Impact of Upadacitinib on Atopic Keratoconjunctivitis Exacerbated by Dupilumab Treatment in Atopic Dermatitis Patients: A Prospective Dermatological and Ophthalmological Clinical Evaluation in Common Clinical Practice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
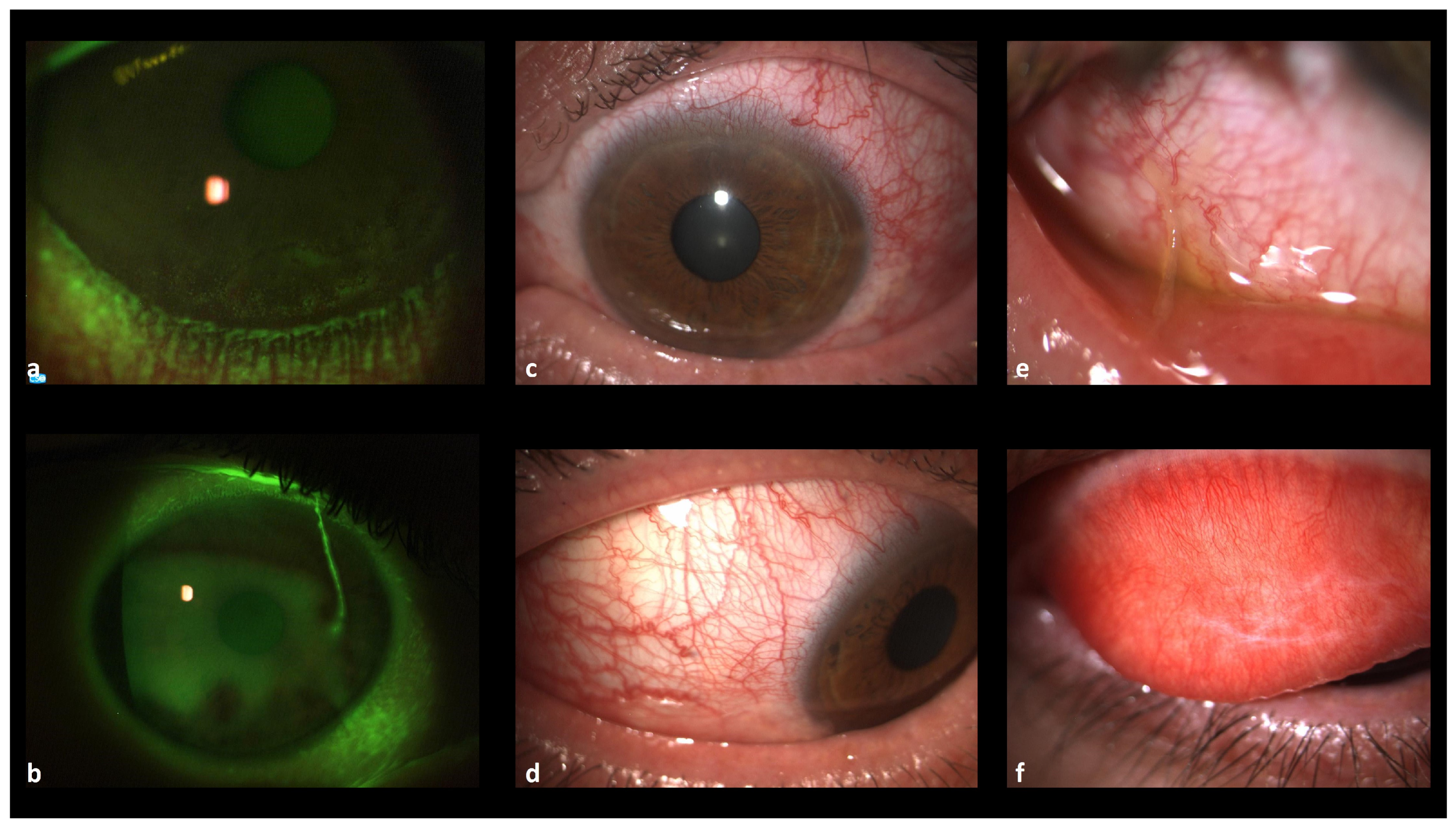
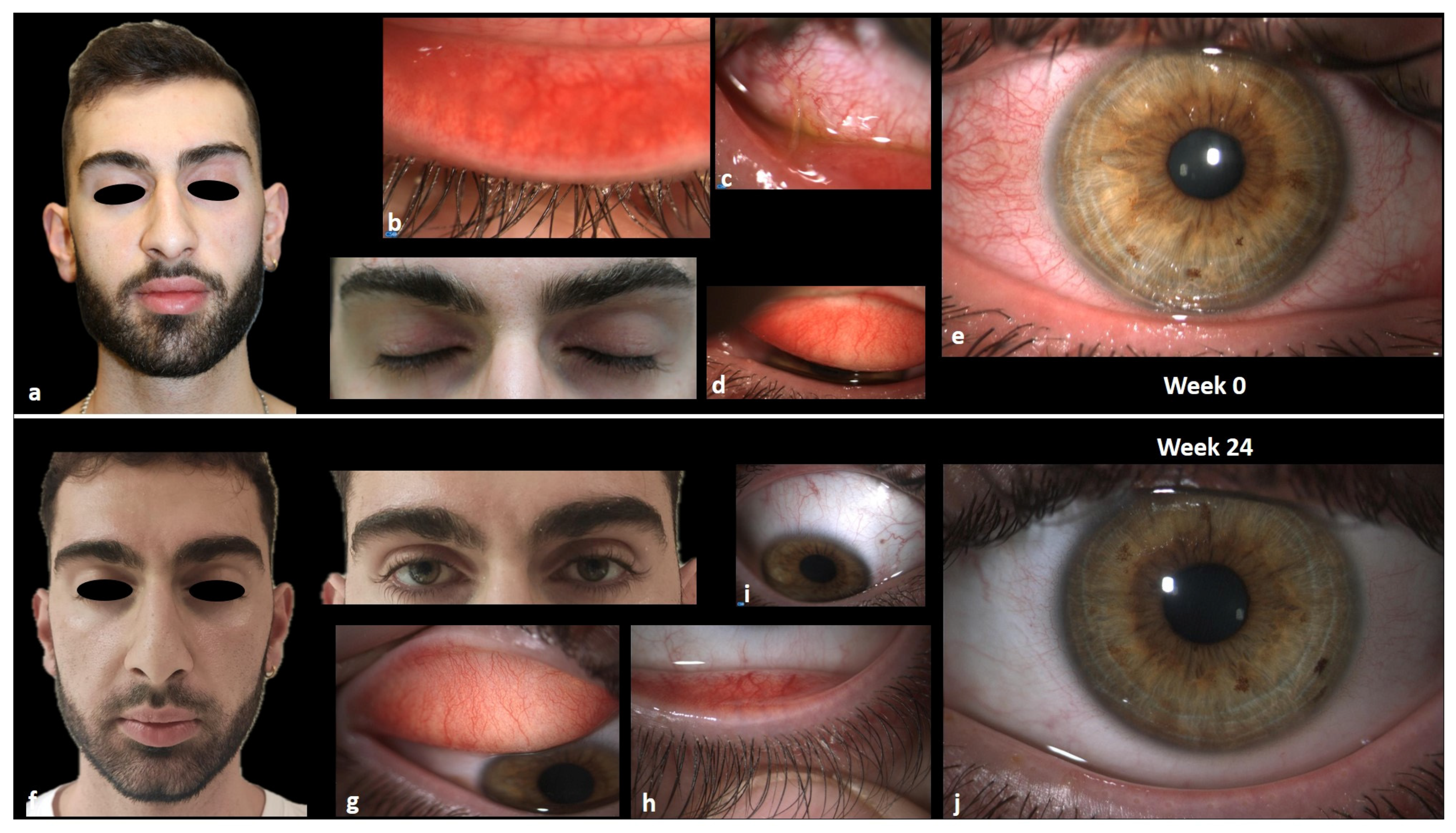
3.1. Clinical Cases
3.1.1. Case 1
3.1.2. Case 2
3.1.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomsen, S.F. Atopic dermatitis: Natural history, diagnosis, and treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Cooper, K.D.; Ho, V.C.; Kang, S.; Krafchik, B.R.; Margolis, D.J.; Schachner, L.A.; Sidbury, R.; Whitmore, S.E.; Sieck, C.K.; et al. Guidelines of care for atopic dermatitis. J. Am. Acad. Dermatol. 2004, 50, 391–404. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Toft, P.B.; Halling-Overgaard, A.-S.; Gislason, G.H.; Skov, L.; Egeberg, A. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J. Am. Acad. Dermatol. 2017, 77, 280–286.e1. [Google Scholar]
- Thyssen, J.; Heegaard, S.; Ivert, L.; Remitz, A.; Agner, T.; Bruin-Weller, M.; Huldt-Nystrøm, T.; Korhonen, L.; Leinonen, P.; Mandelin, J.; et al. Management of Ocular Manifestations of Atopic Dermatitis: A Consensus Meeting Using a Modified Delphi Process. Acta Derm. Venereol. 2020, 100, adv00264. [Google Scholar] [CrossRef] [PubMed]
- Guin, J.D. Eyelid dermatitis: Experience in 203 cases. J. Am. Acad. Dermatol. 2002, 47, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Applebaum, D.S.; Sun, G.S.; Pflugfelder, S.C. Atopic keratoconjunctivitis: A review. J. Am. Acad. Dermatol. 2014, 70, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.; McDonald, J.; Papp, K. Diagnosis and Management of Conjunctivitis for the Dermatologist. J. Cutan. Med. Surg. 2018, 22, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Hanifin, J.M.; Luger, T.A.; Stevens, S.R.; Pride, H.B. Consensus Conference on Pediatric Atopic Dermatitis. J. Am. Acad. Dermatol. 2003, 49, 1088–1095. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Dart, J.K.; Calder, V. Atopic keratoconjunctivitis and atopic dermatitis. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 478–485. [Google Scholar] [CrossRef]
- Miyazaki, D.; Takamura, E.; Uchio, E.; Ebihara, N.; Ohno, S.; Ohashi, Y.; Okamoto, S.; Satake, Y.; Shoji, J.; Namba, K.; et al. Japanese guidelines for allergic conjunctival diseases 2020. Allergol. Int. 2020, 69, 346–355. [Google Scholar]
