Tattoo-Associated Skin Reaction in a Melanoma Patient Receiving B-RAF and MEK Inhibitors: A Case Report with an Emphasis on Etiopathogenic and Histological Features
Abstract
1. Introduction
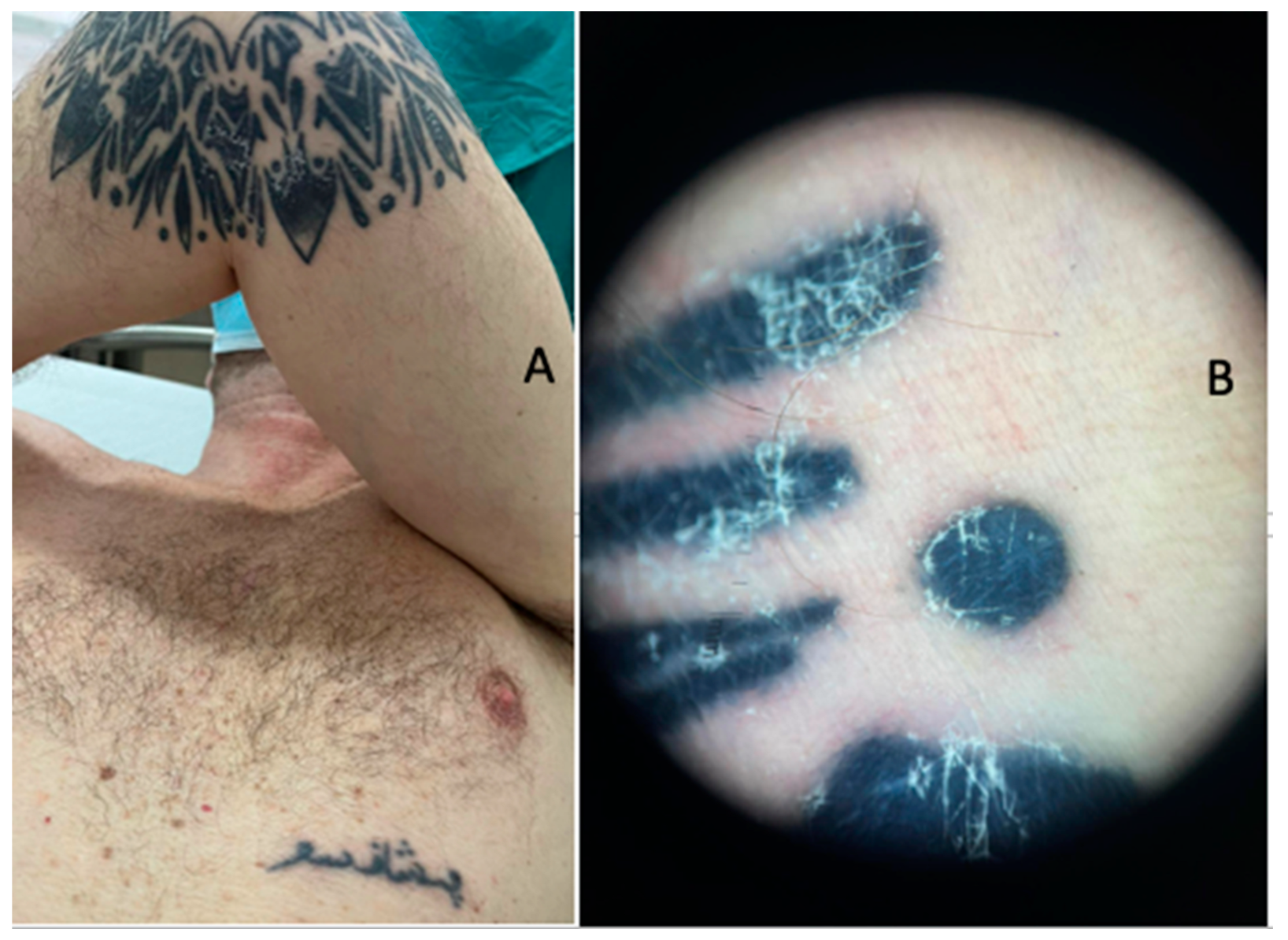
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kluger, N. Epidemiology of tattoos in industrialized countries. Curr. Probl. Dermatol. 2015, 48, 6–20. [Google Scholar] [CrossRef]
- Laux, P.; Tralau, T.; Tentschert, J.; Blume, A.; Dahouk, S.A.; Bäumler, W.; Bernstein, E.; Bocca, B.; Alimonti, A.; Colebrook, H.; et al. A medical-toxicological view of tattooing. Lancet 2016, 387, 395–402. [Google Scholar] [CrossRef]
- Renzoni, A.; Pirrera, A.; Novello, F.; Lepri, A.; Cammarata, P.; Tarantino, C.; D’Ancona, F.; Perra, A. The tattooed population in Italy: A national survey on demography, characteristics and perception of health risks. Ann. Ist. Super. Sanita 2018, 54, 126–136. [Google Scholar] [CrossRef]
- Serup, J.; Carlsen, K.H.; Sepehri, M. Tattoo complaints and complications: Diagnosis and clinical spectrum. Curr. Probl. Dermatol. 2015, 48, 48–60. [Google Scholar] [CrossRef]
- Brungs, C.; Schmid, R.; Wolf, C.; Berg, T.; Korf, A.; Heuckeroth, S.; Hayen, H.; van der Bent, S.; Maijer, K.; Rustemeyer, T.; et al. Tattoo Pigment Identification in Inks and Skin Biopsies of Adverse Reactions by Complementary Elemental and Molecular Bioimaging with Mass Spectral Library Matching. Anal. Chem. 2022, 94, 3581–3589. [Google Scholar] [CrossRef]
- Godinho, M.M.; Aguinaga, F.; Grynszpan, R.; Lima, V.M.; Azulay, D.R.; Cuzzi, T.; Ramos-E-Silva, M.; Manela-Azulay, M. Granulomatous reaction to red tattoo pigment treated with allopurinol. J. Cosmet. Dermatol. 2015, 14, 241–245. [Google Scholar] [CrossRef]
- Cornejo, C.M.; Haun, P.M.; English, J., 3rd; Rosenbach, M. Immune checkpoint inhibitors and the development of granulomatous reactions. J. Am. Acad. Dermatol. 2019, 81, 1165–1175. [Google Scholar] [CrossRef]
- Kluger, N. Tattoo Reactions Associated with Targeted Therapies and Immune Checkpoint Inhibitors for Advanced Cancers: A Brief Review. Dermatology 2019, 235, 522–524. [Google Scholar] [CrossRef]
- Kluger, N. Nickel and tattoos: Where are we? Contact Dermat. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Simunovic, C.; Shinohara, M.M. Complications of decorative tattoos: Recognition and management. Am. J. Clin. Dermatol. 2014, 15, 525–536. [Google Scholar] [CrossRef]
- Mataix, J.; Silvestre, J.F. Reacciones cutáneas adversas por tatuajes y piercings. Actas Dermosifiliogr. 2009, 100, 643–656. [Google Scholar] [CrossRef]
- Serup, J. Diagnostic tools for doctors’ evaluation of tattoo complications. Curr. Probl. Dermatol. 2017, 52, 42–57. [Google Scholar]
