Hypertension and Dental Implants: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Objective
2.2. Search Strategies
2.3. Inclusion and Exclusion Criteria
2.4. Study Selection
2.5. Quality Assessment
2.6. Definitions
2.7. Data Extraction
2.8. Meta-Analysis
3. Results
3.1. Literature Search
3.2. Description of the Studies
3.3. Quality Assessment
3.4. Meta-Analysis
3.5. Meta-Regression

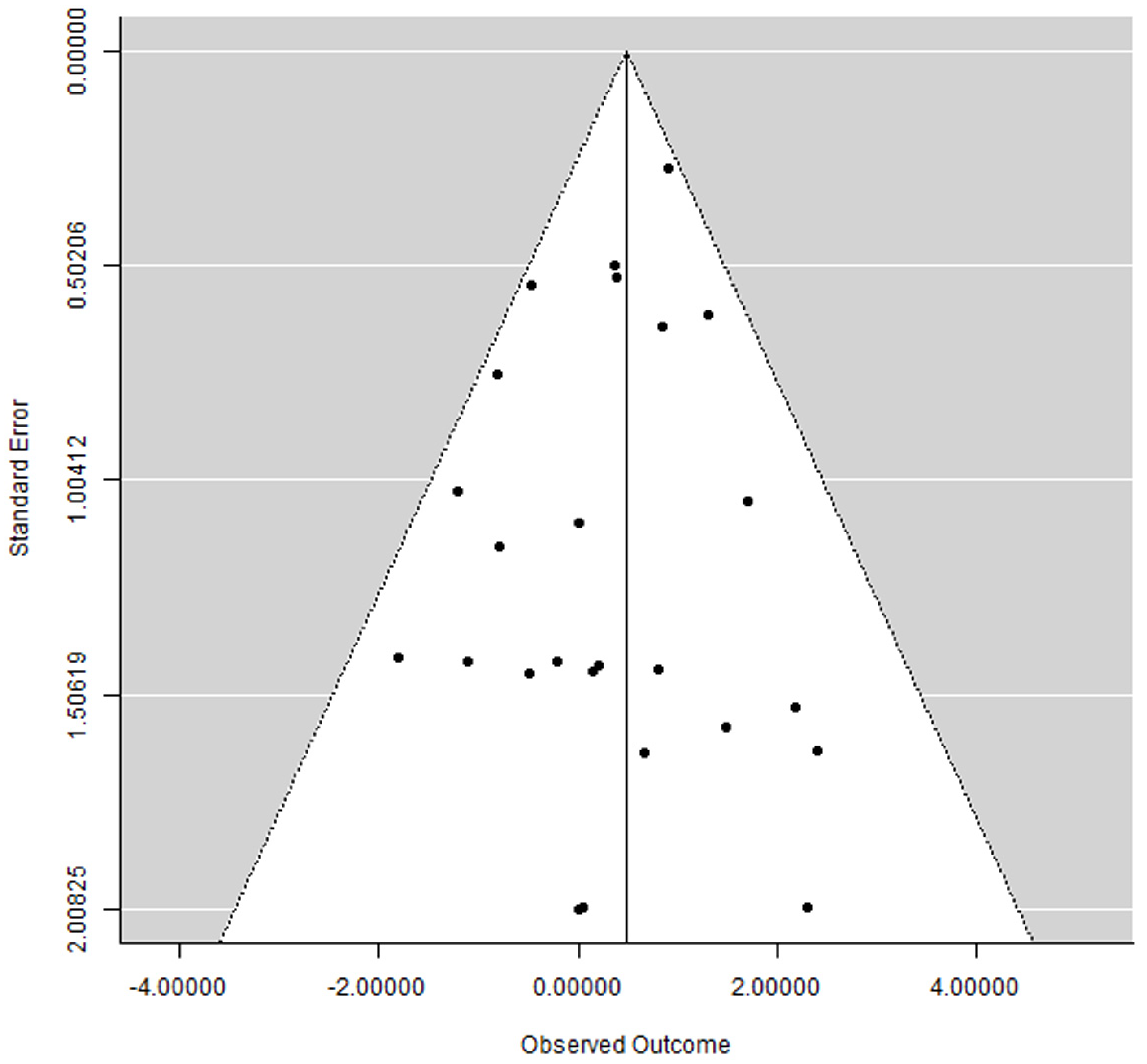
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Hypertension. Available online: https://www.who.int/health-topics/hypertension (accessed on 2 August 2023).
