Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis—Case Report and Literature Review
Abstract
1. Introduction
2. Case Report
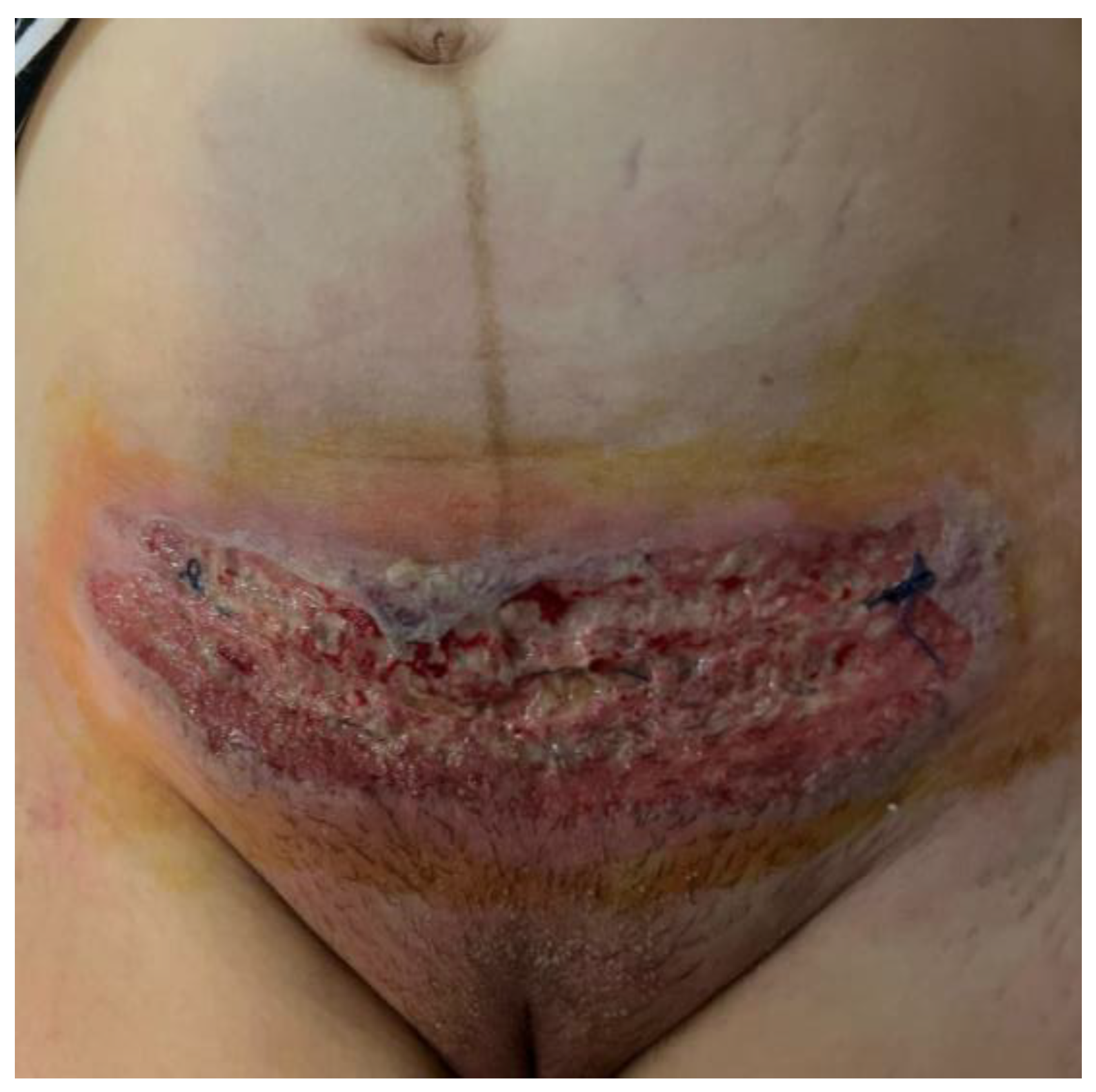
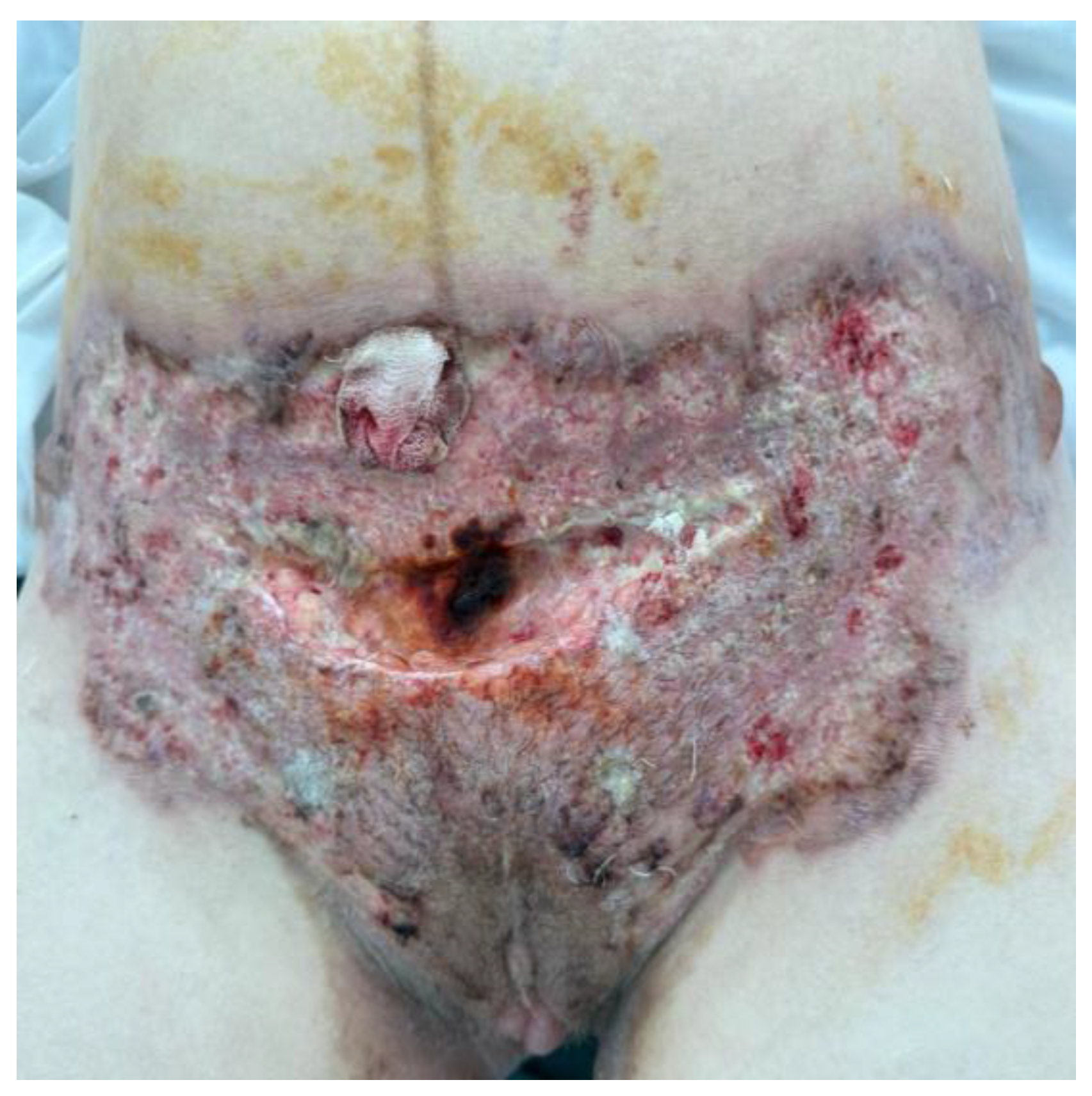
2.1. Patient History and Physical Examination
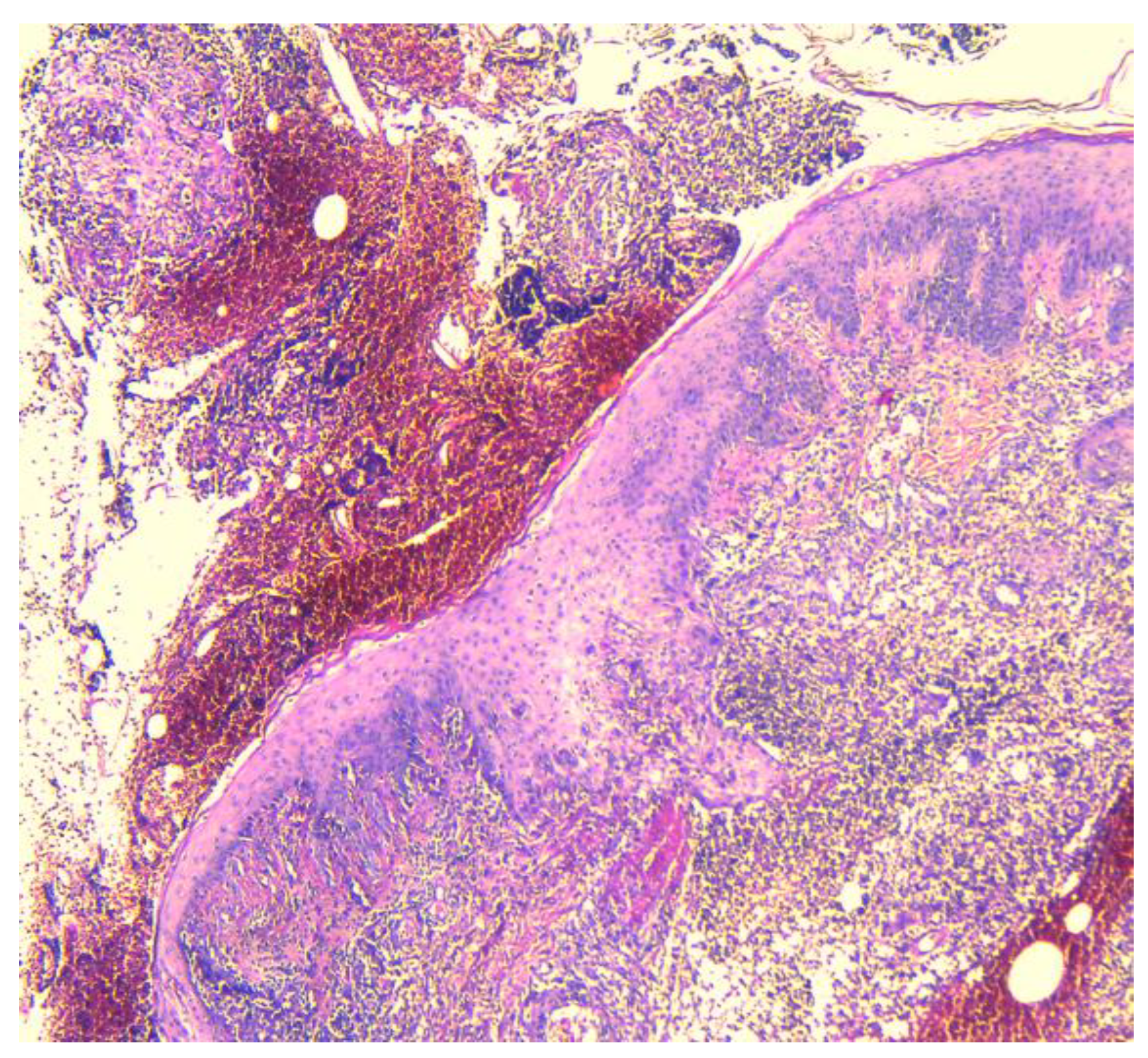
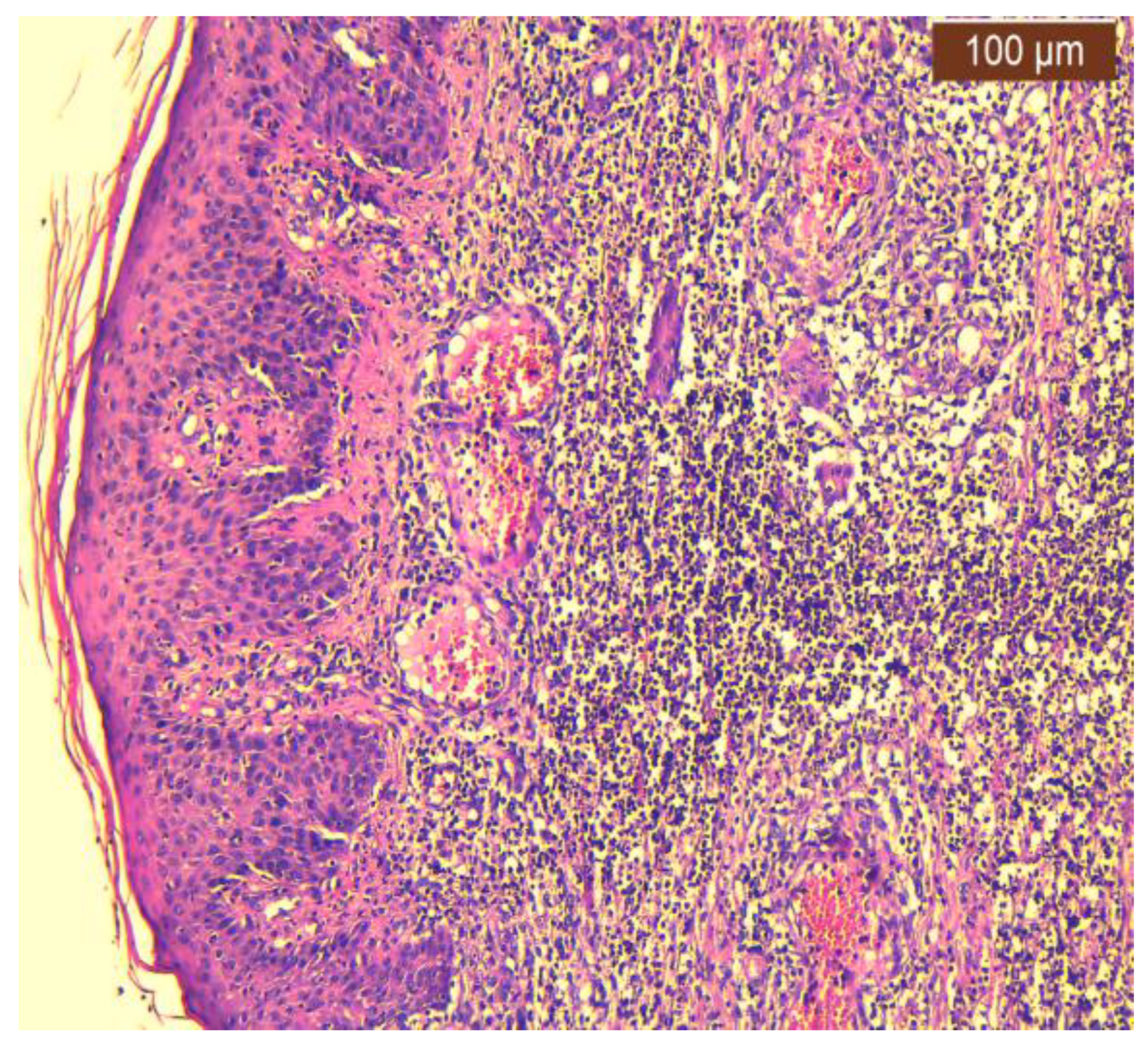
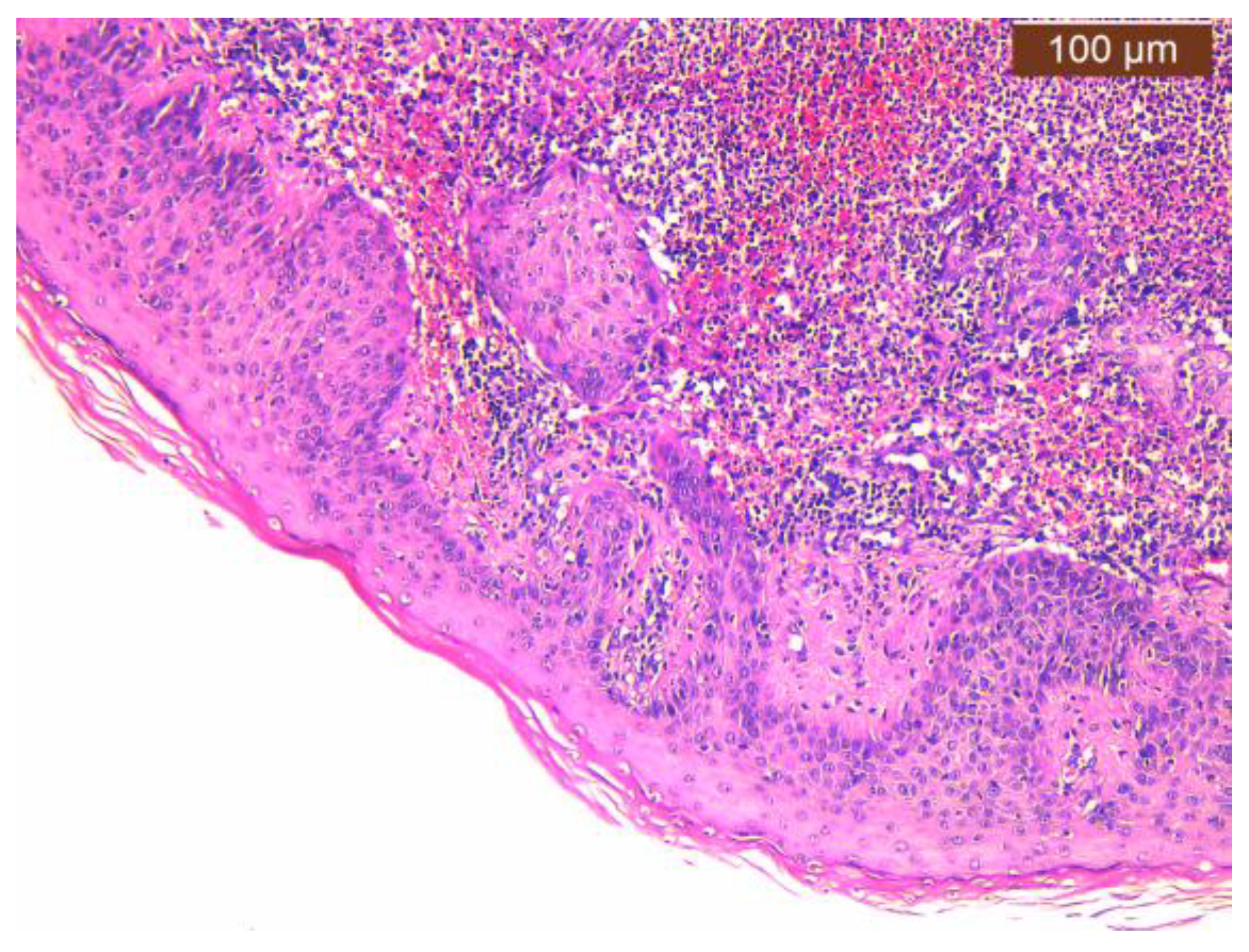
2.2. Histopathology Findings
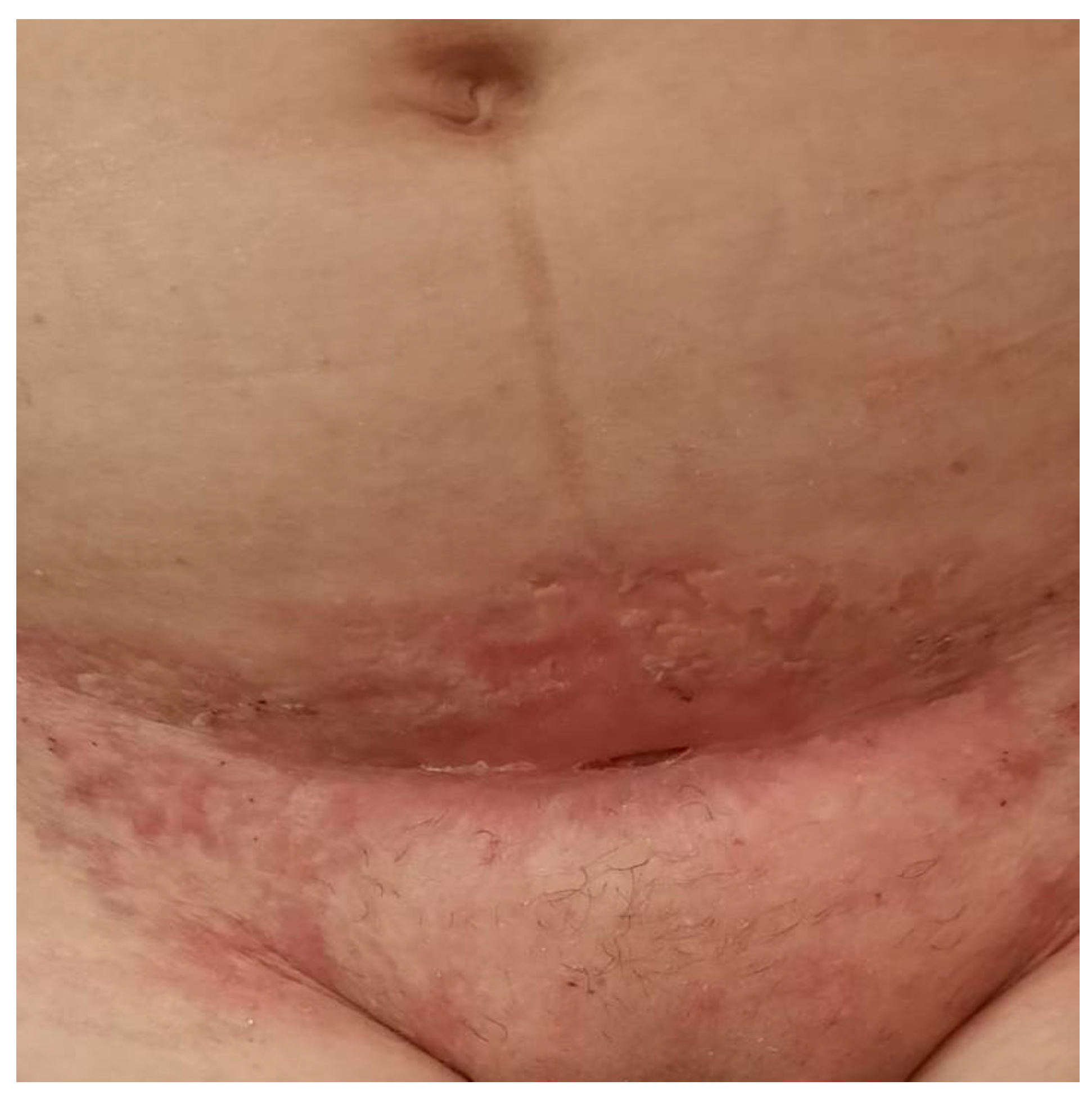
2.3. Dermatological Management
3. Literature Search—Methodology and Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarwar, S.; Sajid, F.; Wasim, A.U.; Waleed, M.S.; Thada, P.K. Pyoderma Gangrenosum Precipitated by Breast Engorgement Following Lactation Discontinuation: A Rare Case Report. Cureus 2023, 15, e42203. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Pereira, N.; Cardoso, J.C.; Gonçalo, M. Pyoderma gangrenosum: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2015, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- George, C.; Deroide, F.; Rustin, M. Pyoderma gangrenosum—A guide to diagnosis and management. Clin. Med. (Lond.) 2019, 19, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, W.; Jiang, X. Pyoderma gangrenosum after cesarean section treated with skin graft: A case report. Medicine 2019, 98, e15380. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Muñoz, C.; Martínez-Morán, C.; Miranda-Romero, A. Pyoderma Gangrenosum Following Cesarean Delivery. Actas Dermosifiliogr. 2008, 99, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Jain, V.; Bhattacharjee, R.; Vishwajeet, V.; Thakur, G. Pyoderma gangrenosum at an episiotomy site in successive pregnancies: A case report. Case Rep. Womens Health 2021, 32, e00365. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Davis, M.D.; Weenig, R.H.; Powell, F.C.; Perry, H.O. Pyoderma gangrenosum: Clinicopathologic correlation and proposed diagnostic criteria. Int. J. Dermatol. 2004, 43, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.L.; Jackson, J.M.; Jorizzo, J.L.; Fleischer, A.B., Jr.; White, W.L.; Callen, J.P. Pyoderma gangrenosum. A comparison of typical and atypical forms with an emphasis on time to remission. Case review of 86 patients from 2 institutions. Medicine 2000, 79, 37–46. [Google Scholar] [CrossRef]
- Suvirya, S.; Pathania, S.; Singhai, A. A case of bullous pyoderma gangrenosum. BMJ Case Rep. 2019, 12, e228772. [Google Scholar] [CrossRef]
- Banga, F.; Schuitemaker, N.; Meijer, P. Pyoderma gangrenosum after caesarean section: A case report. Reprod. Health 2006, 3, 9. [Google Scholar] [CrossRef][Green Version]
- Ahn, C.; Negus, D.; Huang, W. Pyoderma gangrenosum: A review of pathogenesis and treatment. Expert. Rev. Clin. Immunol. 2018, 14, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Zúñiga, M.J.M.; Heath, M.S.; Gontijo, J.R.V.; Ortega-Loayza, A.G. Pyoderma gangrenosum: A review with special emphasis on Latin America literature. An. Bras. Dermatol. 