- González, I.; Silvestre, J.F. Reacciones alérgicas frente a las tintas de los tatuajes. In Tratado Sobre los Tatuajes. Claves Para su Eliminación Con Láser; Muñoz, D., Ed.; Ediciones Journal: Buenos Aires, Argentina, 2017; pp. 69–76. [Google Scholar]
- Høgsberg, T.; Thomsen, B.M.; Serup, J. Histopathology and immune histochemistry of red tattoo reactions. Skin Res. Technol. 2015, 21, 449–458. [Google Scholar] [CrossRef]
- Agarwal, P.; Jagatim, A.; Mehta, R.; Vadher, P.; Rathod, S.; Bodar, P. Histopathological evaluation of cutaneous reactions to tattoos: Study at a tertiary care center. J. Cutan. Pathol. 2021, 48, 870–876. [Google Scholar] [CrossRef]
- Gamba, C.S.; Lambert Smith, F.; Wisell, J.; Brown, M. Tattoo reactions in an HIV patient: Autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015, 1, 395–398. [Google Scholar] [CrossRef]
- McPhie, M.L.; Ren, K.Y.M.; Hendry, J.M.; Molin, S.; Herzinger, T. Delayed-Type Hypersensitivity Reaction to Red Tattoo Ink Triggered by Ledipasvir/Sofosbuvir for Hepatitis C: A Case Report. Case Rep. Dermatol. 2021, 13, 379–383. [Google Scholar] [CrossRef]
- Kluger, N. Sarcoidosis on tattoos: A review of the literature from 1939 to 2011. Sarcoidosis Vasc. Diffuse Lung Dis. 1939, 30, 86–102. [Google Scholar]
- Bachmeyer, C.; Blum, L.; Petitjean, B.; Kemiche, F.; Pertuiset, E. Granulomatous tattoo reaction in a patient treated with etanercept. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 550–552. [Google Scholar] [CrossRef]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. [Google Scholar] [CrossRef]
- Giet, G.; Lebas, E.; Rorive, A.; Arrese, J.E.; Nikkels, A.F. Granulomatous Reactions from Tattoos Following BRAF Inhibitor Therapy. Case Rep. Dermatol. 2019, 11, 101–107. [Google Scholar] [CrossRef]
- Boutros, A.; Schiavi, C.; Cecchi, F.; Spagnolo, F.; Guadagno, A.; Tanda, E.T.; Giusti, F.; Murdaca, G.; Queirolo, P. Case Report: Immune-Related Toxicity During Adjuvant Treatment with BRAF Plus MEK Inhibitors in a Melanoma Patient. Front. Immunol. 2020, 11, 579523. [Google Scholar] [CrossRef]
- Malissen, N.; Magis, Q.; Macagno, N.; Monsetier, S.; Troin, L.; Mallet, S.; Gaudy-Marqueste, C.; Grob, J.-J.; Richard, M.-A. Réactions granulomateuses sur tatouage sous anti-BRAF + anti-MEK révélatrices de fausses progressions. Ann. Dermatol. Vénéréol. 2017, 144, S128–S129. [Google Scholar] [CrossRef]
- Reinhard, R.; Gebhardt, C.; Schmieder, A.; Umansky, V.; Utikal, J. Recurrent tattoo reactions in a patient treated with BRAF and MEK inhibitors. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e375–e377. [Google Scholar] [CrossRef]