- Villegas, B.V.; Benitez-Del-Castillo, J.M. Current Knowledge in Allergic Conjunctivitis. Turk. J. Ophthalmol. 2021, 51, 45–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bharali, A.; Deka, B.; Sarma, H.; Sarma, S.; Ahmed, A.; Bhattacharjee, B.; Das, G.; Das, B.; Upadhyaya, M.; Phukan, M.; et al. Integrating Recommendations to Improve Treatment Outcomes in the Clinical Management of Allergic Conjunctivitis. Pharm. Biosci. J. 2021, 9, 22–40. [Google Scholar] [CrossRef]
- Bonini, S.; Coassin, M.; Aronni, S.; Lambiase, A. Vernal keratoconjunctivitis. Eye 2004, 18, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Bielory, B.; Bielory, L. Atopic dermatitis and keratoconjunctivitis. Immunol. Allergy Clin. N. Am. 2010, 30, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jiang, L.; Morrow, N.C.; Avdic, A.; Fairley, J.A.; Ling, J.J.; Greiner, M.A. Recognition of atopic keratoconjunctivitis during treatment with dupilumab for atopic dermatitis. J Am. Acad. Dermatol. 2021, 85, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J. Atopic keratoconjunctivitis. Am. J. Ophthalmol. 1953, 36, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Gambardella, A.; Licata, G.; Di Brizzi, E.V.; Alfano, R.; Argenziano, G. Dupilumab and conjunctivitis: A case series of twenty patients. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e612–e614. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, D.; Fukushima, A.; Uchio, E.; Shoji, J.; Namba, K.; Ebihara, N.; Takamura, E.; Fukuda, K.; Matsuda, A.; Okamoto, S.; et al. Executive summary: Japanese guidelines for allergic conjunctival diseases 2021. Allergol. Int. 2022, 71, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.M.; Seitzman, G.D.; Yang, E.J.; Sanchez, I.M.; Liao, W. Ocular Co-Morbidities of Atopic Dermatitis. Part I: Associated Ocular Diseases. Am. J. Clin. Dermatol. 2019, 20, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, A.S.; Speedwell, L.; Nischal, K.K. Unilateral keratoconus in a child with chronic and persistent eye rubbing. Am. J. Ophthalmol. 2005, 139, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Najmi, H.; Mobarki, Y.; Mania, K.; Altowairqi, B.; Basehi, M.; Mahfouz, M.S.; Elmahdy, M. The correlation between keratoconus and eye rubbing: A review. Int. J. Ophthalmol. 