2019, 94, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Teagle, A.; Hargest, R. Management of pyoderma gangrenosum. JR Soc. Med. 2014, 107, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, J.; Park, J.S.; Jun, J.K. Successful vaginal birth after prior cesarean section in a patient with pyoderma gangrenosum. Obstet. Gynecol. Sci. 2016, 59, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aydın, Ç.A.; Uğurlucan, F.G.; Yaşa, C.; Dural, Ö. Recurrent pyoderma gangrenosum after cesarean delivery successfully treated with vacuum-assisted closure and split thickness skin graft: A case report. J. Obstet. Gynaecol. Res. 2015, 41, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ahronowitz, I.; Harp, J.; Shinkai, K. Etiology and Management of Pyoderma Gangrenosum. Am. J. Clin. Dermatol. 2012, 13, 191–211. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.M. Seat belt pyoderma gangrenosum: Minor pressure as a causative factor. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1273–1274. [Google Scholar] [CrossRef]
- Diallo, M.; Diop, A.; Diatta, B.A.; Diadie, S.; Ndiaye, M.; Ndiaye, M.T.; Seck, B.; Deh, A.; Diop, K. Post-Partum Pyoderma Gangenosum Following a Cesarean Section. Dermatol. Case Rep. 2017, 2, 129. [Google Scholar]
- Steele, R.B.; Nugent, W.H.; Braswell, S.F.; Frisch, S.; Ferrell, J.; Ortega-Loayza, A.G. Pyoderma gangrenosum and pregnancy: An example of abnormal inflammation and challenging treatment. Br. J. Dermatol. 2016, 174, 77–87. [Google Scholar] [CrossRef]
- Harland, C.C.; Jaffe, W.; Holden, C.A.; Ross, L.D. Pyoderma gangrenosum complicating caesarean section. J. Obstet. Gynaecol. 1993, 13, 115–116. [Google Scholar] [CrossRef]
- Stone, N.; Harland, C.; Ross, L.; Holden, C. Pyoderma gangrenosum complicating caesarian section. Clin. Exp. Dermatol. 1996, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Steadman, U.A.; Brennan, T.E.; Daman, L.A.; Curry, S.L. Pyoderma gangrenosum following cesarean delivery. Obstet. Gynecol. 1998, 91, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Futami, H.; Kodaira, M.; Furuta, T.; Hanai, H.; Kaneko, E. Pyoderma gangrenosum complicating ulcerative colitis: Successful treatment with methylprednisolone pulse therapy and cyclosporine. J. Gastroenterol. 1998, 33, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Ronnau, A.C.; von Schmiedeberg, S. Pyoderma gangrenosum after cesarean delivery. Am. J. Obstet. Gynecol. 2000, 183, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Skinner, A.M.; Mills, S.M. Management of a chronic wound secondary to pyoderma gangrenosum following uncomplicated lower segment Caesarean section incision. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.A.; Ahmed, N.; Salman, T.A.; Craven, N.M. Pyoderma gangrenosum in pregnancy. J. Obstet. Gynaecol. 2006, 26, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Aoussar, A.; Ismaïli, N.; Berbich, L.; Tazi Mezalek, Z.; Aït Ourhrouil, M.; Senouci, K.; Mansouri, F.; Hassam, B. Pyoderma gangrenosum révélant une artérite de Takayasu [Pyoderma gangrenosum revealing Takayasu’s arteritis]. Ann. Dermatol. Venereol. 2007, 134, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, I.C.; Braams-Lisman, B.A.; Mekkes, J.R. Pyoderma gangraenosum na een sectio caesarea [Pyoderma gangrenosum after a cesarean section]. Ned. Tijdschr. Geneeskd. 2009, 153, 722–725. [Google Scholar] [PubMed]
