2015, 38, 5–16. [Google Scholar] [PubMed]
- Pockpa, Z.A.D.; Soueidan, A.; Koffi-Coulibaly, N.T.; Mobio, G.S.; Pere, M.; Badran, Z.; Struillou, X. Association Between Periodontitis and Preterm Birth in a Cohort of Pregnant Women in Ivory Coast. Oral Health Prev. Dent. 2022, 20, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Turton, M.; Africa, C.W.J. Further evidence for periodontal disease as a risk indicator for adverse pregnancy outcomes. Int. Dent. J. 2017, 67, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Alrumayh, A.; Alfuhaid, F.; Sayed, A.J.; Tareen, S.U.K.; Alrumayh, I.; Habibullah, M.A. Maternal Periodontal Disease: A Possible Risk Factor for Adverse Pregnancy Outcomes in the Qassim Region of Saudi Arabia. J. Pharm. Bioallied Sci. 2021, 13, S1723–S1727. [Google Scholar] [CrossRef] [PubMed]
- Rihani, F.B.; Ersheidat, A.A.; Alsmadi, H.F.; Al-Nahar, L.A. Multiple long-standing massive oral mandibular granuloma gravidarum (pregnancy tumour). BMJ Case Rep. 2013, 2013, bcr2013010182. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.A.; Spanemberg, J.C.; Cherubini, K.; Figueiredo, M.A.; Salum, F.G. Oral granuloma gravidarum: A retrospective study of 41 cases in Southern Brazil. J. Appl. Oral Sci. 2013, 21, 215–218. [Google Scholar] [CrossRef]
- Shaimaa; Zainab, H.; Hugar, D.; Sultana, A. A comparative study to assess risk of oral candidiasis in pregnant and nonpregnant women. J. Oral Maxillofac. Pathol. 2021, 25, 118–123. [Google Scholar] [CrossRef]
- Tosal Herrero, B.; Richart Martínez, M.; Luque Plaza, M.; Gutiérrez, L.; Pastor García, R.; Cabrero García, J.; Reig Ferrer, A. Gastrointestinal signs and symptoms during pregnancy and postpartum in a sample of Spanish women. Aten. Primaria 2001, 28, 53–58. [Google Scholar] [CrossRef][Green Version]
- Thomas, N.J.; Middleton, P.F.; Crowther, C.A. Oral and dental health care practices in pregnant women in Australia: A postnatal survey. BMC Pregnancy Childbirth 2008, 8, 13. [Google Scholar] [CrossRef]
- Ismail, A.I.; Sohn, W.; Tellez, M.; Amaya, A.; Sen, A.; Hasson, H.; Pitts, N.B. The International Caries Detection and Assessment System (ICDAS): An integrated system for measuring dental caries. Community Dent. Oral Epidemiol. 2007, 35, 170–178. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Type of Study | Number of Participants and Comparison | Age | Manifestations Studied | Conclusions |
|---|---|---|---|---|---|
| Cornejo et al. (2013) [30] | Transversal | 80 pregnant women. There was no comparison. | 18–39 | Periodontitis Gingivitis Caries | Gingivitis: 93.75% Periodontitis: 2.5% Caries: 92.1% Average DMFT: 12.24 |
| Wu et al. (2013) [29] | Transversal | 176 pregnant women and 578 nonpregnant women. There was comparison. | 20–39 | Periodontitis | Pregnant/nonpregnant Periodontitis: 161 pregnant/478 nonpregnant women. Of the 100% of cases with periodontitis, 74.8% were nonpregnant and 25.2% were pregnant. |
| Chaloupka et al. (2014) [36] | Case–control | 142 pregnant women. 81 cases (high-risk pregnancies) and 61 controls (normal pregnancies). There was no comparison. | - | Caries Periodontitis | Caries: Average DMFT case/control: 12.8/11.5 Periodontitis: CPITN case/control number: 0: 0/1 1: 20/20 2: 43/34 3: 16/5 4: 2/1 |
| Nejad et al. (2014) [24] | Transversal | 923 pregnant women. There was no comparison. | 17–41 | Piogenic granuloma | Pyogenic granuloma: 0.22% (2 pregnant women) |
| Onigbinde et al. (2014) [25] | Transversal | 415 pregnant women. | 20–44 | Periodontal disease | Periodontal disease: CPITN 0: 6.02% 1: 52.53% |
| There was no comparison. | 2: 41.44% | ||||