2019, 12, 1775–1781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahebjada, S.; Al-Mahrouqi, H.H.; Moshegov, S.; Panchatcharam, S.M.; Chan, E.; Daniell, M.; Baird, P.N. Eye rubbing in the aetiology of keratoconus: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M.; Priglinger, S.G.; Kassumeh, S. Aktuelles zur Keratoconjunctivitis vernalis und atopica [Current aspects of vernal and atopic keratoconjunctivitis]. Ophthalmologie 2024, 121, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.F.; Jan, R.L.; Wang, J.J.; Tseng, S.H.; Chang, Y.S. Association between atopic keratoconjunctivitis and the risk of keratoconus. Acta Ophthalmol. 2021, 99, e54–e61. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.H.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Gallelli, L.; Terracciano, R.; Navalesi, P.; Maselli, R.; Pelaia, G. Dupilumab for the treatment of asthma. Expert Opin. Biol. Ther. 2017, 17, 1565–1572. [Google Scholar] [CrossRef]
- Schmieder, G.; Draelos, Z.; Pariser, D.; Banfield, C.; Cox, L.; Hodge, M.; Kieras, E.; Parsons-Rich, D.; Menon, S.; Salganik, M.; et al. Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: Phase II, randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2018, 179, 54–62. [Google Scholar] [CrossRef]
- Parmentier, J.M.; Voss, J.; Graff, C.; Schwartz, A.; Argiriadi, M.; Friedman, M.; Camp, H.S.; Padley, R.J.; George, J.S.; Hyland, D.; et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2018, 2, 23. [Google Scholar] [CrossRef]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef]
- Sweeney, S.E.; Firestein, G.S. Primer: Signal transduction in rheumatic disease--a clinician’s guide. Nat. Clin. Pract. Rheumatol. 2007, 3, 651–660. [Google Scholar] [CrossRef]
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 2014, 57, 5023–5038. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef]
- Bohner, A.; Topham, C.; Strunck, J.; Haynes, D.; Brazil, M.; Clements, J.; Simpson, E.; Winston, C. Dupilumab-Associated Ocular Surface Disease: Clinical Characteristics, Treatment, and Follow-Up. Cornea 2021, 40, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Neagu, N.; Dianzani, C.; Avallone, G.; Dell’aquila, C.; Morariu, S.; Zalaudek, I.; Conforti, C. Dupilumab ocular side effects in patients with atopic dermatitis: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 820–835. [Google Scholar] [CrossRef]