- Pauser, S.; Goerge, T.; Eickelmann, M.; Markus, G.; Luger, T.; Steinoff, M. Pyoderma Gangrenosum After Cesarean Delivery. Clin. Med. Insights Dermatol. 2009, 2, 23–26. [Google Scholar]
- Wierzbicka-Hainaut, E.; Le Naoures, H.; Bonneric-Malthieu, F.; Debiais, P.; Levillain, P.; Pierre, F.; Guillet, G. Recurring Pyoderma gangrenosum in pregnancy. Ann. Dermatol. Venereol. 2010, 137, 225–229. [Google Scholar] [CrossRef]
- Kikuchi, N.; Hanami, Y.; Miura, T.; Kawakami, Y.; Satoh, M.; Ohtsuka, M.; Yamamoto, T. Pyoderma gangrenosum following surgical procedures. Int. J. Dermatol. 2010, 49, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Muresan, M. Successful relactation—A case history. Breastfeed. Med. 2011, 6, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.V.; Bajapai, N.; Pai, A.; Bharatnur, S.; Hebbar, S. Pyoderma gangrenosum in two successive pregnancies complicating caesarean wound. Case Rep. Obstet. Gynecol. 2014, 2014, 654843. [Google Scholar] [CrossRef] [PubMed]
- Radhika, A.G.; Singal, A.; Radhakrishnan, G.; Singh, S. Pyoderma gangrenosum following a routine caesarean section: Pseudo-infection in a caesarean wound. Qatar Med. J. 2015, 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Simmons, P.; Artis, A.; Dwiggins, M.; Gross, T.; LoCoco, S.; Farell, J. 31: A rare case of pyoderma gangrenosum and the effects of pathergy in a postpartum patient. AJOG 2015, 213, P907–P908. [Google Scholar] [CrossRef]
- Cokan, A.; Dovnik, A.; Žebeljan, I.; Mujezinović, F.; Bujas, T.; Marko, P.B. Pyoderma gangrenosum in a caesarean wound following caesarean section with late occurrence of pyoderma gangrenosum of the breast. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 203, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Yoshida, K.; Yamaguchi, M.; Aizawa, A.; Fujiwara, H.; Enomoto, T.; Takakuwa, K. Case with pyoderma gangrenosum abruptly emerging around the wound of cesarean section for placenta previa with placenta accrete. J. Obstet. Gynaecol. Res. 2016, 42, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Alani, A.; Sadlier, M.; Ramsay, B.; Ahmad, K. Pyoderma gangrenosum induced by episiotomy. BMJ Case Rep. 2016, 2016, bcr2015213574. [Google Scholar] [CrossRef] [PubMed]
- Hilton, R.; Berryman, J.; Handoyo, K. Pyoderma Gangrenosum Masquerading as Necrotizing Fasciitis: Stepping Away from Cognitive Shortcuts. Eur. J. Case Rep. Intern. Med. 2017, 4, 000648. [Google Scholar] [CrossRef]
- Satoh, M.; Hiraiwa, T.; Yamamoto, T. Recurrent pyoderma gangrenosum developed after a cesarean section with a 10-year interval. Int. J. Dermatol. 2018, 57, e92–e93. [Google Scholar] [CrossRef]
