How to Manage Advanced Differentiated Thyroid Cancer: Step-by-Step Analysis from Two Italian Tertiary Referral Centers
Abstract
1. Introduction
2. Materials and Methods
- total thyroidectomy (total extracapsular thyroidectomy with standard Kocher incision and high extracapsular dissection of the thyroid gland);
- extended thyroidectomy (total thyroidectomy plus resection of adjacent structures involved by tumor such as larynx, trachea, esophagus, recurrent laryngeal nerve, prevertebral fascia, carotid vessels);
- unilateral central compartment node dissection (lymphectomy of Compartments 6 and 7 on the same side of the tumor);
- bilateral central compartment node dissection (lymphectomy of Compartments 6 and 7 on both sides);
- unilateral modified cervical neck dissection (neck dissection of Compartments 2-3-4-5 on the same side of the tumor sparing spinal accessory nerve and internal jugular vein);
- bilateral modified cervical neck dissection (neck dissection of Compartments 2-3-4-5 on both sides, sparing spinal accessory nerve and internal jugular vein).
- transient hypoparathyroidism (hypocalcemia lasting less than six months)
- permanent hypoparathyroidism (hypocalcemia lasting more than six months)
- transient recurrent laryngeal nerve palsy (vocal cord function recovered within 6 months)
- permanent recurrent laryngeal nerve palsy (vocal cord not functioning after more than 6 months)
- permanent spinal accessory nerve palsy
- other complications.
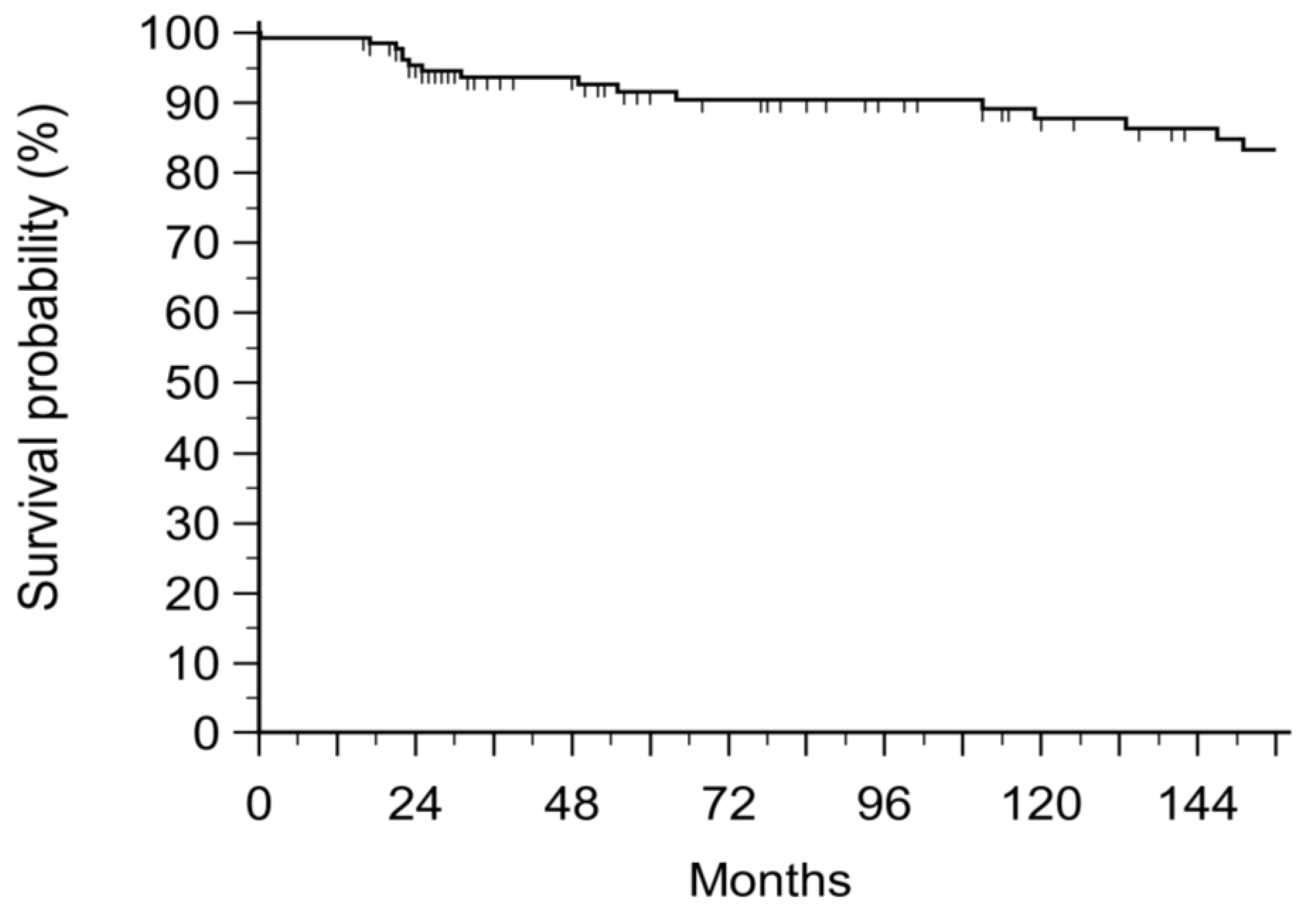
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabanillas, M.E.; Ryder, M.; Jimenez, C. Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocr. Rev. 2019, 40, 1573–1604. [Google Scholar] [CrossRef] [PubMed]
- Maitra, A. The endocrine system. In Robbins and Cotran Pathologic Basis of Disease; Kumar, V., Abbas, A., Fausto, N., Aster, J., Eds.; Elsevier: Philadelphia, PA, USA, 2009; pp. 1119–1124. [Google Scholar]
- LiVolsi, V.A. Papillary thyroid carcinoma: An update. Mod. Pathol. 2011, 24, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Shoup, M.; Stojadinovic, A.; Nissan, A.; Ghossein, R.; Freedman, S.; Brennan, M.F.; Shah, J.P.; Shaha, A.R. Prognostic indicators of outcomes in patients with distant metastases from differentiated thyroid carcinoma. J. Am. Coll. Surgeons. 2003, 197, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Houten, P.; Netea-Maier, R.T.; Smit, J.W. Differentiated thyroid carcinoma: An update. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101687. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Hu, X.; Pan, Z.; Xu, T.; Xu, J.; Jiang, L.; Huang, P.; Zhang, Y.; Ge, M. Radioiodine therapy in advanced differentiated thyroid cancer: Resistance and overcoming strategy. Drug Resist. Updates 2023, 68, 100939. [Google Scholar] [CrossRef]