- Halling, A.-S.; Loft, N.; Silverberg, J.I.; Guttman-Yassky, E.; Thyssen, J.P. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2021, 84, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Fachler, T.; Shreberk-Hassidim, R.; Molho-Pessach, V. Dupilumab-induced ocular surface disease: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Hayama, K.; Fujita, H. Improvement of dupilumab-associated conjunctivitis after switching to upadacitinib in a patient with atopic dermatitis. Dermatol. Ther. 2022, 35, e15575. [Google Scholar] [CrossRef]
- Gelato, F.; Mastorino, L.; Quaglino, P.; Cavaliere, G.; Ortoncelli, M.; Ribero, S. Ocular Adverse Events in Patients With Atopic Dermatitis Treated With Upadacitinib: A Real-Life Experience. Dermatitis 2023, 34, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Kamata, M.; Tada, Y. A Literature Review of Real-World Effectiveness and Safety of Dupilumab for Atopic Dermatitis. JID Innov. 2021, 1, 100042. [Google Scholar]
- Muzumdar, S.; Skudalski, L.; Sharp, K.; Waldman, R.A. Dupilumab Facial Redness/Dupilumab Facial Dermatitis: A Guide for Clinicians. Am. J. Clin. Dermatol. 2022, 23, 61–67. [Google Scholar] [CrossRef]
- Galluzzo, M.; Tofani, L.; Spelta, S.; Talamonti, M.; Micera, A.; Bianchi, L.; Coassin, M.; Bonini, S.; Di Zazzo, A. Dupilumab-associated ocular surface disease or atopic keratoconjunctivitis not improved by dupilumab? Upadacitinib may clarify the dilemma: A case report. Ski. Health Dis. 2024, 4, e354. [Google Scholar] [CrossRef]
- Foley, P.; Kerdraon, Y.A.; Hogden, J.P.; Shumack, S.; Spelman, L.; Sebaratnam, D.F.; Su, C.S.; Katelaris, C.H. Dupilumab-associated ocular surface disease: An interdisciplinary decision framework for prescribers in the Australian setting. Australas. J. Dermatol. 2022, 63, 421–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lapp, T.; Mann, C.; Jakob, T.; Reinhard, T.; Maier, P.C. Atopic Keratoconjunctivitis: Pathophysiology, Clinic, and Potential New Therapeutic Concepts. Klin. Monatsblatter Augenheilkd. 2024, 241, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute. Attività dei Comitati Etici Istituiti ai Sensi del Decreto Ministeriale 18 Marzo 1998. 2002. Available online: https://www.aifa.gov.it/documents/20142/0/Cir_Min_2_Settembre_2002_CE.pdf (accessed on 3 March 2023).