- Naciri, I.; Meziane, M.; Benzekri, L.; Ghaouti, M.; Senouci, K.; Hassam, B. Pyoderma gangrenosum récidivant du post-partum et cardiomyopathie fatale [ecurrent postpartum pyoderma gangrenosum and fatal cardiomyopathy]. Ann. Dermatol. Venereol. 2018, 145, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Kyozuka, H.; Fukuda, T.; Hiraiwa, T.; Yamaguchi, A.; Fujimori, K. Incisional pyoderma gangrenosum after caesarean section: Two case reports. Case Rep. Womens Health 2019, 23, e00128. [Google Scholar] [CrossRef] [PubMed]
- Foessleitner, P.; Just, U.; Kiss, H.; Farr, A. Challenge of diagnosing pyoderma gangrenosum after caesarean section. BMJ Case Rep. 2019, 12, e230315. [Google Scholar] [CrossRef] [PubMed]
- van Donkelaar, C.E.; de Haan, J.M.H.; Lange, J.F.M.; de Vries, M.; Horváth, B. Pseudo-wound infection after a caesarean section: Case report of unrecognized Pyoderma Gangrenosum. Int. J. Surg. Case Rep. 2020, 69, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Gündüz, K.; Gülbaşaran, F.; Hasdemir, P.S.; Temiz, P.; Inanır, I. Successful treatment of severe refractory post-cesarean pyoderma gangrenosum with intravenous immunoglobulin. Dermatol. Ther. 2020, 33, e14121. [Google Scholar] [CrossRef] [PubMed]
- Zolper, E.G.; Harbour, P.W.; Dekker, P.K.; Schwitzer, J.A.; Viramontes, A.; Evans, K.K. Post-Cesarean Section Pyoderma Gangrenosum Presenting with Vasopressor-dependent Shock: Long-term Follow-up after Delayed Primary Closure. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3427. [Google Scholar] [CrossRef] [PubMed]
- Moutos, C.P.; Hoyer, P.; Kelly, B.; Saad, A.F. Pyoderma gangrenosum after cesarean delivery. Am. J. Obstet. Gynecol. 2021, 225, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Ghumra, W.; Gold, A.; Azurdia, R.M. Pyoderma gangrenosum following an unplanned caesarean section: A patient revisited. BMJ Case Rep. 2021, 14, e238702. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Bian, H.; Sun, C.; Zheng, S.; Xiong, B.; Huang, Z.; Liu, Z.; Ma, L.; Li, H.; Chen, H.; et al. Pyoderma gangrenosum secondary to caesarean section treated with negative pressure wound therapy and skin graft. Dermatol. Ther. 2021, 34, e14858. [Google Scholar] [CrossRef]
- Aziret, M.; Kara, Ş.; Yaldız, M.; Köse, N.; Aşıkuzunoğlu, F.; Cevrioğlu, A.S. An extensive pyoderma gangrenosum mimicking necrotizing fasciitis: An unusual case report. Int. J. Surg. Case Rep. 2021, 81, 105697. [Google Scholar] [CrossRef]
- Ishizaki, R.; Yamamoto, M.; Yamamoto, T. Bullous Pyoderma Gangrenosum Occurring on a Cesarean Section Scar. Indian J. Dermatol. 2022, 67, 481. [Google Scholar] [CrossRef] [PubMed]
- Bal, H.; Subramanian, S.; Sharma, Y.K.; Lal, S. A Case of Post-caesarean Pyoderma Gangrenosum. J. Obstet. Gynaecol. India 2022, 72, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.; Sexton, F.; Martin-Smith, J.; Raghallaigh, S.N. Postpartum pyoderma gangrenosum following lactation-induced mastitis and abscess incision. JAAD Case Rep. 2023, 42, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Sangiuliano, S.; Gravina, A.G.; Miranda, A.; Nicoletti, G. Pyoderma gangrenosum: An updated review. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Tosun, Z.; Ucar, C.; Savaci, N. Pyoderma gangrenosum in a battered child. Ann. Plast. Surg. 2006, 57, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Brightman, L.; Venna, S. Pyoderma gangrenosum with pathergy in a pregnant patient without associated systemic disease. Cutis 2008, 81, 255–258. [Google Scholar] [PubMed]
