Sleep Disorders in Connective Tissue Diseases—Coexisting Diseases or Disease Components?
Abstract
1. Introduction
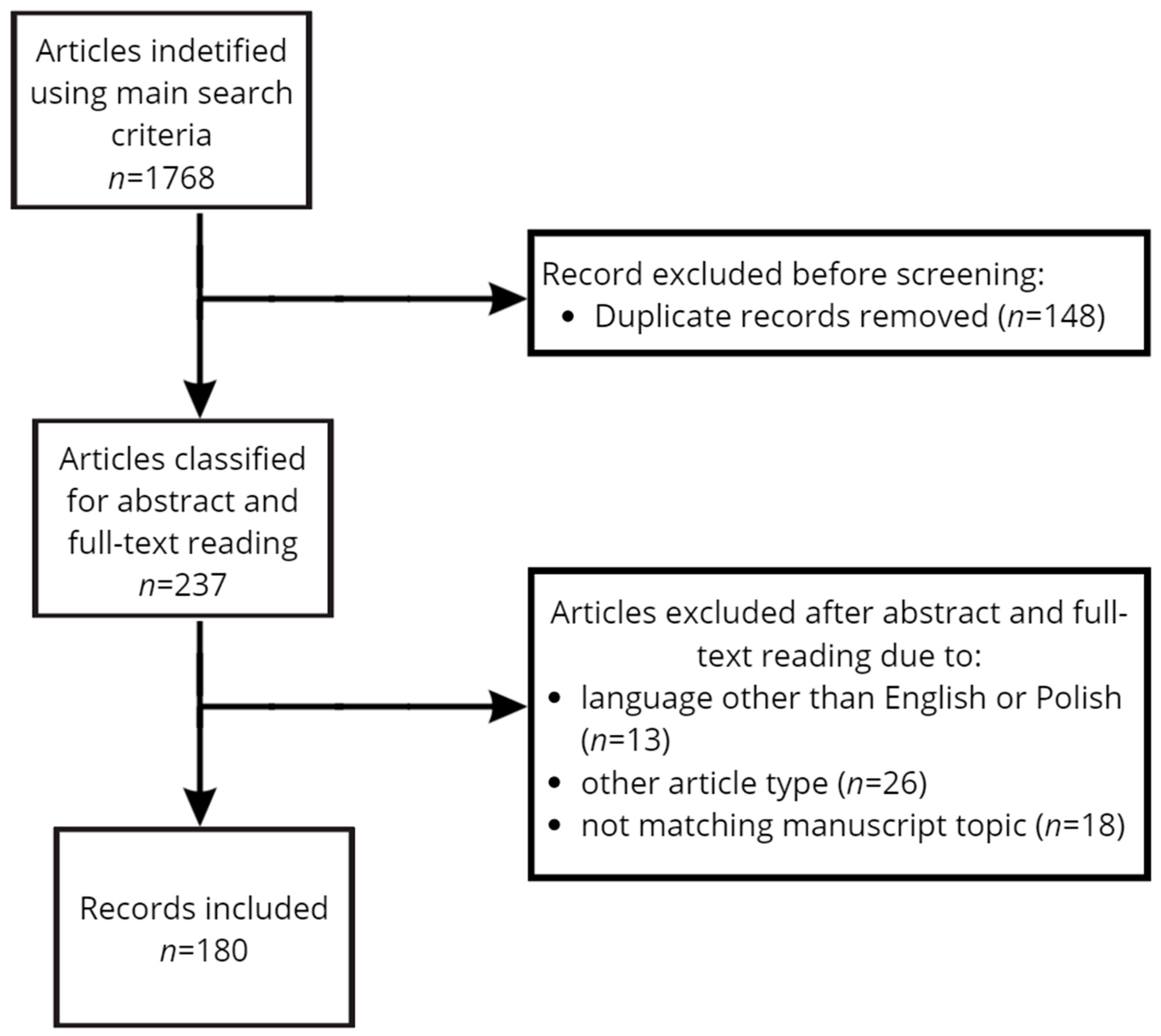
2. Materials and Methods
3. Epidemiology of Sleep Disorders in CTDs
| Disorder | Prevalence of | |||
|---|---|---|---|---|
| Insomnia (%) | EDS (%) | OSA (%) | RLS (%) | |
| RA | 28.6 [10] | NA | 12.7–21 [11,12] | 30 [11] |
| SSc | NA | NA | 32.1–58 [13,14] | 40.7 [15] |
| SLE | 33.3 [16] | 28.5–35.8 [17,18] | 50.0 [17] | 34.2 [19] |
| Sjogren | 71.0 [20] | 15.3–55.0 [20,21] | 45.0 [20] | 15.3 [21] |
| General population | 22.1 [22] | 11.9 [21] | 0.3 [5] | 14.3 [23] |
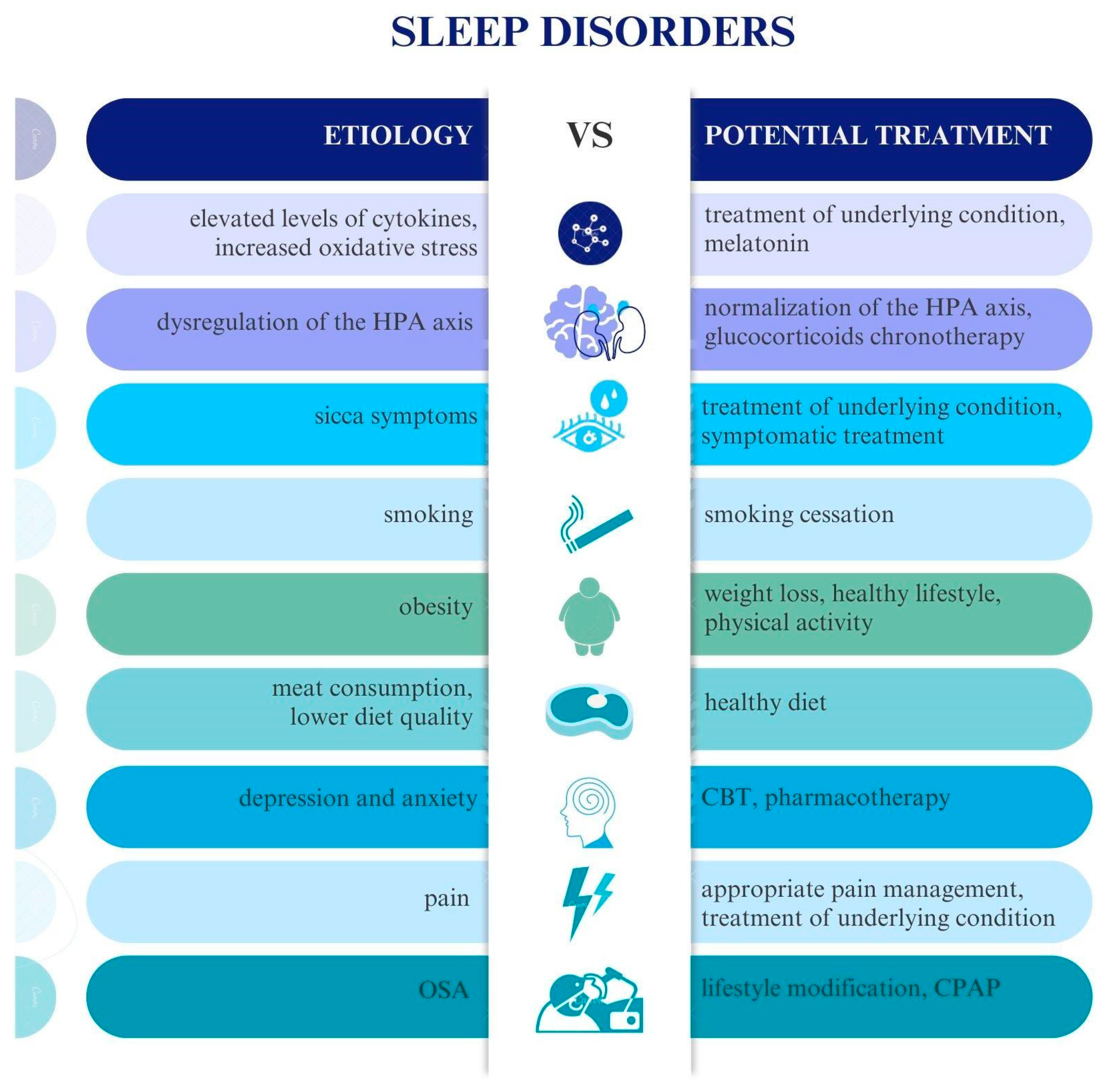
4. Etiology of Sleep Disorders in Connective Tissue Diseases
4.1. Cytokines
4.2. Melatonin
4.3. Hypothalamic–Pituitary–Adrenal Axis
4.4. Obesity
4.5. Diet
4.6. Smoking
4.7. Genetics
4.8. Orexins/Hypocretins
4.9. Depression and Anxiety
4.10. Pain
5. Drug-Induced Sleep Disorders
5.1. Glucocorticoids
5.2. Disease-Modifying Antirheumatic Drugs
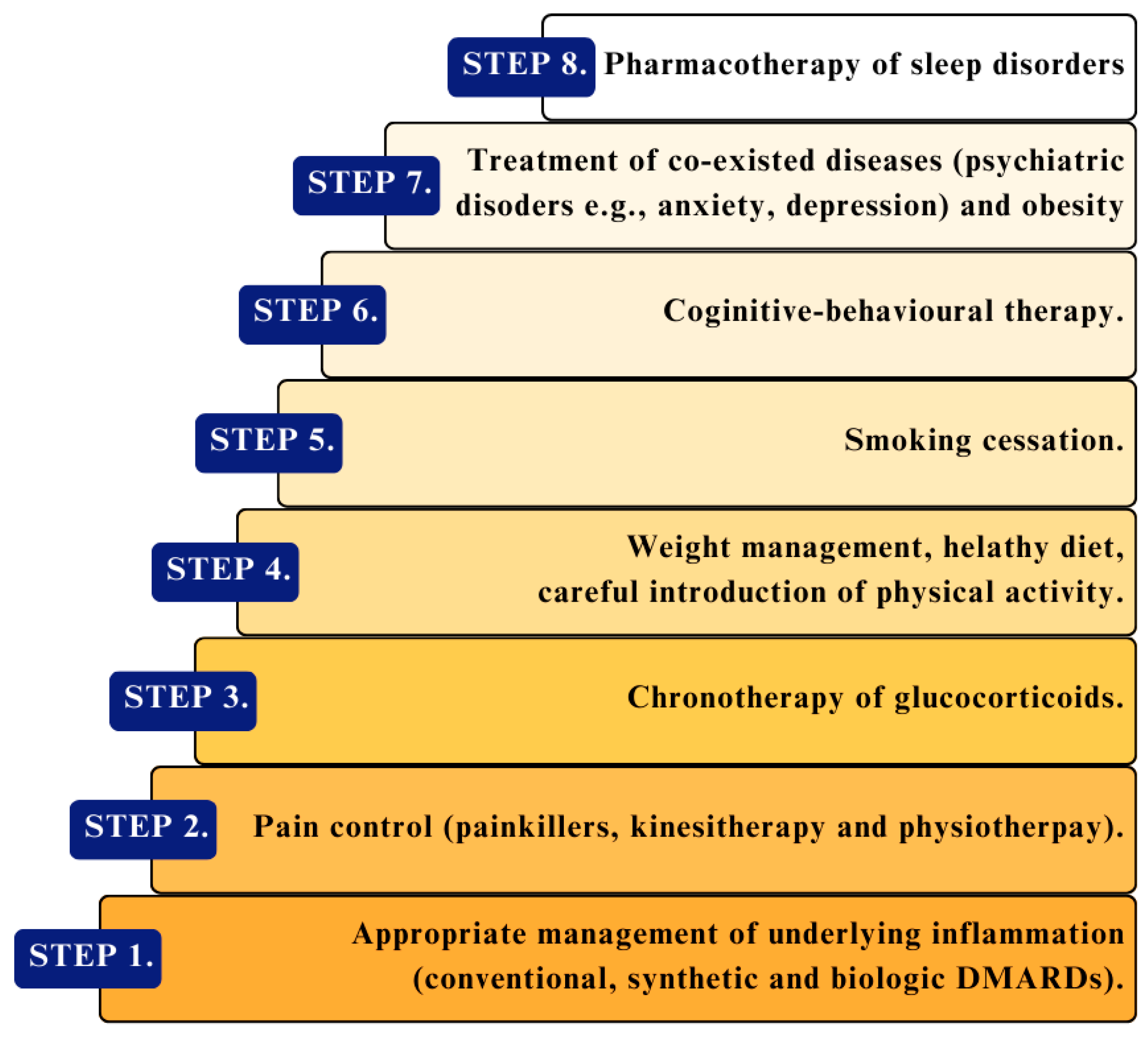
6. Prevention and Treatment of Sleep Disorders in Connective Tissue Diseases
| Disease (Number of Patients) | Medication/Medical Intervention | Influence on Sleep Disorders | Ref. |
|---|---|---|---|
| Healthy (n = 41) | monitored program of ≥150 min of moderate-to-vigorous-intensity physical activity per week for six months | ↓ insomnia symptom severity (evaluated using ISI) | [154] |
| Healthy (n = 110) | pilates-based exercise program | ↓ depression and anxiety scores | [155] |
| RA (n = 26) | relaxation-based yoga intervention | ↑ sleep quality (measured by PSQI score) and FSS questionnaire | [156] |
| SLE (n = 58) | progressive aerobic exercise | ↓ the severity of depression and anxiety | [157] |
| SLE (n = 76) | physical activity counseling | safe and feasible for further investigation | [158] |
| SS (n = 59) | 16-week resistance exercise program | ↓ general fatigue | [159] |
| Chronic primary insomnia (n = 60) | aerobic and resistance exercise trial | no effect on psychological stress, sleep quality, depressive symptoms, or QoL | [160] |
| SLE (n = 50) | 16-week digital therapeutic intervention (focused on dietary, environmental, and lifestyle-triggering factors) | ↑ sleep quality, vitality, and mental health | [161] |
| RA (n = 50) | a 10-week trial of an anti-inflammatory diet | improved fatigue, pain, mental health, vitality, and subjective perception of disease activity | [162] |
| SLE (n = 23) | A 6-week trial of a low-glycemic-index diet | improved sleep quality | [163] |
| Chronic inflammatory arthritis (n = 121) | 1-week whole-body cold mist shower therapy | ↓ pro-inflammatory cytokine concentrations | [164] |
| RA (n = 48) | trial of curcumin supplementation for eight weeks | (the aerobic program potentiates both effects) | [165] |
| RA (n = 60) | 6-month foot reflexology trial | ↑ QoL and ↓ pain and fatigue (assessed FACIT-F, BPI-SF, and LupusQoL) | [166] |
| Patients with mild knee pain (n = 50) | 6-month krill oil supplementation | ↓ activity and symptoms (DAS28-ESR) | [167] |
| Disease (Number of Patients) | DMARD | Influence on Sleep Disorders | Ref. |
|---|---|---|---|
| RA (n = 288) | tocilizumab | ↓ the severity of sleep disorders ↑ sleep quality (evaluated with PSQI) | [168] |
| Healthy individuals (n = 79) | tocilizumab | Prevention of sleep problem development in healthy individuals | [169] |
| Ankylosing spondylitis (n = 60) | anti-TNF-alpha therapy | ↓ in disease activity and fatigue ↑ sleep quality (evaluated with PSQI) | [170] |
| RA (n = 35) | anti-TNF-alpha therapy | ↑ sleep quality (PSQI) | [171] |
| Treatment-resistant major depression with high inflammation (n = 36) | infliximab | ↑ sleep efficiency | [172] |
| Healthy (n = 16) | anakinra (IL-1 receptor antagonist) | ↓ postprandial fatigue (evaluated by Stanford Sleepiness Scale) | [173] |
6.1. Treatment of Coexisting Diseases with Sleep Disorders
6.2. Prevention
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| AHEI-10 | alternative healthy eating index—2010 |
| CBT | cognitive behavioral therapy |
| CD20 | cluster of differentiation 20 |
| CPAP | continuous positive airway pressure |
| CRP | C-reactive protein |
| CSF | cerebrospinal fluid |
| CTD | connective tissue diseases |
| DC | dendritic cell |
| DMARD | disease-modifying antirheumatic drugs |
| EDS | excessive daytime sleepiness |
| FACIT-F | functional assessment of chronic illness therapy-fatigue |
| FSS | fatigue severity scale |
| GC | glucocorticoids |
| GLP-1 | glucagon-like peptide-1 |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| HLA | human leukocyte antigen |
| HPA | axis hypothalamic–pituitary–adrenal axis |
| ICAM-1 | intercellular adhesion molecule 1 |
| IFN-γ | interferon gamma |
| IL | interleukin |
| IIM | idiopathic inflammatory myopathies |
| ISI | insomnia severity index |
| JAK-STAT | janus kinase–signal transducer and activator of transcription |
| LupusQoL | lupus quality of life |
| MTX | methotrexate |
| NO | nitric oxide |
| NREM | non-rapid eye movement |
| OSA | obstructive sleep apnea |
| OSAHS | obstructive sleep apnea/hypopnea syndrome |
| PM/DM | polymyositis/dermatomyositis |
| PSQI | Pittsburgh Sleep Quality Index |
| QoL | quality of life |
| RA | rheumatoid arthritis |
| RLS | restless leg syndrome |
| SAA | serum amyloid protein a |
| SLE | systemic lupus erythematosus |
| SSc | systemic sclerosis |
| SS | Sjögren’s syndrome |
| TNF-α | tumor necrosis factor alpha |
References
- Goldblatt, F.; O’Neill, S.G. Clinical Aspects of Autoimmune Rheumatic Diseases. Lancet 2013, 382, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Boeselt, T.; Koczulla, R.; Nell, C.; Beutel, B.; Guenter, K.; Cassel, W.; Hildebrandt, O.; Koehler, U.; Kroenig, J. Sleep and Rheumatic Diseases. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101434. [Google Scholar] [CrossRef]
- Castrejón, I.; Yazici, Y.; Samuels, J.; Luta, G.; Pincus, T. Discordance of Global Estimates by Patients and Their Physicians in Usual Care of Many Rheumatic Diseases: Association with 5 Scores on a Multidimensional Health Assessment Questionnaire (MDHAQ) That Are Not Found on the Health Assessment Questionnaire (HAQ). Arthritis Care Res. 2014, 66, 934–942. [Google Scholar] [CrossRef]
- Christie, A.P.; Abecasis, D.; Adjeroud, M.; Alonso, J.C.; Amano, T.; Anton, A.; Baldigo, B.P.; Barrientos, R.; Bicknell, J.E.; Buhl, D.A.; et al. Quantifying and Addressing the Prevalence and Bias of Study Designs in the Environmental and Social Sciences. Nat. Commun. 2020, 11, 6377. [Google Scholar] [CrossRef]