- Sánchez-Hernández, M.C.; Navarro, A.M.; Colás, C.; Del Cuvillo, A.; Sastre, J.; Mullol, J.; Valero, A. Validation of the DECA criteria for allergic conjunctivitis severity and control. Clin. Transl. Allergy. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ESPRINT Study Group and Investigators; Valero, A.; Alonso, J.; Antepara, I.; Baró, E.; Colas, C.; del Cuvillo, A.; Ferrer, M.; Herdman, M.; Martí-Guadaño, E.; et al. Development and validation of a new Spanish instrument to measure health-related quality of life in patients with allergic rhinitis: The ESPRINT questionnaire. Value Health 2007, 10, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.S.; Rice, B.A.; Dutt, J.E. Immunopathology of atopic keratoconjunctivitis. Ophthalmology 1991, 98, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Fregona, I.A.; Plebani, M.; Secchi, A.G.; Calder, V.L. Th1-and Th2-type cytokines in chronic ocular allergy. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Licata, G.; Tancredi, V.; Pezzolo, E.; Pertusi, G.; Tolino, E.; Arisi, M.; Gambardella, A. Efficacy and safeness of tralokinumab in patients with atopic dermatitis who developed conjunctivitis under dupilumab: A case series. J. Eur. Acad. Dermatol. Venereol. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiricozzi, A.; Di Nardo, L.; Gori, N.; Antonelli, F.; Pinto, L.; Cuffaro, G.; Piro, G.; Savino, G.; Tortora, G.; Peris, K. Dupilumab-associated ocular adverse events are predicted by low tear break-up time and correlate with high IL-33 tear concentrations in patients with atopic dermatitis. Exp. Dermatol. 2023, 32, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Thormann, K.; Lüthi, A.S.; Deniau, F.; Heider, A.; Cazzaniga, S.; Radonjic-Hoesli, S.; Lehmann, M.; Schlapbach, C.; Herzog, E.L.; Kreuzer, M.; et al. Dupilumab-associated ocular surface disease is characterized by a shift from Th2/Th17 toward Th1/Th17 inflammation. Allergy 2024, 79, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.N.; Gooderham, M.J.; Beecker, J.R.; Hong, C.H.; Jack, C.S.; Jain, V.; Lansang, P.; Lynde, C.W.; Papp, K.A.; Prajapati, V.H.; et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1135–1148. [Google Scholar] [CrossRef]






| Sex | Age | Age of Onset AD | Duration of AD | AD Clinical Phenotype | Head and Neck Involvement | Duration of Treatment with Dupilumab (Months) | Elevated IgE (>ULN) | EASI Baseline Dupilumab | EASI Baseline Upadacitinib | Itch-NRS Baseline-Upadacitinib | Sleep-NRS Baseline-Upadacitinib | EASI W12 Upadacitinib | Itch-NRS W12 Upadacitinib | Sleep-NRS W12 Upadacitinib | EASI W24 Upadacitinib | Itch-NRS W24 Upadacitinib | Sleep-NRS W24 Upadacitinib | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | M | 25 | 5 | 20 | 2 | Yes | 9 | No | 30 | 15 | 7 | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient 2 | F | 54 | 1 | 53 | 3 | Yes | 39 | No | 25 | 16 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient 3 | M | 24 | 16 | 9 | 4 | Yes | 36 | Yes | 40 | 15 | 10 | 4 | 4 | 4 | 0 | 0 | 2 | 0 |
| Patient 4 | M | 19 | 15 | 3 | 1 | Yes | 29 | Yes | 25 | 15 | 10 | 10 | 0 | 4 | 0 | 3 | 5 | 0 |