- Maverakis, E.; Ma, C.; Shinkai, K.; Fiorentino, D.; Callen, J.P.; Wollina, U.; Marzano, A.V.; Wallach, D.; Kim, K.; Schadt, C.; et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol. 2018, 154, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Tomlin, K. A noninfectious “infection” after cesarian delivery. Contemp. OB/GYN 2022, 67, 26–28. [Google Scholar]
- Weenig, R.H.; Davis, M.D.; Dahl, P.R.; Su, W.P. Skin ulcers misdiagnosed as pyoderma gangrenosum. N. Engl. J. Med. 2002, 347, 1412–1418. [Google Scholar] [CrossRef]
- Ashchyan, H.J.; Nelson, C.A.; Stephen, S.; James, W.D.; Micheletti, R.G.; Rosenbach, M. Neutrophilic dermatoses: Pyoderma gangrenosum and other bowel- and arthritis-associated neutrophilic dermatoses. J. Am. Acad. Dermatol. 2018, 79, 1009–1022. [Google Scholar] [CrossRef]
- Morgenstjerne-Schwenck, L.E.T.; Knudsen, J.T.; Prasad, S.C. Efficacy and safety of skin grafting in treatment of vasculitic ulcer and pyoderma gangrenosum-A systematic review. Wound Repair. Regen. 2021, 29, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Okada, Y.; Watanabe, S.; Danno, K.; Yamamoto, S.; Ishiura, R.; Nakayama, Y.; Mitsui, K.; Ueki, A.; Nakai, Y.; et al. Successful Treatment of Ulcerative-Type Pyoderma Gangrenosum with a Combination Therapy of Oral Prednisolone, Vacuum-Assisted Closure, and Skin Grafting. Case Rep. Dermatol. 2021, 13, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, J.D.; Lucas, C.T.; Sami, N. Pyoderma gangrenosum in pregnancy successfully treated with infliximab and prednisone. JAAD Case Rep. 2017, 3, 387–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohmaru-Nakanishi, T.; Goto, H.; Maehara, M.; Oishi, H.; Ueoka, Y. Perineal pyoderma gangrenosum in pregnancy: A case report. Case Rep. Womens Health 2019, 22, e00102. [Google Scholar] [CrossRef] [PubMed]
- Bandoli, G.; Palmsten, K.; Forbess Smith, C.J.; Chambers, C.D. A Review of Systemic Corticosteroid Use in Pregnancy and the Risk of Select Pregnancy and Birth Outcomes. Rheum. Dis. Clin. N. Am. 2017, 43, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Vigl, K.; Posch, C.; Richter, L.; Monshi, B.; Rappersberger, K. Pyoderma gangrenosum during pregnancy—Treatment options revisited. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1981–1984. [Google Scholar] [CrossRef] [PubMed]
- Erfurt-Berge, C.; Herbst, C.; Schuler, G.; Bauerschmitz, J. Successful treatment of pyoderma gangrenosum with intravenous immunoglobulins during pregnancy. J. Cutan. Med. Surg. 2012, 16, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.S.; King, A.D.; Bardhi, R.; Daveluy, S. Targeted therapy with ixekizumab in pyoderma gangrenosum: A case series and a literature overview. JAAD Case Rep. 2023, 37, 49–53. [Google Scholar] [CrossRef]