- Santilli, M.; Manciocchi, E.; D’Addazio, G.; Di Maria, E.; D’Attilio, M.; Femminella, B.; Sinjari, B. Prevalence of Obstructive Sleep Apnea Syndrome: A Single-Center Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 10277. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Huscher, D.; Callhoff, J.; Richter, J.G.; Alexander, T.; Henes, J.; Zink, A. Trends in Idiopathic Inflammatory Myopathies: Cross-Sectional Data from the German National Database. Rheumatol. Int. 2020, 40, 1639–1647. [Google Scholar] [CrossRef]
- Horsley-Silva, J.L.; Umar, S.B.; Vela, M.F.; Griffing, W.L.; Parish, J.M.; DiBaise, J.K.; Crowell, M.D. The Impact of Gastroesophageal Reflux Disease Symptoms in Scleroderma: Effects on Sleep Quality. Dis. Esophagus 2019, 32, doy136. [Google Scholar] [CrossRef] [PubMed]
- Bassel, M.; Hudson, M.; Taillefer, S.S.; Schieir, O.; Baron, M.; Thombs, B.D. Frequency and Impact of Symptoms Experienced by Patients with Systemic Sclerosis: Results from a Canadian National Survey. Rheumatology 2011, 50, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.F.; Allen, R.P.; Trevisani, V.M.F.; Toscano, V.G.; Earley, C.J. Sleep Disruption in Systemic Sclerosis (Scleroderma) Patients: Clinical and Polysomnographic Findings. Sleep Med. 2002, 3, 341–345. [Google Scholar] [CrossRef]
- Urashima, K.; Ichinose, K.; Kondo, H.; Maeda, T.; Kawakami, A.; Ozawa, H. The Prevalence of Insomnia and Restless Legs Syndrome among Japanese Outpatients with Rheumatic Disease: A Cross-Sectional Study. PLoS ONE 2020, 15, e0230273. [Google Scholar] [CrossRef]
- Katz, P.; Pedro, S.; Michaud, K. Sleep Disorders among Individuals with Rheumatoid Arthritis. Arthritis Care Res. 2022, 75, 1250–1260. [Google Scholar] [CrossRef]
- Crowson, C.S.; Gunderson, T.M.; Dykhoff, H.J.; Myasoedova, E.; Atkinson, E.J.; Kronzer, V.L.; Coffey, C.M.; Davis, J.M., III. Comprehensive Assessment of Multimorbidity Burden in a Population-Based Cohort of Patients with Rheumatoid Arthritis. RMD Open 2022, 8, e002022. [Google Scholar] [CrossRef] [PubMed]
- Nokes, B.T.; Raza, H.A.; Cartin-Ceba, R.; Lyng, P.J.; Krahn, L.E.; Wesselius, L.; Jokerst, C.E.; Umar, S.B.; Griffing, W.L.; Neville, M.R.; et al. Individuals With Scleroderma May Have Increased Risk of Sleep-Disordered Breathing. J. Clin. Sleep Med. 2019, 15, 1665–1669. [Google Scholar] [CrossRef]
- Gundogdu, S.; Borekci, S.; Atahan, E.; Musellim, B. Increased Frequency of Obstructive Sleep Apnea in the Patients with Systemic Sclerosis. Sleep Breath. 2021, 25, 237–242. [Google Scholar] [CrossRef]
- Ostojic, P.; Jovic, T.; Stojic, B. Restless Legs Syndrome in Patients with Systemic Sclerosis. Prevalence and Possible Causes. Z. Für Rheumatol. 2013, 72, 590–593. [Google Scholar] [CrossRef]
- Palagini, L.; Tani, C.; Bruno, R.M.; Gemignani, A.; Mauri, M.; Bombardieri, S.; Riemann, D.; Mosca, M. Poor Sleep Quality in Systemic Lupus Erythematosus: Does It Depend on Depressive Symptoms? Lupus 2014, 23, 1350–1357. [Google Scholar] [CrossRef]
- Sahebari, M.; Ravanshad, S.; Ravanshad, Y.; Rezaeitalab, F.; Bayegi, H.R.P.; Asadpour, H.; Javadinia, S.A.; Rezaieyazdi, Z. A Survey on Sleep Disorders and Related Hormones in Patients with Newly Diagnosed Systemic Lupus Erythematosus. Mediterr. J. Rheumatol. 2021, 32, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.R.; Green, S.L.; Trivedi, T.; Kwoh, C.K.; Utset, T.O. Correlates of Sleep Abnormalities in Systemic Lupus: A Cross-Sectional Survey in an Urban, Academic Center. J. Clin. Rheumatol. 2013, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Falup-Pecurariu, C.; Enache, A.; Duca, L.; Fotescu, C.; Falup-Pecurariu, O.; Monescu, V.; Diaconu, Ş.; Sirbu, C.A. Restless Legs Syndrome in Systemic Lupus Erythematosus: A Case-Control Study. Exp. Ther. Med. 2021, 22, 802. [Google Scholar] [CrossRef]
- Goulabchand, R.; Castille, E.; Navucet, S.; Etchecopar-Etchart, D.; Matos, A.; Maria, A.; Gutierrez, L.A.; Le Quellec, A.; de Champfleur, N.M.; Gabelle, A.; et al. The Interplay between Cognition, Depression, Anxiety, and Sleep in Primary Sjogren’s Syndrome Patients. Sci. Rep. 2022, 12, 13176. [Google Scholar] [CrossRef]
- Theander, L.; Strömbeck, B.; Mandl, T.; Theander, E. Sleepiness or Fatigue? Can We Detect Treatable Causes of Tiredness in Primary Sjögren’s Syndrome? Rheumatololgy 2010, 49, 1177–1183. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dopheide, J.A. Insomnia Overview: Epidemiology, Pathophysiology, Diagnosis and Monitoring, and Nonpharmacologic Therapy. Am. J. Manag. Care 2020, 26, S76–S84. [Google Scholar] [PubMed]
- Bjorvatn, B.; Wensaas, K.-A.; Emberland, K.E.; Fadnes, L.T.; Litleskare, S.; Diaz, E.; Ruths, S.; Rørtveit, G.; Waage, S. Restless legs syndrome—A study from general practice. Tidsskr. Den Nor. Legeforening 2021, 141. [Google Scholar] [CrossRef]
- Manson, J.J.; Isenberg, D.A. The Pathogenesis of Systemic Lupus Erythematosus. Neth. J. Med. 2003, 61, 343–346. [Google Scholar] [PubMed]
- Lin, Y.-J.; Anzaghe, M.; Schülke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef]
- Pollard, R.P.E.; Abdulahad, W.H.; Bootsma, H.; Meiners, P.M.; Spijkervet, F.K.L.; Huitema, M.G.; Burgerhof, J.G.M.; Vissink, A.; Kroese, F.G.M. Predominantly Proinflammatory Cytokines Decrease after B Cell Depletion Therapy in Patients with Primary Sjogren’s Syndrome. Ann. Rheum. Dis. 2013, 72, 2048–2050. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.; Rinetti, G.; Redwine, L.; Motivala, S.; Dang, J.; Ehlers, C. Nocturnal Proinflammatory Cytokine-Associated Sleep Disturbances in Abstinent African American Alcoholics. Brain Behav. Immun. 2004, 18, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Chung, J.W. Inflammatory Cytokines and Sleep Disturbance in Patients with Temporomandibular Disorders. J. Oral Facial Pain Headache 2016, 30, 27–33. [Google Scholar] [CrossRef]
- Alt, J.A.; Sautter, N.B.; Mace, J.C.; Detwiller, K.Y.; Smith, T.L. Antisomnogenic Cytokines, Quality of Life, and Chronic Rhinosinusitis: A Pilot Study. Laryngoscope 2014, 124, E107–E114. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Niu, J.-W.; Ge, W.; Chen, J.-T.; Yang, S.; Su, A.-X.; Feng, Y.-Z.; Wang, F.; Chen, G.; et al. Relationships Between a Range of Inflammatory Biomarkers and Subjective Sleep Quality in Chronic Insomnia Patients: A Clinical Study. Nat. Sci. Sleep 2021, 13, 1419–1428. [Google Scholar] [CrossRef]