- Rohmer, E.; Scrivener, J.N.; Schissler, C.; Cribier, B.; Lenormand, C. Hypersensibilité retardée aux tatouages induite par un traitement combiné anti-BRAF/anti-MEK [Tattoo hypersensitivity reaction in a patient receiving combined BRAF and MEK inhibitors]. Ann. Dermatol. Venereol. 2019, 146, 725–729. (In French) [Google Scholar] [CrossRef]
- Laske, J.; Meier, F.; Bauer, A.; Beissert, S.; Garzarolli, M. Tattoo-associated complications of metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2018, 28, 485–487. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Villanova, J.; Laux, P.; Dreiack, N.; Penning, R.; Tucoulou, R.; Cotte, M.; et al. Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci. Rep. 2017, 7, 11395. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, M.; Ge, Y.; Krepler, C.; Belser, E.; Lopez-Coral, A.; Xu, X.; Zhang, G.; Azuma, R.; Liu, Q.; et al. BRAF Inhibition Stimulates Melanoma-Associated Macrophages to Drive Tumor Growth. Clin. Cancer Res. 2015, 21, 1652–1664. [Google Scholar] [CrossRef]
- Linke, M.; Pham, H.T.; Katholnig, K.; Schnöller, T.; Miller, A.; Demel, F.; Schütz, B.; Rosner, M.; Kovacic, B.; Sukhbaatar, N.; et al. Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nat. Immunol. 2017, 18, 293–302. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baratta, S.; Cazzato, G.; Foti, C.; Ingravallo, G.; Lospalluti, L.; Laface, C.; Filotico, R.; Ambrogio, F. Tattoo-Associated Skin Reaction in a Melanoma Patient Receiving B-RAF and MEK Inhibitors: A Case Report with an Emphasis on Etiopathogenic and Histological Features. J. Clin. Med. 2024, 13, 321. https://doi.org/10.3390/jcm13020321
Baratta S, Cazzato G, Foti C, Ingravallo G, Lospalluti L, Laface C, Filotico R, Ambrogio F. Tattoo-Associated Skin Reaction in a Melanoma Patient Receiving B-RAF and MEK Inhibitors: A Case Report with an Emphasis on Etiopathogenic and Histological Features. Journal of Clinical Medicine. 2024; 13(2):321. https://doi.org/10.3390/jcm13020321
Chicago/Turabian StyleBaratta, Silvia, Gerardo Cazzato, Caterina Foti, Giuseppe Ingravallo, Lucia Lospalluti, Carmelo Laface, Raffaele Filotico, and Francesca Ambrogio. 2024. "Tattoo-Associated Skin Reaction in a Melanoma Patient Receiving B-RAF and MEK Inhibitors: A Case Report with an Emphasis on Etiopathogenic and Histological Features" Journal of Clinical Medicine 13, no. 2: 321. https://doi.org/10.3390/jcm13020321
APA StyleBaratta, S., Cazzato, G., Foti, C., Ingravallo, G., Lospalluti, L., Laface, C., Filotico, R., & Ambrogio, F. (2024). Tattoo-Associated Skin Reaction in a Melanoma Patient Receiving B-RAF and MEK Inhibitors: A Case Report with an Emphasis on Etiopathogenic and Histological Features. Journal of Clinical Medicine, 13(2), 321. https://doi.org/10.3390/jcm13020321