| Soroye et al. (2016) [31] | Transversal | 445 pregnant women. There was no comparison. | From 16 | Gingivitis | Gingivitis: 85.2% Mild: 86.3% Moderate: 12.9% Severe: 0.8% |
| Ibrahim et al. (2016) [32] | Transversal | 420 pregnant women. There was no comparison. | 16–44 | Caries Periodontal disease | Caries: 75.5% Periodontal disease: CPTIN 0: 58.6% 1: 12.1% 2: 22.9% 3 and 4: 1.9% |
| Geevarghese et al. (2017) [33] | Transversal | 150 pregnant and 150 nonpregnant women. There was a comparison. | 18–35 | Periodontitis Caries | Pregnant/nonpregnant Periodontitis: (32%/16%) Caries: DMFT > 6 (56%/44.7%), DMFT ≤ 6 (44%/55.3%) |
| Corchuelo-Ojeda et al. (2017) [34] | Transversal | 87 pregnant women and 415 nonpregnant women. There was a comparison. | - | Gingivitis Caries | Pregnant/nonpregnant Gingivitis: 73.6%/58.8% Caries: 82.8%/80.5% |
| Kateeb et al. (2018) [9] | Transversal | 152 pregnant women. There was no comparison. | 17–42 years | Caries | Caries (100%) DMFT (average 15.5)
|
| -High: 6% -Extremely high: 89.3% | |||||
| Adesina et al. (2018) [35] | Transversal | 225 pregnant women and 166 nonpregnant women. There was a comparison. | - | Caries Granuloma Telangiectasia Erosion Ptyalism Gingivitis | Pregnant/nonpregnant
DMFT 1–3: 10.7%/16.9%. DMFT ≥ 4: 1.8/2.4% |
| Xiao et al. (2019) [27] | Transversal | 48 pregnant women and 34 non-pregnant women. There was a comparison. | From 18 | Caries Oral candidiasis | Pregnant/nonpregnant Caries (79.1%/47.1%) Average DMFT: 7.5/6.9 Oral candidiasis: 56% pregnant in tonsils (57%), vestibule (46%), tongue (42%), hard palate (29%)/56% not pregnant |
| Deghatipour et al. (2019) [28] | Transversal | 407 pregnant women. There was no comparison. | 15–44 | Caries Periodontitis | Caries: 67% 15–25 years old: 78% 35–44: 44% Periodontitis: 27% (more in the third trimester than in the second trimester) |
| Africa et al. (2019) [26] | Transversal | 443 pregnant women. There was no comparison. | 18–42 | Caries Granuloma Mobility Candidiasis Canker sores | Caries Average DMFT: 7.18 Granuloma: 8.5% |
| |||||
| Sari et al. (2020) [8] | Transversal | 192 pregnant women. There was no comparison. | 17–42 | Caries Gingivitis Periodontitis | Caries: 93.2% (DMFT—C: 58.9%; A: 59.9%; O: 76%) Gingivitis: 100% Mild: 46.4% (GI 0.1–1.0) Moderate: 46.9% (GI 1.1–2.0) Severe: 6.8% (GI 2.1–3.0) Periodontitis: 46.3% CPITN 1 (bleeding on probing): 11.5% 2 (calculation): 41.1% 3 (4–5 mm bags): 45.8% 4 (bags ≥ 6 mm): 0.5% |
| Caneiro et al. (2020) [23] | Cohorts | 158 pregnant women. There was no comparison. | 18–40 | Bleeding on probing Loss of insertion | Bleeding on probing and insertion loss were higher in patients with periodontitis during all three trimesters than in those without periodontitis. |
| Gil-Montoya et al. (2021) [4] | Longitudinal | 246 pregnant women. There was no comparison. | From 18 | Caries Periodontitis | Periodontitis
|
| Cademartori et al. (2022) [22] | Cohorts | 3100 pregnant women. There was no comparison. | <20 46 | Caries | Caries: 88.2% (DMFT ≥ 1) Severity: 0 to 3 decayed teeth: 91.5% 4 or more decayed teeth: 8.5% |
| Da Silva et al. (2022) [21] | Cohorts | 2481 pregnant women. There was no comparison. | - | Oral mucosal lesions | 409 women had at least one lesion (15.9% had 2 lesions and 2.2% had 3):
|
| Gil-Montoya et al. (2022) [12] | Cohorts | 147 pregnant women. There was no comparison. | From 16 | Periodontitis Gingivitis | Periodontitis
|
| Methods | ||
| Configuration | 1 | Describes the setting, participating locations, and relevant dates (period of recruitment, exposure, follow-up, data collection) |
| Participants | 2 | Gives the inclusion and exclusion criteria (including paired or control groups) |