- Jankovic, B.; Le, K.T.; Hershman, J.M. Hashimoto’s thyroiditis and papillary thyroid carcinoma: Is there a correlation? J. Clin. Endocrinol. Metab. 2013, 98, 474–482. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Tzortzinis, A.A.; Dalieraki-Ott, E.I.; Tsartsalis, A.N.; Syros, P.K.; Karefilakis, C.M.; Papadomanolaki, M.G.; Starakis, I.K. Coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. A retrospective study. Hormones 2010, 9, 312–317. [Google Scholar] [CrossRef]
- Azizi, G.; Malchoff, C.D. Autoimmune thyroid disease: A risk factor for thyroid cancer. Endocr. Pract. 2011, 17, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, Y.; Choi, J.-W.; Kim, Y.-S. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: A meta-analysis. Eur. J. Endocrinol. 2013, 168, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Spitale, A.; Faquin, W.C.; Mazzucchelli, L.; Baloch, Z.W. The Bethesda System for Reporting Thyroid Cytopathology: A meta-analysis. Acta Cytol. 2012, 56, 333–339. [Google Scholar] [CrossRef]
- Russell, M.D.; Kamani, D.; Randolph, G.W. Modern Surgery for Advanced Thyroid Cancer: A Tailored Approach. Gland Surg. 2020, 9 (Suppl. 2), S105–S119. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Bulfamante, A.M.; Lori, E.; Bellini, M.I.; Bolis, E.; Lozza, P.; Castellani, L.; Saibene, A.M.; Pipolo, C.; Fuccillo, E.; Rosso, C.; et al. Advanced Differentiated Thyroid Cancer: A Complex Condition Needing a Tailored Approach. Front. Oncol. 2022, 12, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Namba, H.; Nakashima, M.; Hayashi, T.; Hayashida, N.; Maeda, S.; Rogounovitch, T.I.; Ohtsuru, A.; Saenko, V.A.; Kanematsu, T.; Yamashita, S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2003, 88, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kang, D.W.; Kim, S.H.; Seong, I.O.; Kang, D.Y. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med. J. 2004, 45, 818–821. [Google Scholar] [CrossRef]
- Hyeon, J.; Ahn, S.; Shin, J.H.; Oh, Y.L. The prediction of malignant risk in the category “atypia of undetermined significance/follicular lesion of undetermined significance” of the Bethesda system for reporting thyroid cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014, 122, 368–376. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Ohori, N.P.; Hodak, S.P.; Carty, S.E.; LeBeau, S.O.; Ferris, R.L. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J. Clin. Endocrinol. Metab. 2011, 96, 3390–3397. [Google Scholar] [CrossRef]
- Yip, L.; Gooding, W.E.; Nikitski, A.; Wald, A.I.; Carty, S.E.; Karslioglu-French, E.; Seethala, R.R.; Zandberg, D.P.; Ferris, R.L.; Nikiforova, M.N.; et al. Risk assessment for distant metastasis in differentiated thyroid cancer using molecular profiling: A matched case-control study. Cancer 2021, 127, 1779–1787. [Google Scholar] [CrossRef]