- Taylor-Gjevre, R.M.; Nair, B.V.; Gjevre, J.A. Obstructive Sleep Apnoea in Relation to Rheumatic Disease. Rheumatology 2013, 52, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Campos-Rodriguez, F.; Cordero-Guevara, J.; Asensio-Cruz, M.I.; Sanchez-Armengol, A.; Sanchez-Lopez, V.; Arellano-Orden, E.; Gozal, D.; Martinez-Garcia, M.A. Interleukin 6 as a Marker of Depression in Women with Sleep Apnea. J. Sleep Res. 2021, 30, e13035. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Storari, M.; Scano, A.; Piras, V.; Taibi, R.; Viscuso, D. Obstructive Sleep Apnea, Oxidative Stress, Inflammation and Endothelial Dysfunction—An Overview of Predictive Laboratory Biomarkers. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6939–6948. [Google Scholar] [CrossRef] [PubMed]
- Wali, S.O.; Al-Mughales, J.; Alhejaili, F.; Manzar, M.D.; Alsallum, F.; Almojaddidi, H.; Gozal, D. The Utility of Proinflammatory Markers in Patients with Obstructive Sleep Apnea. Sleep Breath. Schlaf Atm. 2021, 25, 545–553. [Google Scholar] [CrossRef]
- Almendros, I.; Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Huang, L.; Akbarpour, M.; Andrade, J.; Farré, R.; Gozal, D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest 2016, 150, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Lin, H.-C. Obstructive Sleep Apnea and the Risk of Autoimmune Diseases: A Longitudinal Population-Based Study. Sleep Med. 2012, 13, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, K.D.; Mansukhani, M.P.; Silber, M.H.; Kolla, B.P. Excessive Daytime Sleepiness: A Clinical Review. Mayo Clin. Proc. 2021, 96, 1288–1301. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Tanaka, S.; Honda, M.; Toyoda, H.; Kodama, T. Increased Plasma IL-6, IL-8, TNF-Alpha, and G-CSF in Japanese Narcolepsy. Hum. Immunol. 2014, 75, 940–944. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.L.; Licinio, J.; Gold, P.W.; et al. Circadian Interleukin-6 Secretion and Quantity and Depth of Sleep. J. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Lal, C.; Weaver, T.E.; Bae, C.J.; Strohl, K.P. Excessive Daytime Sleepiness in Obstructive Sleep Apnea. Mechanisms and Clinical Management. Ann. Am. Thorac. Soc. 2021, 18, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, E.I.; Theologi, V.; Malakasioti, G.; Maragozidis, P.; Tsilioni, I.; Chrousos, G.; Gourgoulianis, K.; Kaditis, A.G. Obstructive Sleep Apnea, Excessive Daytime Sleepiness, and Morning Plasma TNF-α Levels in Greek Children. Sleep 2013, 36, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.P.; Serpero, L.D.; Barceló, A.; Barbé, F.; Agustí, A.; Gozal, D. Inflammatory Proteins in Patients with Obstructive Sleep Apnea with and without Daytime Sleepiness. Sleep Breath. 2007, 11, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Wang, L.; Andrews, N.; Tang, W.H.W.; Young, J.B.; Javaheri, S.; Foldvary-Schaefer, N. Dissociation of Objective and Subjective Daytime Sleepiness and Biomarkers of Systemic Inflammation in Sleep-Disordered Breathing and Systolic Heart Failure. J. Clin. Sleep Med. 2017, 13, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Serpero, L.D.; Kheirandish-Gozal, L.; Capdevila, O.S.; Gozal, D. TNF-α Gene Polymorphisms and Excessive Daytime Sleepiness in Pediatric Obstructive Sleep Apnea. J. Pediatr. 2011, 158, 77–82. [Google Scholar] [CrossRef]
- Behboudi, A.; Thelander, T.; Yazici, D.; Celik, Y.; Yucel-Lindberg, T.; Thunström, E.; Peker, Y. Association of TNF-α (-308G/A) Gene Polymorphism with Circulating TNF-α Levels and Excessive Daytime Sleepiness in Adults with Coronary Artery Disease and Concomitant Obstructive Sleep Apnea. J. Clin. Med. 2021, 10, 3413. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Gupta, V.; Chandra, H.; Dhyani, M.; Kotwal, A.; Verma, S.K.; Gupta, R. Serum Interleukin-6 Is Not Linked with Sleep-Quality, Restless Legs Syndrome, and Depression, But with 6-Month Survival in Hematological Malignancies. J. Neurosci. Rural Pract. 2019, 10, 94–100. [Google Scholar] [CrossRef]
- Uslu, F.I.; Demir, E.; Güler, E.M.; Koçyiğit, A. Circulating Levels of Cytokines Are Increased in Restless Legs Syndrome. Sleep Breath. 2021, 25, 1581–1585. [Google Scholar] [CrossRef]
- Trotti, L.M.; Rye, D.B.; De Staercke, C.; Hooper, W.C.; Quyyumi, A.; Bliwise, D.L. Elevated C-Reactive Protein Is Associated with Severe Periodic Leg Movements of Sleep in Patients with Restless Legs Syndrome. Brain. Behav. Immun. 2012, 26, 1239–1243. [Google Scholar] [CrossRef]
- Higuchi, T.; Abe, M.; Mizuno, M.; Yamazaki, T.; Suzuki, H.; Moriuchi, M.; Oikawa, O.; Okawa, E.; Ando, H.; Okada, K. Association of Restless Legs Syndrome with Oxidative Stress and Inflammation in Patients Undergoing Hemodialysis. Sleep Med. 2015, 16, 941–948. [Google Scholar] [CrossRef]
- Auvinen, P.; Mäntyselkä, P.; Koponen, H.; Kautiainen, H.; Korniloff, K.; Ahonen, T.; Vanhala, M. Elevation of Tumor Necrosis Factor Alpha Levels Is Associated with Restless Legs Symptoms in Clinically Depressed Patients. J. Psychosom. Res. 2018, 115, 1–5. [Google Scholar] [CrossRef]
- Hennessy, M.D.; Zak, R.S.; Gay, C.L.; Pullinger, C.R.; Lee, K.A.; Aouizerat, B.E. Polymorphisms of Interleukin-1 Beta and Interleukin-17Alpha Genes Are Associated with Restless Legs Syndrome. Biol. Res. Nurs. 2014, 16, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New Perspectives on the Role of Melatonin in Human Sleep, Circadian Rhythms and Their Regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Redwine, L.; Hauger, R.L.; Gillin, J.C.; Irwin, M. Effects of Sleep and Sleep Deprivation on Interleukin-6, Growth Hormone, Cortisol, and Melatonin Levels in Humans. J. Clin. Endocrinol. Metab. 2000, 85, 3597–3603. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, I.J.; Huang, C.-C.; Liu, S.-C.; Tang, C.-H. Reconsidering the Role of Melatonin in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Villaggio, B.; Candido, F.; Valenti, S.; Giusti, M.; Felli, L.; Sulli, A.; Accardo, S. Melatonin Influences Interleukin-12 and Nitric Oxide Production by Primary Cultures of Rheumatoid Synovial Macrophages and THP-1 Cells. Ann. N. Y. Acad. Sci. 1999, 876, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.; Chang, H.W.; Jung, H.-R.; Cho, C.-H.; Hur, J.-A.; Lee, S.-I.; Choi, T.H.; Kim, S.-H.; Ha, E. Melatonin Attenuates Clock Gene Cryptochrome1, Which May Aggravate Mouse Anti-Type II Collagen Antibody-Induced Arthritis. Rheumatol. Int. 2012, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Esalatmanesh, K.; Loghman, A.; Esalatmanesh, R.; Soleimani, Z.; Khabbazi, A.; Mahdavi, A.M.; Mousavi, S.G.A. Effects of Melatonin Supplementation on Disease Activity, Oxidative Stress, Inflammatory, and Metabolic Parameters in Patients with Rheumatoid Arthritis: A Randomized Double-Blind Placebo-Controlled Trial. Clin. Rheumatol. 2021, 40, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, H.; Elmeadawy, S.; Elshinnawi, U.; Anees, M. Is Dietary Melatonin Supplementation a Viable Adjunctive Therapy for Chronic Periodontitis?-A Randomized Controlled Clinical Trial. J. Periodontal Res. 2019, 54, 190–197. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, X.; Wei, M.; Yu, S.; Ding, Y.; Cheng, B. Melatonin Regulates the Immune Response and Improves Sjögren’s Syndrome-like Symptoms in NOD/Ltj Mice. Biochem. Pharmacol. 2022, 201, 115073. [Google Scholar] [CrossRef]