- Djokanovic, N.; Klieger-Grossmann, C.; Pupco, A.; Koren, G. Safety of infliximab use during pregnancy. Reprod. Toxicol. 2011, 32, 93–97. [Google Scholar] [CrossRef]
- Wanberg, L.J.; Fletcher, K.M.; Goldfarb, N.; Alavi, A. Treatment of pyoderma gangrenosum in pregnancy with certolizumab pegol. JEADV Clin. Prac. 2023, 3, 696–698. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Fogh, K.; Bech, R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: A semi-systematic review. Int. Wound J. 2019, 16, 511–521. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Gallagher, C.; Hollywood, A.; Paul, L.; O’Connell, M. Anakinra for recalcitrant pyoderma gangrenosum. Clin. Exp. Dermatol. 2021, 46, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Whitley, M.J.; Balaban, A.; Ellington, K.; Marano, A.L. Pyoderma gangrenosum induced by secukinumab in a patient with psoriasis successfully treated with ustekinumab. JAAD Case Rep. 2020, 6, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.D.; Liu, K.; Lockwood, S.; Butler, D.; Tsiaras, W.G.; Joyce, C.; Mostaghimi, A. Risk of developing pyoderma gangrenosum after procedures in patients with a known history of pyoderma gangrenosum-A retrospective analysis. J. Am. Acad. Dermatol. 2018, 78, 310–314.e1. [Google Scholar] [CrossRef] [PubMed]
- Jockenhöfer, F.; Wollina, U.; Salva, K.A.; Benson, S.; Dissemond, J. The PARACELSUS score: A novel diagnostic tool for pyoderma gangrenosum. Br. J. Dermatol. 2019, 180, 615–620. [Google Scholar] [CrossRef]
| Report (Author and Year of Publications) | Onset of the Disease | Site of the Lesion | Age (Years) | Recurrence | Associated Pathology | Treatment |
|---|---|---|---|---|---|---|
| Harlaand et al. (1993) [20] | Postpartum (29 weeks) | C-section | 36 | No | No | corticotherapy |
| Stone et al. (1996) [21] | Postpartum (29 weeks) | C-section | 36 | No | No | corticotherapy surgery |
| Steadman et al. (1998) [22] | Postpartum (37 weeks) | C-section | 32 | No | No | corticotherapy |
| Futami et al. (1998) [23] | Postpartum (vaginal birth) | Face, neck, upper arm, and thigh | 32 | Relapse | Ulcerative colitis | corticotherapy cyclosporine |
| Ronnau et al. (2000) [24] | Postpartum (not specified) | C-section | 24 | Yes (third episode) | Chronic type C hepatitis | corticotherapy cyclosporine |
| Skinner et al. (2006) [25] | Postpartum (39 weeks) | C-section | 36 | Yes After the vaginal delivery that occurred a year ago, she experienced a delayed healing perineal ulcer. | No | corticotherapy cyclosporine |
| Karim et al. (2006) [26] | Postpartum (37 weeks) | C-section | 29 | No | No | corticotherapy cyclosporine |
| Banga et al. (2007) [10] | Postpartum (29 weeks) | C-section | 32 | No | No | corticotherapy tacrolimus cream |
| Aoussar et al. (2007) [27] | During pregnancy (16 weeks) Postpartum (not specified) | On the torso and on the limbs | 26 | Yes After one year during pregnancy (30 weeks of gestation) and after two years in postpartum (on the right arm—intramuscular injection site). | Takayasu arteritis, type B hepatitis | corticotherapy |