- NCD Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- El-Bikai, R.; Tahir, M.R.; Tremblay, J.; Joffres, M.; Šeda, O.; Šedová, L.; Awadalla, P.; Laberge, C.; Knoppers, B.M.; Dumas, P.; et al. Association of age-dependent height and bone mineral density decline with increased arterial stiffness and rate of fractures in hypertensive individuals. J. Hypertens. 2015, 33, 727–735; discussion 735. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.M.; Hsu, J.F.; Jee, W.S.; Matthews, J.L. Evidence for reduced cancellous bone mass in the spontaneously hypertensive rat. Bone Miner. 1993, 20, 251–264. [Google Scholar] [CrossRef]
- Humar, R.; Zimmerli, L.; Battegay, E. Angiogenesis and hypertension: An update. J. Hum. Hypertens. 2009, 23, 773–782. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontol. 2000 2017, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part I: Review of titanium alloys, surface characteristics and treatments. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e514–e525. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; Amato, A.; Chiacchio, A.; Sisalli, L.; Giordano, F. Do Systemic Diseases and Medications Influence Dental Implant Osseointegration and Dental Implant Health? An Umbrella Review. Dent. J. 2023, 11, 146. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Ioannidis, J.P.A. Conducting umbrella reviews. BMJ Med. 2022, 1, e000071. [Google Scholar] [CrossRef]
- Mishra, S.K.; Sonnahalli, N.K.; Chowdhary, R. Do antihypertensive medications have an effect on dental implants? A systematic review. Oral Maxillofac. Surg. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- NIH. Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 January 2020).
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Schmid, J. Pathogenesis of implant failures. Periodontol. 2000 1994, 4, 127–138. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Factors influencing the fracture of dental implants. Clin. Implant. Dent. Relat. Res. 2018, 20, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D. Principles of and procedures for systematic reviews. In Systematic Reviews in Health Care: Meta-Analysis in Context; Egger, M., Smith, G.D., Altman, D.G., Eds.; BMJ Books: London, UK, 2003; pp. 23–42. [Google Scholar]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Alsaadi, G.; Quirynen, M.; Komarek, A.; van Steenberghe, D. Impact of local and systemic factors on the incidence of late oral implant loss. Clin. Oral Implant. Res. 2008, 19, 670–676. [Google Scholar] [CrossRef]
- Alsaadi, G.; Quirynen, M.; Michiles, K.; Teughels, W.; Komarek, A.; van Steenberghe, D. Impact of local and systemic factors on the incidence of failures up to abutment connection with modified surface oral implants. J. Clin. Periodontol. 2008, 35, 51–57. [Google Scholar] [CrossRef]
- Altay, M.A.; Tozoğlu, S.; Yıldırımyan, N.; Özarslan, M.M. Is History of Periodontitis a Risk Factor for Peri-implant Disease? A Pilot Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 152–160. [Google Scholar] [CrossRef]
- Bertl, K.; Ebner, M.; Knibbe, M.; Pandis, N.; Kuchler, U.; Ulm, C.; Stavropoulos, A. How old is old for implant therapy in terms of early implant losses? J. Clin. Periodontol. 2019, 46, 1282–1293. [Google Scholar] [CrossRef]
- Cabrera-Domínguez, J.; Castellanos-Cosano, L.; Torres-Lagares, D.; Machuca-Portillo, G. A Prospective Case-Control Clinical Study of Titanium-Zirconium Alloy Implants with a Hydrophilic Surface in Patients with Type 2 Diabetes Mellitus. Int. J. Oral Maxillofac. Implant. 2017, 32, 1135–1144. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Analysis of risk factors for cluster behavior of dental implant failures. Clin. Implant. Dent. Relat. Res. 2017, 19, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Coskunses, F.M.; Tak, Ö. Clinical performance of narrow-diameter titanium-zirconium implants in immediately loaded fixed full-arch prostheses: A 2-year clinical study. Int. J. Implant. Dent. 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Grandi, T.; Guazzi, P.; Samarani, R.; Garuti, G.; Grandi, G. Immediate loading of two unsplinted implants retaining the existing complete mandibular denture in elderly edentulous patients: 1-year results from a multicentre prospective cohort study. Eur. J. Oral Implantol. 2012, 5, 61–68. [Google Scholar]