- Nabatian-Asl, M.; Ghorbanihaghjo, A.; Malek Mahdavi, A.; Khabbazi, A.; Hajialilo, M.; Ghojazadeh, M. Effects of Melatonin Supplementation on Serum Oxidative Stress Markers and Disease Activity in Systemic Lupus Erythematosus Patients: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Clin. Pract. 2021, 75, e14246. [Google Scholar] [CrossRef] [PubMed]
- Bhake, R.C.; Kluckner, V.; Stassen, H.; Russell, G.M.; Leendertz, J.; Stevens, K.; Linthorst, A.C.E.; Lightman, S.L. Continuous Free Cortisol Profiles-Circadian Rhythms in Healthy Men. J. Clin. Endocrinol. Metab. 2019, 104, 5935–5947. [Google Scholar] [CrossRef] [PubMed]
- Gumenyuk, V.; Roth, T.; Drake, C.L. Circadian Phase, Sleepiness, and Light Exposure Assessment in Night Workers with and without Shift Work Disorder. Chronobiol. Int. 2012, 29, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.A.; García, M.E.; Rodriguez, J.A.; Mardonez, G.; Jacobelli, S.; Rivero, S. Hypothalamic-Pituitary-Adrenal Axis Function in Patients with Active Rheumatoid Arthritis: A Controlled Study Using Insulin Hypoglycemia Stress Test and Prolactin Stimulation. J. Rheumatol. 1999, 26, 277–281. [Google Scholar] [PubMed]
- Johnson, E.O.; Kostandi, M.; Moutsopoulos, H.M. Hypothalamic-Pituitary-Adrenal Axis Function in Sjögren’s Syndrome: Mechanisms of Neuroendocrine and Immune System Homeostasis. Ann. N. Y. Acad. Sci. 2006, 1088, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Pham, G.S.; Mathis, K.W. Lipopolysaccharide Challenge Reveals Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Murine Systemic Lupus Erythematosus. Brain Sci. 2018, 8, 184. [Google Scholar] [CrossRef]
- Straub, R.H.; Detert, J.; Dziurla, R.; Fietze, I.; Loeschmann, P.-A.; Burmester, G.R.; Buttgereit, F. Inflammation Is an Important Covariate for the Crosstalk of Sleep and the HPA Axis in Rheumatoid Arthritis. Neuroimmunomodulation 2017, 24, 11–20. [Google Scholar] [CrossRef]
- Crofford, L.J.; Kalogeras, K.T.; Mastorakos, G.; Magiakou, M.A.; Wells, J.; Kanik, K.S.; Gold, P.W.; Chrousos, G.P.; Wilder, R.L. Circadian Relationships between Interleukin (IL)-6 and Hypothalamic-Pituitary-Adrenal Axis Hormones: Failure of IL-6 to Cause Sustained Hypercortisolism in Patients with Early Untreated Rheumatoid Arthritis. J. Clin. Endocrinol. Metab. 1997, 82, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Guyon, A.; Balbo, M.; Morselli, L.L.; Tasali, E.; Leproult, R.; L’Hermite-Balériaux, M.; Van Cauter, E.; Spiegel, K. Adverse Effects of Two Nights of Sleep Restriction on the Hypothalamic-Pituitary-Adrenal Axis in Healthy Men. J. Clin. Endocrinol. Metab. 2014, 99, 2861–2868. [Google Scholar] [CrossRef]
- Brady, E.M.; Bodicoat, D.H.; Hall, A.P.; Khunti, K.; Yates, T.; Edwardson, C.; Davies, M.J. Sleep Duration, Obesity and Insulin Resistance in a Multi-Ethnic UK Population at High Risk of Diabetes. Diabetes Res. Clin. Pract. 2018, 139, 195–202. [Google Scholar] [CrossRef]
- Freitas, W.R.; Oliveira, L.V.F.; Perez, E.A.; Ilias, E.J.; Lottenberg, C.P.; Silva, A.S.; Urbano, J.J.; Oliveira, M.C.; Vieira, R.P.; Ribeiro-Alves, M.; et al. Systemic Inflammation in Severe Obese Patients Undergoing Surgery for Obesity and Weight-Related Diseases. Obes. Surg. 2018, 28, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Yao-Borengasser, A.; Rasouli, N.; Bodles, A.M.; Phanavanh, B.; Lee, M.-J.; Starks, T.; Kern, L.M.; Spencer, H.J.; McGehee, R.E.; et al. Human Visfatin Expression: Relationship to Insulin Sensitivity, Intramyocellular Lipids, and Inflammation. J. Clin. Endocrinol. Metab. 2007, 92, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Covassin, N.; Singh, P.; McCrady-Spitzer, S.K.; St Louis, E.K.; Calvin, A.D.; Levine, J.A.; Somers, V.K. Effects of Experimental Sleep Restriction on Energy Intake, Energy Expenditure, and Visceral Obesity. J. Am. Coll. Cardiol. 2022, 79, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Dahani, A.; Arain, S.R.; Khan, F. Metabolic Syndrome In Young Rheumatoid Arthritis Patients. J. Ayub Med. Coll. Abbottabad 2020, 32, 318–322. [Google Scholar] [PubMed]
- Yottasan, P.; Kerr, S.J.; Veeravigrom, M.; Siripen, N.; Rianthavorn, P. Sleep Impairments and Quality of Life in Thai Adolescents with Systemic Lupus Erythematosus. J. Pediatr. Nurs. 2022, 67, e58–e64. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Saarinen, A.; Mikkola, T.M.; Tenhunen, J.; Martinmäki, S.; Rahikainen, A.; Cheng, S.; Eklund, N.; Pekkala, S.; Wiklund, P.; et al. Effects of Exercise and Diet Interventions on Obesity-Related Sleep Disorders in Men: Study Protocol for a Randomized Controlled Trial. Trials 2013, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Rochette, E.; Del Sordo, G.; Doré, É.; Merlin, É.; Walrand, S.; Duché, P. Eucaloric Balanced Diet Improved Objective Sleep in Adolescents with Obesity. Nutrients 2021, 13, 3550. [Google Scholar] [CrossRef] [PubMed]
- Guagnano, M.T.; D’Angelo, C.; Caniglia, D.; Di Giovanni, P.; Celletti, E.; Sabatini, E.; Speranza, L.; Bucci, M.; Cipollone, F.; Paganelli, R. Improvement of Inflammation and Pain after Three Months’ Exclusion Diet in Rheumatoid Arthritis Patients. Nutrients 2021, 13, 3535. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, Inflammation and Diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef]
- Vahid, F.; Bourbour, F.; Gholamalizadeh, M.; Shivappa, N.; Hébert, J.R.; Babakhani, K.; Mosavi Jarrahi, A.; Mirzaei Dahka, S.; Doaei, S. A Pro-Inflammatory Diet Increases the Likelihood of Obesity and Overweight in Adolescent Boys: A Case–Control Study. Diabetol. Metab. Syndr. 2020, 12, 29. [Google Scholar] [CrossRef]
- Kronholm, E.; Aunola, S.; Hyyppä, M.T.; Kaitsaari, M.; Koskenvuo, M.; Mattlar, C.E.; Rönnemaa, T. Sleep in Monozygotic Twin Pairs Discordant for Obesity. J. Appl. Physiol. 1996, 80, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.-C.E.; van Vliet, S.; Cao, C.; Patterson, B.W.; Reeds, D.N.; Laforest, R.; Gropler, R.J.; Ju, Y.-E.S.; Mittendorfer, B. Effect of Obstructive Sleep Apnea on Glucose Metabolism. Eur. J. Endocrinol. 2022, 186, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-S.; Park, S.-H. Metformin as a Treatment Strategy for Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 7231. [Google Scholar] [CrossRef] [PubMed]