| Patient 5 | F | 43 | 0 | 43 | 4 | Yes | 6 | No | 40 | 30 | 10 | 10 | 8 | 8 | 7 | 5 | 3 | 1 |
| Patient 6 | M | 42 | 19 | 23 | 1 | Yes | 29 | Yes | 52 | 30 | 8 | 7 | 0 | 0 | 0 | 2 | 3 | 0 |
| W0 | W12 | W24 | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MF | CNV | CH | TP | TF | BUT | NEI | CO | MF | CNV | CH | TP | TF | BUT | NEI | CO | MF | CNV | CH | TP | TF | BUT | NEI | CO | |||||||||||||||||||||||||
| RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | RE | LE | |
| Patient 1 | 1 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 5 | 6 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 3 | 0 | 0 | 0 | 0 |
| Patient 2 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 | 3 | 0 | 0 | 0 | 0 |
| Patient 3 | 1 | 1 | 1 | 1 | 4 | 4 | 2 | 2 | 1 | 1 | 12 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 13 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 10 | 8 | 0 | 0 | 0 | 0 |
| Patient 4 | 1 | 1 | 1 | 1 | 4 | 4 | 3 | 3 | 0 | 0 | 2 | 4 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 0 | 0 | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 7 | 8 | 1 | 0 | 0 | 0 |
| Patient 5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | 6 | 0 | 0 | 0 | 0 |
| Patient 6 | 1 | 1 | 3 | 2 | 3 | 3 | 1 | 1 | 1 | 1 | 3 | 4 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 5 | 6 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 | 7 | 0 | 0 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paganini, C.; Spelta, S.; Tofani, L.; Talamonti, M.; Bianchi, L.; Coassin, M.; Di Zazzo, A.; Galluzzo, M. Impact of Upadacitinib on Atopic Keratoconjunctivitis Exacerbated by Dupilumab Treatment in Atopic Dermatitis Patients: A Prospective Dermatological and Ophthalmological Clinical Evaluation in Common Clinical Practice. J. Clin. Med. 2024, 13, 3818. https://doi.org/10.3390/jcm13133818
Paganini C, Spelta S, Tofani L, Talamonti M, Bianchi L, Coassin M, Di Zazzo A, Galluzzo M. Impact of Upadacitinib on Atopic Keratoconjunctivitis Exacerbated by Dupilumab Treatment in Atopic Dermatitis Patients: A Prospective Dermatological and Ophthalmological Clinical Evaluation in Common Clinical Practice. Journal of Clinical Medicine. 2024; 13(13):3818. https://doi.org/10.3390/jcm13133818
Chicago/Turabian StylePaganini, Claudia, Sara Spelta, Lorenzo Tofani, Marina Talamonti, Luca Bianchi, Marco Coassin, Antonio Di Zazzo, and Marco Galluzzo. 2024. "Impact of Upadacitinib on Atopic Keratoconjunctivitis Exacerbated by Dupilumab Treatment in Atopic Dermatitis Patients: A Prospective Dermatological and Ophthalmological Clinical Evaluation in Common Clinical Practice" Journal of Clinical Medicine 13, no. 13: 3818. https://doi.org/10.3390/jcm13133818
APA StylePaganini, C., Spelta, S., Tofani, L., Talamonti, M., Bianchi, L., Coassin, M., Di Zazzo, A., & Galluzzo, M. (2024). Impact of Upadacitinib on Atopic Keratoconjunctivitis Exacerbated by Dupilumab Treatment in Atopic Dermatitis Patients: A Prospective Dermatological and Ophthalmological Clinical Evaluation in Common Clinical Practice. Journal of Clinical Medicine, 13(13), 3818. https://doi.org/10.3390/jcm13133818