| Sanz-Munoz et al. (2008) [5] | During pregnancy postpartum (not specified) | Anterior side of the left leg 4 days earlier C-section | 30 | No | No | corticotherapy |
| Wiersma et al. (2009) [28] | Postpartum (not specified) | C-section | 31 | No | No | corticotherapy |
| Pauser et al. (2009) [29] | Postpartum (not specified) | C-section | 39 | No | No | corticotherapy cyclosporine dapsone |
| Wierzbicka-Hainaut et al. (2010) [30] | During pregnancy (third trimester) Postpartum (37 weeks) | Left calf C-section | 25 | Yes During her first and second pregnancy. | No | corticotherapy |
| Kikuchi et al. (2010) [31] | Postpartum (not specified) | C-section | 28 | No | No | corticotherapy |
| Muresan et al. (2010) [32] | Postpartum (term) | C-section | 27 | Yes | No | corticotherapy |
| Amin et al. (2014) [33] | Postpartum (32 weeks) | C-section | 32 | Yes Third recurrence, after every one of the three C-sections. | No | corticotherapy dapsone |
| Aydin et al. (2015) [15] | Postpartum (37 weeks) | C-section | 32 | Yes Previous C-section and left hand at the site of intravenous catheterization. | No | corticotherapy azathioprine—unresponsive vacuum-assisted closure split-thickness skin graft |
| Radhika et al. (2015) [34] | Postpartum (term) | C-section | 22 | Yes After an intramuscular injection on her buttock. | No | corticotherapy cyclosporin dapsone |
| Simmons et al. (2015) [35] | Postpartum (27 weeks) | C-section | 43 | No | No | corticotherapy |
| Cokan et al. (2016) [36] | Postpartum (not specified abruptio placentae) | C-section Left breast | 23 | No | No | corticotherapy |
| Park et al. (2016) [14] | Postpartum (term) | C-section | 33 | No | No (antiphospholipid antigen—weakly positive) | corticotherapy cyclosporine |
| Nonaka et al. (2016) [37] | Postpartum (36 weeks) | C-section | 39 | No | Antiphospholipid syndrome (lupus anticoagulant weakly positive but with 3 spontaneous abortions) | corticotherapy minocycline hydrochloride |
| Alani et al. (2016) [38] | Postpartum (not specified) | Episiotomy | 29 | No | Hepatitis C | Corticotherapy cyclosporine mycophenolate mofetil dapsone |
| Hilton et al. (2017) [39] | Postpartum (not specified) | C-section | 28 | No | No | corticotherapy dapsone |
| Satoh et al. (2018) [40] | Postpartum (33 weeks) | C-section | 27 | Yes First episode after appendectomy 10 years previously. | No | corticotherapy |
| Diallo et al. (2017) [18] | Postpartum (not specified) | C-section | 25 | No | No | corticotherapy |
| Naciri et al. (2018) [41] | Postpartum (term) | C-section | 23 | Yes Abscess on her left breast prior to pregnancy | Peripartum/Postpartum cardiomyopathy | corticotherapy |
| Murata et al. (2019) [42] | Postpartum (37 weeks) | C-section | 29 | Yes | No | corticotherapy |
| Postpartum (33 weeks) | C-section | 27 | Yes | No | corticotherapy | |
| Foessleitner et al. (2019) [43] | Postpartum (30 weeks) | C-section | 34 | No | No | negative pressure wound therapy high-dose corticosteroids therapy |