- Grandi, T.; Guazzi, P.; Samarani, R.; Grandi, G. Immediate loading of four (all-on-4) post-extractive implants supporting mandibular cross-arch fixed prostheses: 18-month follow-up from a multicentre prospective cohort study. Eur. J. Oral Implantol. 2012, 5, 277–285. [Google Scholar] [PubMed]
- Krennmair, S.; Weinländer, M.; Forstner, T.; Krennmair, G.; Stimmelmayr, M. Factors affecting peri-implant bone resorption in four Implant supported mandibular full-arch restorations: A 3-year prospective study. J. Clin. Periodontol. 2016, 43, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Tran, D.; Jeng, M.D.; Shen, Y.T. Survival rates of hybrid rough surface implants and their alveolar bone level alterations. J. Periodontol. 2018, 89, 1390–1399. [Google Scholar] [CrossRef]
- Lee, K.J.; Cha, J.K.; Sanz-Martin, I.; Sanz, M.; Jung, U.W. A retrospective case series evaluating the outcome of implants with low primary stability. Clin. Oral Implant. Res. 2019, 30, 861–871. [Google Scholar] [CrossRef]
- Lobato, R.P.B.; Kinalski, M.A.; Martins, T.M.; Agostini, B.A.; Bergoli, C.D.; Dos Santos, M.B.F. Influence of low-level laser therapy on implant stability in implants placed in fresh extraction sockets: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2020, 22, 261–269. [Google Scholar] [CrossRef]
- Malo, P.; de Araujo Nobre, M.; Lopes, A.; Ferro, A.; Botto, J. The All-on-4 treatment concept for the rehabilitation of the completely edentulous mandible: A longitudinal study with 10 to 18 years of follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 565–577. [Google Scholar] [CrossRef]
- Malo, P.; de Araujo Nobre, M.; Lopes, A.; Ferro, A.; Nunes, M. The All-on-4 concept for full-arch rehabilitation of the edentulous maxillae: A longitudinal study with 5-13 years of follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 538–549. [Google Scholar] [CrossRef]
- Moy, P.K.; Medina, D.; Shetty, V.; Aghaloo, T.L. Dental implant failure rates and associated risk factors. Int. J. Oral Maxillofac. Implant. 2005, 20, 569–577. [Google Scholar]
- Park, J.C.; Baek, W.S.; Choi, S.H.; Cho, K.S.; Jung, U.W. Long-term outcomes of dental implants placed in elderly patients: A retrospective clinical and radiographic analysis. Clin. Oral Implant. Res. 2017, 28, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Song, Y.W.; Sanz-Martin, I.; Cha, J.K.; Lee, J.S.; Jung, U.W. Clinical benefits of ridge preservation for implant placement compared to natural healing in maxillary teeth: A retrospective study. J. Clin. Periodontol. 2020, 47, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Arad, D.; Ofec, R.; Eliyahu, G.; Ruban, A.; Sterer, N. Long Term Follow-Up of Dental Implants Placed in Autologous Onlay Bone Graft. Clin. Implant. Dent. Relat. Res. 2016, 18, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Hasuike, A.; Iwano, Y.; Hagiwara, Y. Influence of antihypertensive medications on the clinical parameters of anodized dental implants: A retrospective cohort study. Int. J. Implant. Dent. 2020, 6, 32. [Google Scholar] [CrossRef]
- Simons, W.F.; De Smit, M.; Duyck, J.; Coucke, W.; Quirynen, M. The proportion of cancellous bone as predictive factor for early marginal bone loss around implants in the posterior part of the mandible. Clin. Oral Implant. Res. 2015, 26, 1051–1059. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, A.S.; Vaibhav, V.; Kumar, K.; Singh, R.; Jerry, J.J. A 10 years retrospective study of assessment of prevalence and risk factors of dental implants failures. J. Fam. Med. Prim. Care 2020, 9, 1617–1619. [Google Scholar] [CrossRef]
- Wang, J.; Lerman, G.; Bittner, N.; Fan, W.; Lalla, E.; Papapanou, P.N. Immediate versus delayed temporization at posterior single implant sites: A randomized controlled trial. J. Clin. Periodontol. 2020, 47, 1281–1291. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Eimar, H.; Arekunnath Madathil, S.; Abi-Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Antihypertensive Medications and the Survival Rate of Osseointegrated Dental Implants: A Cohort Study. Clin. Implant. Dent. Relat. Res. 2016, 18, 1171–1182. [Google Scholar] [CrossRef]