| 3 | Describes the disease studied | |
| Variables | 4 | Clearly defines the diagnostic criteria (ICDAS, light, Rx, etc.) |
| Data/measurement sources | 5 | Measure of caries by itself |
| Study size | 6 | Explains how the study sample size was arrived at |
| Statistical methods | 7 | Describes the statistical methods, including those used to control for confounders |
| 8 | Describes any methods used to examine subgroups and interactions | |
| Results | ||
| Descriptive data | 9 | Provides characteristics of study participants (eg, demographic, clinical, social) and reports on exposures and potential confounders |
| Result data | 10 | Measures and presents exposure data |
| Cornejo et al., 2013 [30] | Wu et al., 2013 [29] | Chaloupka et al., 2014 [36] | Nejad et al., 2014 [24] | Onigbind e et al., 2014 [25] | Soroye et al., 2016 [31] | Ibrahim et al., 2016 [32] | Geevarg Hese et al., 2017 [33] | Corchuel o- Ojeda et al., 2017 [34] | Kateeb et al., 2018 [9] | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2 | X | ✓ | X | ✓ | X | X | ✓ | ✓ | ✓ | ✓ |
| 3 | ✓ | X | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4 | X | ✓ | ✓ | ✓ | X | ✓ | X | ✓ | X | X |
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ | X | ✓ |
| 6 | ✓ | X | X | ✓ | ✓ | X | ✓ | ✓ | X | X |
| 7 | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 8 | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 9 | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ |
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Total risk of bias | 9 Low | 8 Low | 8 Low | 9 Low | 8 Low | 9 Low | 9 Low | 11 Low | 7 Moderate | 9 Low |
| Adesin et al., 2018 [35] | Xiao et al., 2019 [27] | Deghatipour et al., 2019 [28] | Africa et al., 2019 [26] | Sari et al., 2020 [8] | Caneiro et al., 2020 [23] | Gil-Monto ya et al., 2021 [4] | Cademartori et al., 2021 [22] | Da Silva et al., 2022 [21] | Gil-Montoya et al., 2022 [12] | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | X | X | ✓ | X | X | ✓ | ✓ | ✓ | ✓ | ✓ |
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | X | X | ✓ |
| 3 | ✓ | X | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 4 | X | ✓ | X | X | ✓ | ✓ | ✓ | X | X | ✓ |
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 6 | ✓ | ✓ | ✓ | ✓ | ✓ | X | X | ✓ | X | ✓ |
| 7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 11 | ✓ | ✓ | ✓ | ✓ | ✓ | X | ✓ | ✓ | ✓ | ✓ |
| Total Risk of bias | 9 Low | 9 Low | 10 Low | 9 Low | 9 Low | 9 Low | 10 Low | 9 Low | 8 Low | 11 Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecci-Lloret, M.P.; Linares-Pérez, C.; Pecci-Lloret, M.R.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Oral Manifestations in Pregnant Women: A Systematic Review. J. Clin. Med. 2024, 13, 707. https://doi.org/10.3390/jcm13030707
Pecci-Lloret MP, Linares-Pérez C, Pecci-Lloret MR, Rodríguez-Lozano FJ, Oñate-Sánchez RE. Oral Manifestations in Pregnant Women: A Systematic Review. Journal of Clinical Medicine. 2024; 13(3):707. https://doi.org/10.3390/jcm13030707
Chicago/Turabian StylePecci-Lloret, María Pilar, Covadonga Linares-Pérez, Miguel Ramón Pecci-Lloret, Francisco Javier Rodríguez-Lozano, and Ricardo Elías Oñate-Sánchez. 2024. "Oral Manifestations in Pregnant Women: A Systematic Review" Journal of Clinical Medicine 13, no. 3: 707. https://doi.org/10.3390/jcm13030707
APA StylePecci-Lloret, M. P., Linares-Pérez, C., Pecci-Lloret, M. R., Rodríguez-Lozano, F. J., & Oñate-Sánchez, R. E. (2024). Oral Manifestations in Pregnant Women: A Systematic Review. Journal of Clinical Medicine, 13(3), 707. https://doi.org/10.3390/jcm13030707