| Shen et al. (2019) [4] | Postpartum (>7 months of gestation) | C-section | 32 | No | No | corticotherapy human immunoglobulin wound debridement vacuum sealing negative pressure drainage skin grafting hyperbaric oxygen therapy |
| van Donkelaar et al. (2020) [44] | Postpartum (32 weeks) | C-section | 21 | No | No | corticotherapy |
| Gunduz et al. (2020) [45] | Postpartum (not specified) | C-section Bilateral gluteal regions post NSAIDs administration | 32 | No | No | intravenous immunoglobulin |
| Zolper et al. (2021) [46] | Postpartum (not specified) | C-section | 28 | Yes First episode (post-vaginal delivery) | No | corticotherapy cyclosporine incisional negative pressure wound therapy |
| Moutos et al. (2021) [47] | Postpartum (28 weeks) | C-section | 23 | No | No | corticotherapy cyclosporine |
| Ghumra et al. (2021) [48] | Postpartum (31 weeks) | C-section | 35 | Yes 14 years prior, skin grafts for ulcers on her left lower leg | No | corticotherapy cyclosporine |
| Luo et al. (2021) [49] | Postpartum (not specified) | C-section | 38 | No | Hepatitis B | corticotherapy intravenous immunoglobulin |
| Rao et al. (2021) [6] | Postpartum (not specified) | Episiotomy | 28 | No | No | corticotherapy |
| Aziret et al. (2021) [50] | Postpartum (term) | C-section | 36 | No | No | corticotherapy |
| Ishizaki et al. (2022) [51] | Postpartum (36 weeks) | C-section | 34 | No | Thrombocytopenia early in pregnancy | corticotherapy |
| Bal et al. (2022) [52] | Postpartum (not specified abruptio placentae) | C-section | 25 | Yes 2 years prior—injection abscess right gluteal region healed with a large scar | No | corticotherapy |
| Lyons et al. (2023) [53] | Postpartum | Right breast | 32 | No | No | corticotherapy cyclosporine skin graft |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matasariu, D.R.; Bujor, I.E.; Mihălceanu, E.; Gîscă, T.C.; Stâncanu, A.; Andriescu, E.C.; Popescu, I.; Socolov, D.; Vasiluță, C.; Ursache, A. Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis—Case Report and Literature Review. J. Clin. Med. 2024, 13, 3653. https://doi.org/10.3390/jcm13133653
Matasariu DR, Bujor IE, Mihălceanu E, Gîscă TC, Stâncanu A, Andriescu EC, Popescu I, Socolov D, Vasiluță C, Ursache A. Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis—Case Report and Literature Review. Journal of Clinical Medicine. 2024; 13(13):3653. https://doi.org/10.3390/jcm13133653
Chicago/Turabian StyleMatasariu, Daniela Roxana, Iuliana Elena Bujor, Elena Mihălceanu, Tudor Cătălin Gîscă, Alina Stâncanu, Elena Corina Andriescu, Ioana Popescu, Demetra Socolov, Ciprian Vasiluță, and Alexandra Ursache. 2024. "Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis—Case Report and Literature Review" Journal of Clinical Medicine 13, no. 13: 3653. https://doi.org/10.3390/jcm13133653
APA StyleMatasariu, D. R., Bujor, I. E., Mihălceanu, E., Gîscă, T. C., Stâncanu, A., Andriescu, E. C., Popescu, I., Socolov, D., Vasiluță, C., & Ursache, A. (2024). Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis—Case Report and Literature Review. Journal of Clinical Medicine, 13(13), 3653. https://doi.org/10.3390/jcm13133653