- Perez-Castrillon, L.J.; Justo, I.; Sanz-Cantalapiedra, A.; Pueyo, C.; Hernandez, G.; Duenas, A. Effect of the Antihypertensive Treatment on the Bone Mineral Density and Osteoporotic Fracture. Curr. Hypertens. Rev. 2005, 1, 61–66. [Google Scholar] [CrossRef]
- Bellido, T.; Saini, V.; Pajevic, P.D. Effects of PTH on osteocyte function. Bone 2013, 54, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Schleithoff, S.S.; Koerfer, R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br. J. Nutr. 2005, 94, 483–492. [Google Scholar] [CrossRef]
- London, G.M.; Guérin, A.P.; Verbeke, F.H.; Pannier, B.; Boutouyrie, P.; Marchais, S.J.; Mëtivier, F. Mineral metabolism and arterial functions in end-stage renal disease: Potential role of 25-hydroxyvitamin D deficiency. J. Am. Soc. Nephrol. 2007, 18, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, L. Vitamin D and the elderly. Clin. Endocrinol. 2005, 62, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, K.; Hu, Z.; Li, M.; Wei, H.; Tang, Z.; Chen, B.; Su, C.; Cai, D.; Xu, J. Determining the association between hypertension and bone metabolism markers in osteoporotic patients. Medicine 2021, 100, e26276. [Google Scholar] [CrossRef]
- Manrique, N.; Pereira, C.C.; Luvizuto, E.R.; Sánchez Mdel, P.; Okamoto, T.; Okamoto, R.; Sumida, D.H.; Antoniali, C. Hypertension modifies OPG, RANK, and RANKL expression during the dental socket bone healing process in spontaneously hypertensive rats. Clin. Oral Investig. 2015, 19, 1319–1327. [Google Scholar] [CrossRef]
- Shalhoub, V.; Faust, J.; Boyle, W.J.; Dunstan, C.R.; Kelley, M.; Kaufman, S.; Scully, S.; Van, G.; Lacey, D.L. Osteoprotegerin and osteoprotegerin ligand effects on osteoclast formation from human peripheral blood mononuclear cell precursors. J. Cell Biochem. 1999, 72, 251–261. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Prewitt, R.L.; Chen, I.I.; Dowell, R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 1982, 243, H243–H251. [Google Scholar] [CrossRef]
- Hutchins, P.M.; Darnell, A.E. Observations of a decreased number of small arterioles in spontaneously hypertensive rats. Circ. Res. 1974, 34–35, 161–165. [Google Scholar]
- Sokolova, I.A.; Manukhina, E.B.; Blinkov, S.M.; Koshelev, V.B.; Pinelis, V.G.; Rodionov, I.M. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc. Res. 1985, 30, 1–9. [Google Scholar] [CrossRef]
- Antonios, T.F.; Singer, D.R.; Markandu, N.D.; Mortimer, P.S.; MacGregor, G.A. Structural skin capillary rarefaction in essential hypertension. Hypertension 1999, 33, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Smith, F.M.; Morris, L.W.; Greene, A.S.; Lombard, J.H. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ. Res. 1996, 79, 324–330. [Google Scholar] [CrossRef]
- Serné, E.H.; Gans, R.O.; ter Maaten, J.C.; Tangelder, G.J.; Donker, A.J.; Stehouwer, C.D. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 2001, 38, 238–242. [Google Scholar] [CrossRef]
- Kang, K.Y.; Kang, Y.; Kim, M.; Kim, Y.; Yi, H.; Kim, J.; Jung, H.R.; Park, S.H.; Kim, H.Y.; Ju, J.H.; et al. The effects of antihypertensive drugs on bone mineral density in ovariectomized mice. J. Korean Med. Sci. 2013, 28, 1139–1144. [Google Scholar] [CrossRef]
- Ma, L.; Ji, J.L.; Ji, H.; Yu, X.; Ding, L.J.; Liu, K.; Li, Y.Q. Telmisartan alleviates rosiglitazone-induced bone loss in ovariectomized spontaneous hypertensive rats. Bone 2010, 47, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Ames, R.W.; Orr-Walker, B.J.; Clearwater, J.M.; Horne, A.M.; Evans, M.C.; Murray, M.A.; McNeil, A.R.; Gamble, G.D. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: A randomized controlled trial. Am. J. Med. 2000, 109, 362–370. [Google Scholar] [CrossRef]
- Ott, S.M.; LaCroix, A.Z.; Scholes, D.; Ichikawa, L.E.; Wu, K. Effects of three years of low-dose thiazides on mineral metabolism in healthy elderly persons. Osteoporos. Int. 2008, 19, 1315–1322. [Google Scholar] [CrossRef]