- Gharib, M.; Elbaz, W.; Darweesh, E.; Sabri, N.A.; Shawki, M.A. Efficacy and Safety of Metformin Use in Rheumatoid Arthritis: A Randomized Controlled Study. Front. Pharmacol. 2021, 12, 726490. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.J.; Symmons, D.P.M.; Lunt, M.; Welch, A.; Luben, R.; Bingham, S.A.; Khaw, K.-T.; Day, N.E.; Silman, A.J. Dietary Risk Factors for the Development of Inflammatory Polyarthritis: Evidence for a Role of High Level of Red Meat Consumption. Arthritis Rheum. 2004, 50, 3804–3812. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Feng, M.; Zhang, X.; Jin, Y.-B.; Li, X.; Su, L.-C.; Liu, S.; Wang, A.-X.; Chen, X.-M.; et al. Dietary Intake and Risk of Rheumatoid Arthritis—A Cross Section Multicenter Study. Clin. Rheumatol. 2016, 35, 2901–2908. [Google Scholar] [CrossRef] [PubMed]
- Lana, A.; Struijk, E.A.; Arias-Fernandez, L.; Graciani, A.; Mesas, A.E.; Rodriguez-Artalejo, F.; Lopez-Garcia, E. Habitual Meat Consumption and Changes in Sleep Duration and Quality in Older Adults. Aging Dis. 2019, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.; Maras, J.E.; Shea, S.; Wood, A.C.; Castro-Diehl, C.; Johnson, D.A.; Huang, T.; Jacobs, D.R.; Crawford, A.; St-Onge, M.-P.; et al. Association between Diet Quality and Sleep Apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 42, zsy194. [Google Scholar] [CrossRef]
- Cheng, F.W.; Li, Y.; Winkelman, J.W.; Hu, F.B.; Rimm, E.B.; Gao, X. Probable Insomnia Is Associated with Future Total Energy Intake and Diet Quality in Men. Am. J. Clin. Nutr. 2016, 104, 462–469. [Google Scholar] [CrossRef]
- Charoenwoodhipong, P.; Harlow, S.D.; Marder, W.; Hassett, A.L.; McCune, W.J.; Gordon, C.; Helmick, C.G.; Barbour, K.E.; Wang, L.; Mancuso, P.; et al. Dietary Omega Polyunsaturated Fatty Acid Intake and Patient-Reported Outcomes in Systemic Lupus Erythematosus: The Michigan Lupus Epidemiology and Surveillance Program. Arthritis Care Res. 2020, 72, 874–881. [Google Scholar] [CrossRef]
- Tibuakuu, M.; Kamimura, D.; Kianoush, S.; DeFilippis, A.P.; Al Rifai, M.; Reynolds, L.M.; White, W.B.; Butler, K.R.; Mosley, T.H.; Turner, S.T.; et al. The Association between Cigarette Smoking and Inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) Study. PLoS ONE 2017, 12, e0184914. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.K.; Palmer, J.R.; Rosenberg, L.; McAlindon, T.E. Smoking, Alcohol Consumption, and Risk of Systemic Lupus Erythematosus in the Black Women’s Health Study. J. Rheumatol. 2003, 30, 1222–1226. [Google Scholar] [PubMed]
- Saevarsdottir, S.; Rezaei, H.; Geborek, P.; Petersson, I.; Ernestam, S.; Albertsson, K.; Forslind, K.; van Vollenhoven, R.F.; SWEFOT Study Group. Current Smoking Status Is a Strong Predictor of Radiographic Progression in Early Rheumatoid Arthritis: Results from the SWEFOT Trial. Ann. Rheum. Dis. 2015, 74, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Safy-Khan, M.; de Hair, M.J.H.; Welsing, P.M.J.; van Laar, J.M.; Jacobs, J.W.G.; Society for Rheumatology Research Utrecht (SRU). Current Smoking Negatively Affects the Response to Methotrexate in Rheumatoid Arthritis in a Dose-Responsive Way, Independently of Concomitant Prednisone Use. J. Rheumatol. 2021, 48, 1504–1507. [Google Scholar] [CrossRef] [PubMed]
- Schletzbaum, M.; Wang, X.; Greenlee, R.; Piper, M.E.; Bartels, C.M. Predictors of Smoking Cessation in Patients With Rheumatoid Arthritis in Two Cohorts: Most Predictive Health Care Factors. Arthritis Care Res. 2021, 73, 633–639. [Google Scholar] [CrossRef] [PubMed]
- AlRyalat, S.A.; Kussad, S.; El Khatib, O.; Hamad, I.; Al-Tanjy, A.; Alshnneikat, M.; AbuMahfouz, B. Assessing the Effect of Nicotine Dose in Cigarette Smoking on Sleep Quality. Sleep Breath. 2021, 25, 1319–1324. [Google Scholar] [CrossRef]
- Purani, H.; Friedrichsen, S.; Allen, A.M. Sleep Quality in Cigarette Smokers: Associations with Smoking-Related Outcomes and Exercise. Addict. Behav. 2019, 90, 71–76. [Google Scholar] [CrossRef]
- Peters, E.N.; Fucito, L.M.; Novosad, C.; Toll, B.A.; O’Malley, S.S. Effect of Night Smoking, Sleep Disturbance, and Their Co-Occurrence on Smoking Outcomes. Psychol. Addict. Behav. 2011, 25, 312–319. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Li, Q.-Y.; Zhang, X.-J. Interaction between Smoking and Obstructive Sleep Apnea: Not Just Participants. Chin. Med. J. 2012, 125, 3150–3156. [Google Scholar] [PubMed]
- Esen, A.D.; Akpinar, M. Relevance of Obstructive Sleep Apnea and Smoking: Obstructive Sleep Apnea and Smoking. Fam. Pract. 2021, 38, 181–186. [Google Scholar] [CrossRef]
- Stipelman, B.A.; Augustson, E.; McNeel, T. The Relationship among Smoking, Sleep, and Chronic Rheumatic Conditions Commonly Associated with Pain in the National Health Interview Survey. J. Behav. Med. 2013, 36, 539–548. [Google Scholar] [CrossRef]
- Reveille, J.D.; Moulds, J.M.; Ahn, C.; Friedman, A.W.; Baethge, B.; Roseman, J.; Straaton, K.V.; Alarcón, G.S. Systemic Lupus Erythematosus in Three Ethnic Groups: I. The Effects of HLA Class II, C4, and CR1 Alleles, Socioeconomic Factors, and Ethnicity at Disease Onset. LUMINA Study Group. Lupus in Minority Populations, Nature versus Nurture. Arthritis Rheum. 1998, 41, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- du Montcel, S.T.; Michou, L.; Petit-Teixeira, E.; Osorio, J.; Lemaire, I.; Lasbleiz, S.; Pierlot, C.; Quillet, P.; Bardin, T.; Prum, B.; et al. New Classification of HLA-DRB1 Alleles Supports the Shared Epitope Hypothesis of Rheumatoid Arthritis Susceptibility. Arthritis Rheum. 2005, 52, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Miglioranza Scavuzzi, B.; van Drongelen, V.; Kaur, B.; Fox, J.C.; Liu, J.; Mesquita-Ferrari, R.A.; Kahlenberg, J.M.; Farkash, E.A.; Benavides, F.; Miller, F.W.; et al. The Lupus Susceptibility Allele DRB1*03:01 Encodes a Disease-Driving Epitope. Commun. Biol. 2022, 5, 751. [Google Scholar] [CrossRef] [PubMed]
- Bolsius, Y.G.; Zurbriggen, M.D.; Kim, J.K.; Kas, M.J.; Meerlo, P.; Aton, S.J.; Havekes, R. The Role of Clock Genes in Sleep, Stress and Memory. Biochem. Pharmacol. 2021, 191, 114493. [Google Scholar] [CrossRef]
- Mahoney, C.E.; Cogswell, A.; Koralnik, I.J.; Scammell, T.E. The Neurobiological Basis of Narcolepsy. Nat. Rev. Neurosci. 2019, 20, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Akashiba, T.; Kurashina, K.; Otsuka, K.; Horie, T. Genetics and Obstructive Sleep Apnea Syndrome: A Study of Human Leukocyte Antigen (HLA) Typing. Intern. Med. 1993, 32, 94–97. [Google Scholar] [CrossRef][Green Version]