- Hay, I.D.; McConahey, W.M.; Goellner, J.R. Managing patients with papillary thyroid carcinoma: Insights gained from the Mayo Clinic’s experience of treating 2512 consecutive patients during 1940 through 2000. Trans. Am. Clin. Climatol. Assoc. 2002, 113, 241–260. [Google Scholar]
- Simpson, W.J.; McKinney, S.E.; Carruthers, J.S.; Gospodarowicz, M.K.; Sutcliffe, S.B.; Panzarella, T. Papillary and follicular thyroid cancer. Prognostic factors in 1578 patients. Am. J. Med. 1987, 83, 479–488. [Google Scholar] [CrossRef]
- Docimo, G.; Ruggiero, R.; Casalino, G.; Del Genio, G.; Docimo, L.; Tolone, S. Risk factors for postoperative hypocalcemia. Updates Surg. 2017, 69, 255–260. [Google Scholar] [CrossRef]
- Chapman, D.B.; French, C.C.; Leng, X.; Browne, J.D.; Waltonen, J.D.; Sullivan, C.A. Parathyroid hormone early percent change: An individualized approach to predict postthyroidectomy hypocalcemia. Am. J. Otolaryngol. 2012, 33, 216–220. [Google Scholar] [CrossRef]
- Del Rio, P.; Rossini, M.; Montana, C.M.; Viani, L.; Pedrazzi, G.; Loderer, T.; Cozzani, F. Postoperative hypocalcemia: Analysis of factors influencing early hypocalcemia development following thyroid surgery. BMC Surg. 2019, 18 (Suppl. 1), 25. [Google Scholar] [CrossRef] [PubMed]
- McMurran, A.E.L.; Blundell, R.; Kim, V. Predictors of post-thyroidectomy hypocalcaemia: A systematic and narrative review. J. Laryngol. Otol. 2020, 134, 541–552. [Google Scholar] [CrossRef]
- Privitera, F.; Gioco, R.; Fazio, I.; Volpicelli, A.; Cannizzaro, M.T.; Costa, S.; Cannizzaro, M.A.; Veroux, M. Risk Factors for Low Levels of Parathyroid Hormone after Surgery for Thyroid Cancer: A Single Center Study. J. Clin. Med. 2021, 10, 4113. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, G.B.; Diamantino, L.R.; Schiaveto, L.F.; Forster, C.H.Q.; Shiguemori, É.H.; Hirata, D.; Kohler, H.F.; Lira, R.B.; Vartanian, J.G.; Matieli, J.E.; et al. Identification of secondary predictive factors for acute hypocalcemia following thyroidectomy in patients with low postoperative parathyroid hormone levels without overt calcium deficiency: A cohort study. Am. J. Otolaryngol. 2021, 42, 103115. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, M.; Emadi-Jamali, S.M.; Azadbakht, S. Hypocalcemia following total and subtotal thyroidectomy and associated factors. Ann. Med. Surg. 2021, 66, 102417. [Google Scholar] [CrossRef] [PubMed]
- Edafe, O.; Antakia, R.; Laskar, N.; Uttley, L.; Balasubramanian, S.P. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br. J. Surg. 2014, 101, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.; Jimeno, J.; Miquel, J.; Iglesias, M.; Munné, A.; Sancho, J.J.; Sitges-Serra, A. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005, 138, 1095–1101. [Google Scholar] [CrossRef]