- Bolland, M.J.; Ames, R.W.; Horne, A.M.; Orr-Walker, B.J.; Gamble, G.D.; Reid, I.R. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos. Int. 2007, 18, 479–486. [Google Scholar] [CrossRef]
- de Vries, F.; Pouwels, S.; Bracke, M.; Leufkens, H.G.; Cooper, C.; Lammers, J.W.; van Staa, T.P. Use of beta-2 agonists and risk of hip/femur fracture: A population-based case-control study. Pharmacoepidemiol. Drug Saf. 2007, 16, 612–619. [Google Scholar] [CrossRef]
- Levasseur, R.; Dargent-Molina, P.; Sabatier, J.P.; Marcelli, C.; Bréart, G. Beta-blocker use, bone mineral density, and fracture risk in older women: Results from the Epidemiologie de l’Osteoporose prospective study. J. Am. Geriatr. Soc. 2005, 53, 550–552. [Google Scholar] [CrossRef]
- Reid, I.R.; Gamble, G.D.; Grey, A.B.; Black, D.M.; Ensrud, K.E.; Browner, W.S.; Bauer, D.C. beta-Blocker use, BMD, and fractures in the study of osteoporotic fractures. J. Bone Miner. Res. 2005, 20, 613–618. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Kassem, M.; Christoffersen, B.R.; Kolthoff, N.; Brixen, K.; Mosekilde, L. Fracture risk in perimenopausal women treated with beta-blockers. Calcif. Tissue Int. 2004, 75, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Canoy, D.; Harvey, N.C.; Prieto-Alhambra, D.; Cooper, C.; Meyer, H.E.; Åsvold, B.O.; Nazarzadeh, M.; Rahimi, K. Elevated blood pressure, antihypertensive medications and bone health in the population: Revisiting old hypotheses and exploring future research directions. Osteoporos. Int. 2022, 33, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Al Ansari, Y.; Shahwan, H.; Chrcanovic, B.R. Diabetes Mellitus and Dental Implants: A Systematic Review and Meta-Analysis. Materials 2022, 15, 3227. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental Implants in Patients Receiving Chemotherapy: A Meta-Analysis. Implant. Dent. 2016, 25, 261–271. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Is the intake of selective serotonin reuptake inhibitors associated with an increased risk of dental implant failure? Int. J. Oral Maxillofac. Surg. 2017, 46, 782–788. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Intake of Proton Pump Inhibitors Is Associated with an Increased Risk of Dental Implant Failure. Int. J. Oral Maxillofac. Implant. 2017, 32, 1097–1102. [Google Scholar] [CrossRef]
- Häggman-Henrikson, B.; Ali, D.; Aljamal, M.; Chrcanovic, B.R. Bruxism and dental implants: A systematic review and meta-analysis. J. Oral Rehabil. 2023, 51, 202–217. [Google Scholar] [CrossRef]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.; Fadhul, F.; Chrcanovic, B.R. Bisphosphonates and Dental Implants: A Systematic Review and Meta-Analysis. Materials 2023, 16, 6078. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants in irradiated versus nonirradiated patients: A meta-analysis. Head Neck 2016, 38, 448–481. [Google Scholar] [CrossRef] [PubMed]
- Bredberg, C.; Vu, C.; Häggman-Henrikson, B.; Chrcanovic, B.R. Marginal bone loss around dental implants: Comparison between matched groups of bruxer and non-bruxer patients: A retrospective case-control study. Clin. Implant. Dent. Relat. Res. 2023, 25, 124–132. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. Impact of Different Surgeons on Dental Implant Failure. Int. J. Prosthodont. 2017, 30, 445–454. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamadé, L.; El-Disoki, S.; Chrcanovic, B.R. Hypertension and Dental Implants: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 499. https://doi.org/10.3390/jcm13020499
Hamadé L, El-Disoki S, Chrcanovic BR. Hypertension and Dental Implants: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(2):499. https://doi.org/10.3390/jcm13020499
Chicago/Turabian StyleHamadé, Liljan, Salma El-Disoki, and Bruno Ramos Chrcanovic. 2024. "Hypertension and Dental Implants: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 2: 499. https://doi.org/10.3390/jcm13020499
APA StyleHamadé, L., El-Disoki, S., & Chrcanovic, B. R. (2024). Hypertension and Dental Implants: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(2), 499. https://doi.org/10.3390/jcm13020499