- Schenck, C.H.; Garcia-Rill, E.; Segall, M.; Noreen, H.; Mahowald, M.W. HLA Class II Genes Associated with REM Sleep Behavior Disorder. Ann. Neurol. 1996, 39, 261–263. [Google Scholar] [CrossRef]
- Wu, W.; Li, Z.; Tang, T.; Wu, J.; Liu, F.; Gu, L. 5-HTR2A and IL-6 Polymorphisms and Obstructive Sleep Apnea-Hypopnea Syndrome. Biomed. Rep. 2016, 4, 203–208. [Google Scholar] [CrossRef]
- Toor, B.; Ray, L.B.; Pozzobon, A.; Fogel, S.M. Sleep, Orexin and Cognition. Front. Neurol. Neurosci. 2021, 45, 38–51. [Google Scholar] [CrossRef]
- Martínez-Orozco, F.J.; Vicario, J.L.; Villalibre-Valderrey, I.; De Andrés, C.; Fernández-Arquero, M.; Peraita-Adrados, R. Narcolepsy with Cataplexy and Comorbid Immunopathological Diseases. J. Sleep Res. 2014, 23, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Inutsuka, A.; Yamashita, A.; Chowdhury, S.; Nakai, J.; Ohkura, M.; Taguchi, T.; Yamanaka, A. The Integrative Role of Orexin/Hypocretin Neurons in Nociceptive Perception and Analgesic Regulation. Sci. Rep. 2016, 6, 29480. [Google Scholar] [CrossRef] [PubMed]
- Jahangirvand, M.; Yazdi, F.; Moradi, M.; Haghparast, A. Intra-Accumbal Orexin-1 Receptors Are Involved in Antinociception Induced by Stimulation of the Lateral Hypothalamus in the Formalin Test as an Animal Model of Persistent Inflammatory Pain. Iran. J. Pharm. Res. IJPR 2016, 15, 851–859. [Google Scholar] [PubMed]
- Mohamed, A.R.; El-Hadidy, W.F. Effect of Orexin-A (Hypocretin-1) on Hyperalgesic and Cachectic Manifestations of Experimentally Induced Rheumatoid Arthritis in Rats. Can. J. Physiol. Pharmacol. 2014, 92, 813–820. [Google Scholar] [CrossRef]
- Sun, M.; Wang, W.; Li, Q.; Yuan, T.; Weng, W. Orexin A May Suppress Inflammatory Response in Fibroblast-like Synoviocytes. Biomed. Pharmacother. 2018, 107, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Miyamoto, M.; Miyamoto, T.; Matsubara, T.; Inoue, Y.; Iijima, M.; Mizuno, S.; Horie, J.; Hirata, K.; Shimizu, T.; et al. Cerebrospinal Fluid Orexin-A Levels in Systemic Lupus Erythematosus Patients Presenting with Excessive Daytime Sleepiness. Lupus 2018, 27, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Bårdsen, K.; Brede, C.; Kvivik, I.; Kvaløy, J.T.; Jonsdottir, K.; Tjensvoll, A.B.; Ruoff, P.; Omdal, R. Interleukin-1-Related Activity and Hypocretin-1 in Cerebrospinal Fluid Contribute to Fatigue in Primary Sjögren’s Syndrome. J. Neuroinflammation 2019, 16, 102. [Google Scholar] [CrossRef]
- Manglick, M.; Rajaratnam, S.M.; Taffe, J.; Tonge, B.; Melvin, G. Persistent Sleep Disturbance Is Associated with Treatment Response in Adolescents with Depression. Aust. N. Z. J. Psychiatry 2013, 47, 556–563. [Google Scholar] [CrossRef]
- Alfano, C.A.; Ginsburg, G.S.; Kingery, J.N. Sleep-Related Problems among Children and Adolescents with Anxiety Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 224–232. [Google Scholar] [CrossRef]
- Ryan, S.; McGuire, B. Psychological Predictors of Pain Severity, Pain Interference, Depression, and Anxiety in Rheumatoid Arthritis Patients with Chronic Pain. Br. J. Health Psychol. 2016, 21, 336–350. [Google Scholar] [CrossRef]
- Tench, C.; Bentley, D.; Vleck, V.; McCurdie, I.; White, P.; D’Cruz, D. Aerobic Fitness, Fatigue, and Physical Disability in Systemic Lupus Erythematosus. J. Rheumatol. 2002, 29, 474–481. [Google Scholar] [PubMed]
- Magro, R.; Borg, A.A. Characterisation of Patients with Systemic Lupus Erythematosus in Malta: A Population Based Cohort Cross-Sectional Study. BioMed Res. Int. 2018, 2018, 2385386. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xia, L.; Li, L.; Zhao, Q.; Chen, S.; Gu, Z. Anxiety and Depression in Primary Sjögren’s Syndrome: A Cross-Sectional Study. BMC Psychiatry 2018, 18, 131. [Google Scholar] [CrossRef]
- Enns, M.W.; Bernstein, C.N.; Kroeker, K.; Graff, L.; Walker, J.R.; Lix, L.M.; Hitchon, C.A.; El-Gabalawy, R.; Fisk, J.D.; Marrie, R.A. The Association of Fatigue, Pain, Depression and Anxiety with Work and Activity Impairment in Immune Mediated Inflammatory Diseases. PLoS ONE 2018, 13, e0198975. [Google Scholar] [CrossRef] [PubMed]
- Heffner, K.L.; France, C.R.; Ashrafioun, L.; Quiñones, M.; Walsh, P.; Maloney, M.D.; Giordano, B.D.; Pigeon, W.R. Clinical Pain-Related Outcomes and Inflammatory Cytokine Response to Pain Following Insomnia Improvement in Adults With Knee Osteoarthritis. Clin. J. Pain 2018, 34, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated Inflammatory Markers in Response to Prolonged Sleep Restriction Are Associated with Increased Pain Experience in Healthy Volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Griffin, S.C.; Ravyts, S.G.; Bourchtein, E.; Ulmer, C.S.; Leggett, M.K.; Dzierzewski, J.M.; Calhoun, P.S. Sleep Disturbance and Pain in U.S. Adults over 50: Evidence for Reciprocal, Longitudinal Effects. Sleep Med. 2021, 86, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Schütz, T.C.B.; Andersen, M.L.; Tufik, S. Sleep Alterations in an Experimental Orofacial Pain Model in Rats. Brain Res. 2003, 993, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Goes, A.C.J.; Reis, L.A.B.; Silva, M.B.G.; Kahlow, B.S.; Skare, T.L. Rheumatoid Arthritis and Sleep Quality. Rev. Bras. Reumatol. 2017, 57, 294–298. [Google Scholar] [CrossRef]
- Kasitanon, N.; Achsavalertsak, U.; Maneeton, B.; Wangkaew, S.; Puntana, S.; Sukitawut, W.; Louthrenoo, W. Associated Factors and Psychotherapy on Sleep Disturbances in Systemic Lupus Erythematosus. Lupus 2013, 22, 1353–1360. [Google Scholar] [CrossRef]
- Gudbjörnsson, B.; Broman, J.E.; Hetta, J.; Hällgren, R. Sleep Disturbances in Patients with Primary Sjögren’s Syndrome. Br. J. Rheumatol. 1993, 32, 1072–1076. [Google Scholar] [CrossRef]
- Boers, M.; Hartman, L.; Opris-Belinski, D.; Bos, R.; Kok, M.R.; Da Silva, J.A.; Griep, E.N.; Klaasen, R.; Allaart, C.F.; Baudoin, P.; et al. Low Dose, Add-on Prednisolone in Patients with Rheumatoid Arthritis Aged 65+: The Pragmatic Randomised, Double-Blind Placebo-Controlled GLORIA Trial. Ann. Rheum. Dis. 2022, 81, 925–936. [Google Scholar] [CrossRef]
- Curtis, J.R.; Westfall, A.O.; Allison, J.; Bijlsma, J.W.; Freeman, A.; George, V.; Kovac, S.H.; Spettell, C.M.; Saag, K.G. Population-Based Assessment of Adverse Events Associated with Long-Term Glucocorticoid Use. Arthritis Rheum. 2006, 55, 420–426. [Google Scholar] [CrossRef]
- Huscher, D.; Thiele, K.; Gromnica-Ihle, E.; Hein, G.; Demary, W.; Dreher, R.; Zink, A.; Buttgereit, F. Dose-Related Patterns of Glucocorticoid-Induced Side Effects. Ann. Rheum. Dis. 2009, 68, 1119–1124. [Google Scholar] [CrossRef]