- Raffaelli, M.; DeCrea, C.; Sessa, L.; Giustacchini, P.; Revelli, L.; Bellantone, C.; Lombardi, C.P. Prospective evaluation of total thyroidectomy versus ipsilateral versus bilateral central neck dissection in patients with clinically node–negative papillary thyroid carcinoma. Surgery 2012, 152, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Barczyński, M.; Konturek, A.; Stopa, M.; Nowak, W. Nodal recurrence in the lateral neck after total thyroidectomy with prophylactic central neck dissection for papillary thyroid cancer. Langenbeck’s Arch. Surg. 2014, 399, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bergenfelz, A.; Barczynski, M.; Heie, A.; Muth, A.; Passler, C.; Schneider, M.; Wierzbicka, P.; Konturek, A.; Brauckhoff, K.; Elf, A.K.; et al. Impact of autofluorescence for detection of parathyroid glands during thyroidectomy on postoperative parathyroid hormone levels: Parallel multicentre randomized clinical trial. Br. J. Surg. 2023, 110, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Dionigi, G.; Barczynski, M.; Chiang, F.Y.; Dralle, H.; Schneider, R.; Al-Quaryshi, Z.; Angelos, P.; Brauckhoff, K.; Brooks, J.A.; et al. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope 2018, 128 (Suppl. 3), S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Kamani, D.; Randolph, G.W. Surgical management of the compromised recurrent laryngeal nerve in thyroid cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101282. [Google Scholar] [CrossRef] [PubMed]
- Pacini, F.; Basolo, F.; Bellantone, R.; Boni, G.; Cannizzaro, M.A.; De Palma, M.; Durante, C.; Elisei, R.; Fadda, G.; Frasoldati, A.; et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: Joint statements of six Italian societies. J. Endocrinol. Invest. 2018, 41, 849–876. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Yip, L.; Lubitz, C.C.; Grubbs, E.G.; Miller, B.S.; Shen, W.; Angelos, P.; Chen, H.; Doherty, G.M.; Fahey, T.J., 3rd; et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann. Surg. 2020, 271, e21–e93. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.; Kim, S.B.; Choi, D.G.; Cha, H.K.; Park, J.S.; Jun, S.; Lee, K.D. Shaving Papillary Thyroid Carcinoma Involving Functioning Recurrent Laryngeal Nerve: Safety of Incomplete Tumor Resection and Nerve Sparing. Ann. Surg. Oncol. 2023, 30, 7157–7164. [Google Scholar] [CrossRef]
- Trybek, T.; Kowalska, A.; Lesiak, J.; Młynarczyk, J. The role of 18F-Fluorodeoxyglucose Positron Emission Tomography in patients with suspected recurrence or metastatic differentiated thyroid carcinoma with elevated serum thyroglobulin and negative I-131 whole body scan. Nucl. Med. Rev. Cent. East. Eur. 2014, 17, 87–93. [Google Scholar] [CrossRef]
- Aashiq, M.; Silverman, D.A.; Na’ara, S.; Takahashi, H.; Amit, M. Radioiodine-Refractory Thyroid Cancer: Molecular Basis of Redifferentiation Therapies, Management, and Novel Therapies. Cancers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Lorenz, K.; Raffaeli, M.; Barczyński, M.; Lorente-Poch, L.; Sancho, J. Volume, outcomes, and quality standards in thyroid surgery: An evidence-based analysis-European Society of Endocrine Surgeons (ESES) positional statement. Langenbeck’s Arch. Surg. 2020, 405, 401–425, Erratum in Langenbeck’s Arch. Surg. 2022, 407, 3913. [Google Scholar] [CrossRef] [PubMed]
| Number | % | |
|---|---|---|
| TUMOR TYPE | ||
| Papillary | 268 | 89.3 |
| Follicular | 32 | 10.7 |
| LOCAL INVASION | 22 | |
| Nerve | 12 | |
| Trachea | 6 | |
| Esophagus | 1 | |
| Larynx | 2 | |
| Subcutaneous | 1 | |
| NODAL INVASION | 260 | |
| Central compartment | 121 | 46.5 |
| Laterocervical | 139 | 53.5 |
| METASTASES | 18 | |
| STAGE | ||
| I | 227 | |
| II | 54 | |
| III | 9 | |
| IVA | 1 | |
| IVB | 9 |
| Procedure | N° Patients | % | Complications | N° Patients | % |
|---|---|---|---|---|---|
| TT + UCL + BCC | 165 | 55 | IPOPTH Tot | 112 | 74.17 |
| TT + BCC | 79 | 26.33 | Transient | 40 | 26.49 |
| Permanent | 72 | 47.68 | |||
| TT + BCL + BCC | 13 | 4.33 | TRLNP | 6 | 3.97 |
| BCL | 28 | 9.33 | DRLNP | 17 | 11.25 |
| UCC | 11 | 3.67 | SPINAL ACC D | 3 | 1.98 |
| LOB + UCL + BCC | 1 | 0.33 | OTHERS | 13 | 8.6 |
| LOB + BCL + BCC | 1 | 0.33 | |||
| MET.RES. | 2 | 0.67 | |||
| TOTAL | 300 | 100 | TOTAL | 151 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartori, P.V.; Andreani, S.; De Pasquale, L.; Pauna, I.; Bulfamante, A.M.; Aiello, P.S.L.; Melcarne, R.; Giacomelli, L.; Boniardi, M. How to Manage Advanced Differentiated Thyroid Cancer: Step-by-Step Analysis from Two Italian Tertiary Referral Centers. J. Clin. Med. 2024, 13, 708. https://doi.org/10.3390/jcm13030708
Sartori PV, Andreani S, De Pasquale L, Pauna I, Bulfamante AM, Aiello PSL, Melcarne R, Giacomelli L, Boniardi M. How to Manage Advanced Differentiated Thyroid Cancer: Step-by-Step Analysis from Two Italian Tertiary Referral Centers. Journal of Clinical Medicine. 2024; 13(3):708. https://doi.org/10.3390/jcm13030708
Chicago/Turabian StyleSartori, Paola Vincenza, Sara Andreani, Loredana De Pasquale, Iuliana Pauna, Antonio Mario Bulfamante, Paolo Salvatore Lorenzo Aiello, Rossella Melcarne, Laura Giacomelli, and Marco Boniardi. 2024. "How to Manage Advanced Differentiated Thyroid Cancer: Step-by-Step Analysis from Two Italian Tertiary Referral Centers" Journal of Clinical Medicine 13, no. 3: 708. https://doi.org/10.3390/jcm13030708
APA StyleSartori, P. V., Andreani, S., De Pasquale, L., Pauna, I., Bulfamante, A. M., Aiello, P. S. L., Melcarne, R., Giacomelli, L., & Boniardi, M. (2024). How to Manage Advanced Differentiated Thyroid Cancer: Step-by-Step Analysis from Two Italian Tertiary Referral Centers. Journal of Clinical Medicine, 13(3), 708. https://doi.org/10.3390/jcm13030708