- Jiang, Y.; Gen, N.; Wang, P.; Feng, N.; Lu, X. Prednisolone Induces Sleep Disorders via Inhibition of Melatonin Secretion by the Circadian Rhythm in Zebrafish. Biomed. Pharmacother. 2022, 147, 112590. [Google Scholar] [CrossRef]
- Galbo, H.; Kall, L. Circadian Variations in Clinical Symptoms and Concentrations of Inflammatory Cytokines, Melatonin, and Cortisol in Polymyalgia Rheumatica before and during Prednisolone Treatment: A Controlled, Observational, Clinical Experimental Study. Arthritis Res. Ther. 2016, 18, 174. [Google Scholar] [CrossRef]
- Arvidson, N.G.; Gudbjörnsson, B.; Larsson, A.; Hällgren, R. The Timing of Glucocorticoid Administration in Rheumatoid Arthritis. Ann. Rheum. Dis. 1997, 56, 27–31. [Google Scholar] [CrossRef]
- Alten, R.; Döring, G.; Cutolo, M.; Gromnica-Ihle, E.; Witte, S.; Straub, R.; Buttgereit, F. Hypothalamus-Pituitary-Adrenal Axis Function in Patients with Rheumatoid Arthritis Treated with Nighttime-Release Prednisone. J. Rheumatol. 2010, 37, 2025–2031. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W. Sleep Disorders in Patients with Psoriatic Arthritis and Psoriasis. Reumatologia 2018, 56, 301–306. [Google Scholar] [CrossRef]
- Gezer, O.; Batmaz, İ.; Sariyildiz, M.A.; Sula, B.; Ucmak, D.; Bozkurt, M.; Nas, K. Sleep Quality in Patients with Psoriatic Arthritis. Int. J. Rheum. Dis. 2017, 20, 1212–1218. [Google Scholar] [CrossRef]
- Guan, P.; Sun, C.; Chen, Z.; Chen, J.; Ran, R. Long-Term Hydroxychloroquine Therapy Improves the Quality of Sleep in Patients with Primary Sjögren’s Syndrome: A Real-World Study. Ann. Palliat. Med. 2020, 9, 2203–2210. [Google Scholar] [CrossRef]
- Omair, M.A.; Alahmadi, A.; Johnson, S.R. Safety and Effectiveness of Mycophenolate in Systemic Sclerosis. A Systematic Review. PLoS ONE 2015, 10, e0124205. [Google Scholar] [CrossRef]
- Casal-Dominguez, M.; Pinal-Fernandez, I.; Huapaya, J.; Albayda, J.; Paik, J.J.; Johnson, C.; Silhan, L.; Mammen, A.L.; Danoff, S.K.; Christopher-Stine, L. Efficacy and Adverse Effects of Methotrexate Compared with Azathioprine in the Antisynthetase Syndrome. Clin. Exp. Rheumatol. 2019, 37, 858–861. [Google Scholar]
- Wang, Y.; Lin, S.; Li, C.; Shi, Y.; Guan, W. Sleep Apnea-Hypopnea Syndrome Caused by Ankylosing Spondylitis: A Case Report. Medicine 2020, 99, e20055. [Google Scholar] [CrossRef]
- Nessaibia, I.; Siciliano, D.; Tahraoui, A. Why Nobody Discusses the Adverse Psychiatric Effects of Chloroquine in Case It Might Become the Future Treatment against COVID-19? Int. J. Health Plann. Manag. 2020, 35, 1311–1313. [Google Scholar] [CrossRef]
- Detert, J.; Dziurla, R.; Hoff, P.; Gaber, T.; Klaus, P.; Bastian, H.; Braun, T.; Schellmann, S.; Penzel, T.; Fietze, I.; et al. Effects of Treatment with Etanercept versus Methotrexate on Sleep Quality, Fatigue and Selected Immune Parameters in Patients with Active Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2016, 34, 848–856. [Google Scholar]
- Garber, K. Pfizer’s First-in-Class JAK Inhibitor Pricey for Rheumatoid Arthritis Market. Nat. Biotechnol. 2013, 31, 3–4. [Google Scholar] [CrossRef]
- Wang, F.; Lin, X.; Zhao, Q.; Li, J. Adverse Symptoms with Anti-TNF-Alpha Therapy in Inflammatory Bowel Disease: Systematic Review and Duration-Response Meta-Analysis. Eur. J. Clin. Pharmacol. 2015, 71, 911–919. [Google Scholar] [CrossRef]
- Kasi, P.M.; Tawbi, H.A.; Oddis, C.V.; Kulkarni, H.S. Clinical Review: Serious Adverse Events Associated with the Use of Rituximab—A Critical Care Perspective. Crit. Care 2012, 16, 231. [Google Scholar] [CrossRef]
- Ogata, A.; Kato, Y.; Higa, S.; Yoshizaki, K. IL-6 Inhibitor for the Treatment of Rheumatoid Arthritis: A Comprehensive Review. Mod. Rheumatol. 2019, 29, 258–267. [Google Scholar] [CrossRef]
- Kok, V.C.; Horng, J.-T.; Hung, G.-D.; Xu, J.-L.; Hung, T.-W.; Chen, Y.-C.; Chen, C.-L. Risk of Autoimmune Disease in Adults with Chronic Insomnia Requiring Sleep-Inducing Pills: A Population-Based Longitudinal Study. J. Gen. Intern. Med. 2016, 31, 1019–1026. [Google Scholar] [CrossRef]
- Rossman, J. Cognitive-Behavioral Therapy for Insomnia: An Effective and Underutilized Treatment for Insomnia. Am. J. Lifestyle Med. 2019, 13, 544–547. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European Guideline for the Diagnosis and Treatment of Insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Hartescu, I.; Morgan, K.; Stevinson, C.D. Increased Physical Activity Improves Sleep and Mood Outcomes in Inactive People with Insomnia: A Randomized Controlled Trial. J. Sleep Res. 2015, 24, 526–534. [Google Scholar] [CrossRef]
- Aibar-Almazán, A.; Hita-Contreras, F.; Cruz-Díaz, D.; de la Torre-Cruz, M.; Jiménez-García, J.D.; Martínez-Amat, A. Effects of Pilates Training on Sleep Quality, Anxiety, Depression and Fatigue in Postmenopausal Women: A Randomized Controlled Trial. Maturitas 2019, 124, 62–67. [Google Scholar] [CrossRef]
- Ward, L.; Stebbings, S.; Athens, J.; Cherkin, D.; David Baxter, G. Yoga for the Management of Pain and Sleep in Rheumatoid Arthritis: A Pilot Randomized Controlled Trial. Musculoskelet. Care 2018, 16, 39–47. [Google Scholar] [CrossRef]
- Gavilán-Carrera, B.; Vargas-Hitos, J.A.; Morillas-de-Laguno, P.; Rosales-Castillo, A.; Sola-Rodríguez, S.; Callejas-Rubio, J.L.; Sabio, J.M.; Soriano-Maldonado, A. Effects of 12-Week Aerobic Exercise on Patient-Reported Outcomes in Women with Systemic Lupus Erythematosus. Disabil. Rehabil. 2022, 44, 1863–1871. [Google Scholar] [CrossRef]
- Wu, M.-L.; Tsai, J.-C.; Yu, K.-H.; Chen, J.-J. Effects of Physical Activity Counselling in Women with Systemic Lupus Erythematosus: A Randomized Controlled Trial. Int. J. Nurs. Pract. 2019, 25, e12770. [Google Scholar] [CrossRef]
- Dardin, L.P.; Garcia, A.B.A.; Minali, P.A.; Pinto, A.C.P.N.; Trevisani, V.F.M. The Effects of Resistance Training in Patients with Primary Sjogren’s Syndrome. Clin. Rheumatol. 2022, 41, 1145–1152. [Google Scholar] [CrossRef]
- Al-Jiffri, O.H.; Abd El-Kader, S.M. Aerobic versus Resistance Exercises on Systemic Inflammation and Sleep Parameters in Obese Subjects with Chronic Insomnia Syndrome. Afr. Health Sci. 2021, 21, 1214–1222. [Google Scholar] [CrossRef]
- Khan, F.; Granville, N.; Malkani, R.; Chathampally, Y. Health-Related Quality of Life Improvements in Systemic Lupus Erythematosus Derived from a Digital Therapeutic Plus Tele-Health Coaching Intervention: Randomized Controlled Pilot Trial. J. Med. Internet Res. 2020, 22, e23868. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-Inflammatory Diet In Rheumatoid Arthritis (ADIRA)—A Randomized, Controlled Crossover Trial Indicating Effects on Disease Activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef]
- Davies, R.J.; Lomer, M.C.E.; Yeo, S.I.; Avloniti, K.; Sangle, S.R.; D’Cruz, D.P. Weight Loss and Improvements in Fatigue in Systemic Lupus Erythematosus: A Controlled Trial of a Low Glycaemic Index Diet versus a Calorie Restricted Diet in Patients Treated with Corticosteroids. Lupus 2012, 21, 649–655. [Google Scholar] [CrossRef]
- Hinkka, H.; Väättänen, S.; Ala-Peijari, S.; Nummi, T. Effects of Cold Mist Shower on Patients with Inflammatory Arthritis: A Crossover Controlled Clinical Trial. Scand. J. Rheumatol. 2017, 46, 206–209. [Google Scholar] [CrossRef][Green Version]
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A.-A. Effects of Curcumin Supplementation on Metabolic Parameters, Inflammatory Factors and Obesity Values in Women with Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 2022, 36, 1797–1806. [Google Scholar] [CrossRef]
- Bakir, E.; Baglama, S.S.; Gursoy, S. The Effects of Reflexology on Pain and Sleep Deprivation in Patients with Rheumatoid Arthritis: A Randomized Controlled Trial. Complement. Ther. Clin. Pract. 2018, 31, 315–319. [Google Scholar] [CrossRef]
- Suzuki, Y.; Fukushima, M.; Sakuraba, K.; Sawaki, K.; Sekigawa, K. Krill Oil Improves Mild Knee Joint Pain: A Randomized Control Trial. PLoS ONE 2016, 11, e0162769. [Google Scholar] [CrossRef]
- Bazzichi, L.; Nacci, F.; Sinigaglia, L.; Bianchino, L.; Caporali, R. Subcutaneous Tocilizumab Alone or with a csDMARD in Rheumatoid Arthritis Patients: Subanalysis of Italian Data from a Multicenter Phase IIIb/IV Trial. Clin. Rheumatol. 2019, 38, 841–849. [Google Scholar] [CrossRef]
- Hunt, C.A.; Smith, M.T.; Mun, C.J.; Irwin, M.R.; Finan, P.H. Trait Positive Affect Buffers the Association between Experimental Sleep Disruption and Inflammation. Psychoneuroendocrinology 2021, 129, 105240. [Google Scholar] [CrossRef]
- Coksevim, N.H.; Durmus, D.; Kuru, O. Effects of Global Postural Reeducation Exercise and Anti-TNF Treatments on Disease Activity, Function, Fatigue, Mobility, Sleep Quality and Depression in Patients with Active Ankylosing Spondylitis: A Prospective Follow-up Study. J. Back Musculoskelet. Rehabil. 2018, 31, 1005–1012. [Google Scholar] [CrossRef]
- Karatas, G.; Bal, A.; Yuceege, M.; Yalcin, E.; Firat, H.; Dulgeroglu, D.; Karataş, F.; Sahin, S.; Cakci, A.; Ardic, S. The Evaluation of Sleep Quality and Response to Anti-Tumor Necrosis Factor α Therapy in Rheumatoid Arthritis Patients. Clin. Rheumatol. 2017, 36, 45–50. [Google Scholar] [CrossRef]
- Weinberger, J.F.; Raison, C.L.; Rye, D.B.; Montague, A.R.; Woolwine, B.J.; Felger, J.C.; Haroon, E.; Miller, A.H. Inhibition of Tumor Necrosis Factor Improves Sleep Continuity in Patients with Treatment Resistant Depression and High Inflammation. Brain. Behav. Immun. 2015, 47, 193–200. [Google Scholar] [CrossRef]
- Lehrskov, L.L.; Dorph, E.; Widmer, A.M.; Hepprich, M.; Siegenthaler, J.; Timper, K.; Donath, M.Y. The Role of IL-1 in Postprandial Fatigue. Mol. Metab. 2018, 12, 107–112. [Google Scholar] [CrossRef]
- Kapsimalis, F.; Richardson, G.; Opp, M.R.; Kryger, M. Cytokines and Normal Sleep. Curr. Opin. Pulm. Med. 2005, 11, 481–484. [Google Scholar] [CrossRef]
- Kushikata, T.; Fang, J.; Wang, Y.; Krueger, J.M. Interleukin-4 Inhibits Spontaneous Sleep in Rabbits. Am. J. Physiol. 1998, 275, R1185–R1191. [Google Scholar] [CrossRef]
- Ni, G.; Wang, T.; Walton, S.; Zhu, B.; Chen, S.; Wu, X.; Wang, Y.; Wei, M.Q.; Liu, X. Manipulating IL-10 Signalling Blockade for Better Immunotherapy. Cell. Immunol. 2015, 293, 126–129. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Baerwald, C.; Ablin, J.; Häuser, W. Efficacy, Tolerability and Safety of Cannabinoids in Chronic Pain Associated with Rheumatic Diseases (Fibromyalgia Syndrome, Back Pain, Osteoarthritis, Rheumatoid Arthritis): A Systematic Review of Randomized Controlled Trials. Schmerz 2016, 30, 47–61. [Google Scholar] [CrossRef]
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef]
- Kaur, T.; Shyu, B.-C. Melatonin: A New-Generation Therapy for Reducing Chronic Pain and Improving Sleep Disorder-Related Pain. In Advances in Pain Research: Mechanisms and Modulation of Chronic Pain; Shyu, B.-C., Tominaga, M., Eds.; Springer: Singapore, 2018; pp. 229–251. ISBN 9789811317569. [Google Scholar]
- Andersen, L.P.H.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Tasali, E.; Wroblewski, K.; Kahn, E.; Kilkus, J.; Schoeller, D.A. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults With Overweight in Real-Life Settings: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 365–374. [Google Scholar] [CrossRef]
- Gudbergsen, H.; Henriksen, M.; Wæhrens, E.E.; Overgaard, A.; Bliddal, H.; Christensen, R.; Boesen, M.P.; Knop, F.K.; Astrup, A.; Rasmussen, M.U.; et al. Effect of Liraglutide on Body Weight and Pain in Patients with Overweight and Knee Osteoarthritis: Protocol for a Randomised, Double-Blind, Placebo-Controlled, Parallel-Group, Single-Centre Trial. BMJ Open 2019, 9, e024065. [Google Scholar] [CrossRef]
- Meurot, C.; Martin, C.; Sudre, L.; Breton, J.; Bougault, C.; Rattenbach, R.; Bismuth, K.; Jacques, C.; Berenbaum, F. Liraglutide, a Glucagon-like Peptide 1 Receptor Agonist, Exerts Analgesic, Anti-Inflammatory and Anti-Degradative Actions in Osteoarthritis. Sci. Rep. 2022, 12, 1567. [Google Scholar] [CrossRef]
- Berenbaum, F.; Meurot, C.; Sudre, L.; Bismuth, K.; Rattenbach, R.; Denefle, P.; Martin, C.; Jacques, C. Pos0373 Liraglutide Has Potent Anti-Inflammatory and Anti-Catabolic in Vitro Activities in Osteoarthritis. Ann. Rheum. Dis. 2021, 80, 417. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cholerzyńska, H.; Zasada, W.; Tselios, K.; Grygiel-Górniak, B. Sleep Disorders in Connective Tissue Diseases—Coexisting Diseases or Disease Components? J. Clin. Med. 2024, 13, 3656. https://doi.org/10.3390/jcm13133656
Cholerzyńska H, Zasada W, Tselios K, Grygiel-Górniak B. Sleep Disorders in Connective Tissue Diseases—Coexisting Diseases or Disease Components? Journal of Clinical Medicine. 2024; 13(13):3656. https://doi.org/10.3390/jcm13133656
Chicago/Turabian StyleCholerzyńska, Hanna, Wiktoria Zasada, Konstantinos Tselios, and Bogna Grygiel-Górniak. 2024. "Sleep Disorders in Connective Tissue Diseases—Coexisting Diseases or Disease Components?" Journal of Clinical Medicine 13, no. 13: 3656. https://doi.org/10.3390/jcm13133656
APA StyleCholerzyńska, H., Zasada, W., Tselios, K., & Grygiel-Górniak, B. (2024). Sleep Disorders in Connective Tissue Diseases—Coexisting Diseases or Disease Components? Journal of Clinical Medicine, 13(13), 3656. https://doi.org/10.3390/jcm13133656






